Abstract
Small intestinal bacterial overgrowth (SIBO) can partly explain irritable bowel syndrome (IBS), and rifaximin has been observed to improve abdominal symptoms in nonconstipated IBS patients. However, there are few reports on the association of the rifaximin treatment periods with the results of a lactulose breath test (LBT). Therefore, we performed a retrospective review of patient charts to investigate the relation between the rifaximin treatment periods with LBT results in nonconstipated IBS patients. We also evaluated the time to achieve a symptomatic improvement in the IBS patients as compared to the changes in the LBT. We reviewed the charts for patients who showed IBS symptoms with documented positive results for LBT during their initial visit and who had a follow-up LBT after treatment with rifaximin. The LBT values were compared to the subjects' symptom scores. A total of 102 subjects had a follow-up LBT to assess LBT normalization. The subjects were divided into groups according to treatment periods of 4 weeks (n = 36), 8 weeks (n = 43), and 12 weeks (n = 23). The groups with a longer treatment exhibited an increase in the hydrogen gas value at 90 min and its sum during 90 min at the initial LBT. There were significant differences in hydrogen gas value at 90 min and in its sum during 90 min at the initial LBT between the groups treated for 4 and 12 weeks. The most significant treatment response was observed during the first 4 weeks for all treatment groups. Symptomatic improvement occurred earlier than LBT normalization in the treatment period over 4 weeks. The results indicate that different rifaximin treatment periods are needed in accordance with LBT levels to effectively eradicate SIBO.
Graphical Abstract
Keywords: Irritable Bowel Syndrome, Small Intestinal Bacterial Overgrowth, Lactulose Breath Test, Rifaximin
INTRODUCTION
Irritable bowel syndrome (IBS) is characterized by changes in the bowel habits and abdominal discomfort or pain without organic disease. Primary care physicians and gastroenterologists commonly encounter IBS, and the prevalence has been estimated to be as high as 12% during visits to a primary care physician and 28% on referrals to a gastroenterologist (1). Although the precise etiology of IBS is still unknown, emerging evidence suggests that gut flora have a pathological role (2). The corollary to this theory is that small intestinal bacterial overgrowth (SIBO) can partly explain IBS (3). SIBO is in fact associated with IBS like symptoms, such as bloating, abdominal pain, and a change in bowel habits (4).
The major treatment for IBS includes life style modification and symptomatic control, but many studies have demonstrated that rifaximin improved patients' abdominal symptoms in nonconstipated IBS with a SIBO origin (2, 5, 6). Rifaximin is highly active against enteric bacteria, including anaerobes, due to the lack of intestinal absorption (7), and it is reportedly without side effects and is suitable to treat acute as well as chronic conditions (8). Recent randomized double blind studies have shown that rifaximin resulted in a greater improvement in abdominal symptoms and produced fewer side effects in nonconstipated IBS subjects (5, 6).
Prior studies on rifaximin in nonconstipated IBS patients with SIBO indicated that the rifaximin treatment period was of only 7-14 days in most cases (2, 8, 9), regardless of results of a lactulose breath test (LBT). In addition, a prospective large-scale study may also have difficulty in determining treatment durations according to the LBT values and not according to the symptoms. Furthermore, such a prospective study would be not easy since follow-up tests and long-term compliance would be difficult to achieve. In our practice, we have used rifaximin in nonconstipated IBS patients with SIBO since 2012, and we now report on our experience with the treatment period to manage abnormal LBT results with rifaximin.
In this study, we performed a retrospective chart review to investigate the appropriate treatment period in accordance with the LBT level in nonconstipated IBS patients with SIBO. In addition, this study compared the timing of the improvements in abdominal symptoms and the results of the LBT. Furthermore, we examined whether the gas levels assessed via LBT had any relation with global abdominal symptoms.
MATERIALS AND METHODS
Patients
Patients with abdominal symptoms, such as bloating, abdominal pain or discomfort, and abnormal bowel habit changes who visited the Health Promotion Center and the department of family medicine at Ajou University Hospital, Suwon, Korea from September 2012 through December 2013 were selected as the subjects of this study. The subjects were identified through an electronic search of the scheduling database of the clinic for the period between September 2012 and December 2013. We included subjects aged over 20 yr who had undergone gastroduodenoscopy, colonoscopy, and abdomen ultrasonography within the previous 2 yr as well as those with current IBS symptoms (assessed according to the Rome III diagnostic criteria for IBS) (10) at the time of the hospital visit.
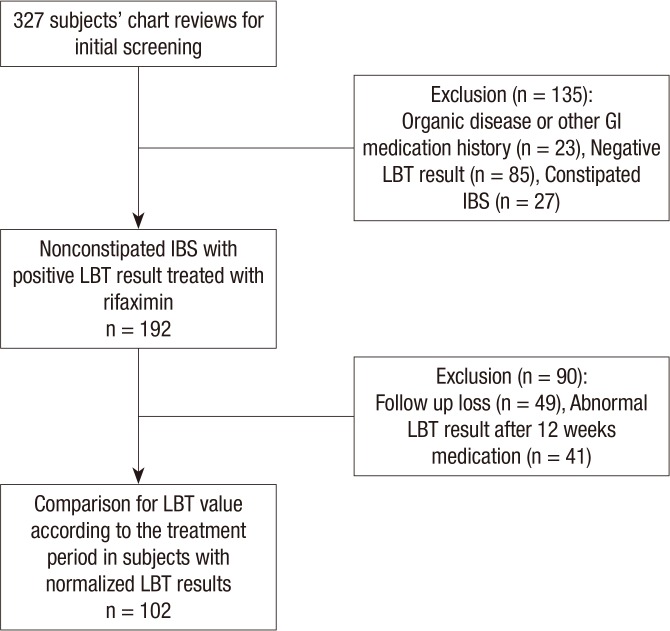
A total of 327 subjects with IBS symptoms had an LBT, and these subjects were included if they were described as having diarrhea, or mixed IBS and were excluded if they had history of, or symptoms consistent with, constipation-predominant IBS. We also excluded subjects with a history of other gastrointestinal disorders (such as inflammatory bowel disease (IBD) or peptic ulcer disease), allergic reaction to rifaximin, or who for 1 month prior to the study used antibiotics, probiotics or any other drug that could influence bowel function. In addition, patients with evidence of other organic, metabolic or psychiatric diseases that could impact the patient's compliance and those with poor medication compliance were also excluded. We selected 192 nonconstipated IBS patients with documented positive results for LBT during the initial visit and 102 subjects had negative results during the follow-up LBT after treatment with rifaximin (1,200 mg/day) (Fig. 1).
Fig. 1. Flow diagram of subject inclusion and exclusion for this study. LBT, Lactulose breath test.
Lactulose breath test
LBT was performed as follows: after measuring the baseline breath sample, the subjects ingested 10 g of lactulose (10 g lactulose in 15 mL water solution). The breath samples were then measured thrice at 20-min (min) intervals during the first 1 hr and 15-min interval 4 times during the following 1 hr. The samples were analyzed for hydrogen gas (H2) and methane gas (CH4), using a model Breath Tracker SC Quintron gas chromatograph (Quintron Instrument Company, Milwaukee, WI, USA) and the results were noted recorded in parts per million (ppm). A normal LBT was defined as 1) the baseline level at under 20 ppm or 2) the absence of a >20 ppm rise in H2 or CH4 excretion over the baseline within 90 min. A subsequent gradual rise in H2 or CH4 was considered to be physiological given the non-absorbable nature of lactulose, its expected colonic fermentation, and the normal 90-100 min orocecal transit time in humans (6, 11, 12).
The patient's baseline information included age, sex, bowel symptoms, medical history and global abdominal symptoms on a 7-point Likert scale (0, hardly any; 1, hardly; 2, somewhat; 3, moderately; 4, a good deal; 5, a great deal; and 6, a very great deal), and stool formation was checked using the Bristol stool scales. We classified subjects into 2 groups according to stool formation on the first visit to the hospital. The Likert score and Bristol stool scales were recorded for every patient visit. The date of every rifaximin (Normix, Alfa Wassermann, Bologna, Italy) treatment and LBT were recorded.
Study design
We investigated 1) the duration of effective treatment for SIBO patients, as assessed by the LBT level; and 2) the improvement in global symptoms reported by the subjects. If the abdominal symptoms at follow up decreased relative to those during the initial visit, we assessed the response to treatment via the subjects' symptom score. Although patients who had received initial rifaximin reported an improvement in abdominal symptoms, they underwent a follow-up LBT to assess the normalization. The subjects typically received an initial treatment with rifaximin for 4 weeks and subsequently retook an LBT every 4 weeks until it was normalized with maintenance medication. Even though an abnormal LBT could still be observed after 3 months of rifaximin treatment in some subjects with IBS, treatment was discontinued at this time since most patients felt improvements in their abdominal symptoms. Therefore, the maximal treatment duration was of 12 weeks. The 3 groups were then divided according to treatment duration: 4 weeks, 8 weeks, and 12 weeks.
Statistic analysis
The data were analyzed in order to determine adequate relief of global IBS symptoms during every visit, and the duration to achieve success for the treatment (defined as the duration in weeks between treatment periods with rifaximin) with a normalized LBT result. Statistical analyses were performed to compare the mean value of the initial LBT according to the treatment duration. We used a chi-square test to compare categorical variables and a t-test to compare continuous variables. We used a one-way ANOVA test to compare among treatment groups. All analyses were carried out in IBM SPSS 19.0 (IBM, Chicago, IL, USA).
Ethics statements
This study was approved by the institutional review board of the Ajou University Hospital (IRB No. MED-MDB-14-313). Informed consent was waived by the board.
RESULTS
A total of 102 subjects had normalized LBT results and symptomatic improvement. Of them, 36 received treatment for 4 weeks, 43 for 8 weeks, and 23 for 12 weeks. Fifty-eight subjects (57.4%) were male and 44 were females, and the mean age was 46.62± 10.91 yr (Table 1). Most subjects had suffered from their symptoms for more than 2 yr (2.8±1.5, range 5 months to 9 yr). Major symptoms included abdominal pain or discomfort, bloating, and diarrhea in both sexes. Bloating (93.2%), and fatigue (51.9%) were more frequent in females and abdominal pain or discomfort (88.2%), and diarrhea (77.6%) were more frequent in males (Table 2).
Table 1. General characteristics of the subjects according to treatment period.
| Parameters | 4 weeks | 8 weeks | 12 weeks |
|---|---|---|---|
| No. (%) of subjects | 36 (35) | 43 (42) | 23 (23) |
| Sex (M/F) | 23/13 | 27/16 | 8/15 |
| Age (yr)* | 46.53 ± 10.05 | 47.51 ± 8.88 | 45.05 ± 15.40 |
*There was no statistical difference in age among groups.
No., number; M, male; F, female.
Table 2. Major symptoms of subjects by gender.
| Symptoms | Male (n = 58) | Female (n = 44) |
|---|---|---|
| Abdominal pain/discomfort [n, (%)] | 51 (88.2) | 38 (86.4) |
| Stool formation [n, (%)] | ||
| Diarrhea | 45 (77.6) | 32 (72.7) |
| Mixed | 13 (22.4) | 12 (27.3) |
| Bloating [n, (%)] | 54 (92.4) | 41 (93.2) |
| Fatigue [n, (%)] | 25 (42.6) | 23 (51.9) |
n, number.
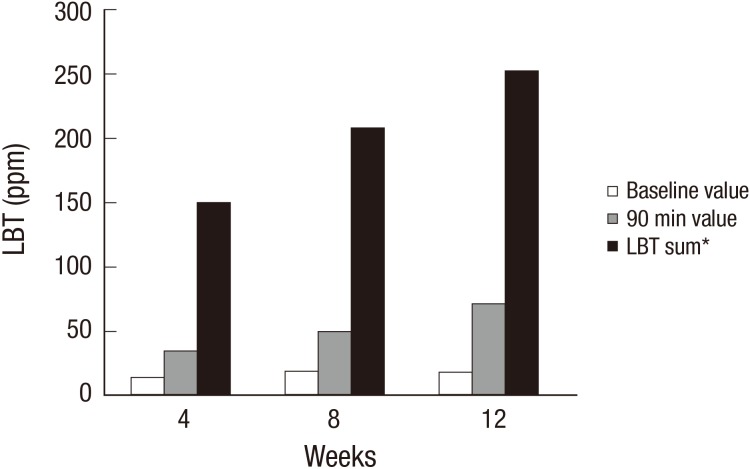
The mean H2 values from the LBT results during the initial examination of the 3 groups are shown in Fig. 2. The mean baseline values of H2 were 14.47±16.44 ppm (4 weeks), 19.30±21.85 ppm (8 weeks) and 18.10±16.70 ppm (12 weeks), and those at 90 min were 35.27±15.28 ppm (4 weeks), 50.47±24.47 ppm (8 weeks) and 71.85±40.33 ppm (12 weeks). The sum of the H2 values in the LBT during 90 min were 150.61±81.55 ppm (4 weeks), 208.81±110.92 ppm (8 weeks) and 253.35±141.00 ppm (12 weeks). The results of the ANOVA indicated that the H2 values at 90 min and the sum of the H2 values in the LBT during 90 min were statistically significant (P value<0.001), but the baseline group was not (P value=0.039). A post hoc analysis between the treatment period for the 4 weeks group and the 12 weeks groups were statistically significant in terms of the H2 value at 90 min and its sum during 90 min. With respect to methanogenic infections, only 5 of the 102 nonconstipated IBS subjects had an abnormal CH4 gas result. Of these subjects, 2 patients were from the 4 weeks group, 1 from 8 weeks and 3 from 12 weeks. After treatment with rifaximin, the methoanogenic infection was normalized in 4 weeks for all groups.
Fig. 2. Mean values of the lactulose breath test (LBT) according to treatment duration. Between the group treated for 4 weeks group and that for 12 weeks, statistically significant differences were found in the hydrogen value at 90 min (P < 0.05) and its sum (P < 0.05). The baseline values were not significantly different among all groups. *LBT sum means a sum of lactulose hydrogen breath test data during 90 min.
A symptomatic evaluation of the 8 and 12 weeks treatment period groups showed improvement earlier than the time at which the LBT values had normalized (Table 3). Most of the subjects exhibited noticeably improved abdominal symptoms after 4 weeks of treatment. The most significant treatment response was observed in the first 4 weeks and the symptoms gradually improved during the treatment period. A correlation analysis between the initial subjective symptoms and LBT results showed no significant relationship between the global symptoms and the LBT values during the initial visit (data not shown). On the other hand, the 102 subjects of this study confirmed normalization in their LBT results, and 41 subjects had abnormal LBT results after 12 weeks of medication. However, relative to the initial results, the results at the 12 weeks follow-up showed a decrease in the LBT values and in the symptom scores in these groups with an abnormal LBT.
Table 3. Relationship between personal symptom score and treatment period.
| Treatment duration | Stool type | Initial visit | 4 weeks | 8 weeks | 12 weeks | P* | |
|---|---|---|---|---|---|---|---|
| 4 weeks group | Diarrhea | Likert score | 2.4 ± 1.0 | 4.6 ± 1.1 | - | - | <0.05 |
| Bristol scale | 6.1 ± 0.8 | 4.3 ± 0.9 | - | - | <0.05 | ||
| Mixed | Likert score | 2.2 ± 0.8 | 5.1 ± 0.7 | - | - | <0.05 | |
| Bristol scale | 2.3 ± 0.8 | 4.1 ± 0.8 | - | - | <0.05 | ||
| 8 weeks group | Diarrhea | Likert score | 2.2 ± 1.2 | 4.3 ± 1.1 | 5.1 ± 0.8 | - | <0.05 |
| Bristol scale | 6.3 ± 0.7 | 4.1 ± 1.3 | 3.9 ± 1.0 | - | <0.05 | ||
| Mixed | Likert score | 2.0 ± 0.9 | 4.3 ± 1.2 | 5.2 ± 1.0 | - | <0.05 | |
| Bristol scale | 2.0 ± 1.1 | 3.7 ± 0.9 | 3.9 ± 0.7 | - | <0.05 | ||
| 12 weeks group | Diarrhea | Likert score | 1.9 ± 1.1 | 4.2 ± 1.3 | 4.8 ± 1.2 | 5.3 ± 1.5 | <0.05 |
| Bristol scale | 6.4 ± 1.2 | 4.9 ± 1.1 | 4.5 ± 1.5 | 4.2 ± 1.1 | <0.05 | ||
| Mixed | Likert score | 1.8 ± 0.9 | 3.9 ± 0.9 | 4.5 ± 1.3 | 5.4 ± 0.9 | <0.05 | |
| Bristol scale | 2.8 ± 0.9 | 3.2 ± 1.1 | 3.4 ± 1.3 | 4.9 ± 0.9 | <0.05 |
*There were statistically significant between initial and last scores.
DISCUSSION
Previous studies have shown that rifaximin can effectively improve abdominal symptoms, such as bowel habit changes or bloating (6, 13). However, few studies had investigated the treatment period to manage abnormal LBT results with rifaximin. In general, a 7-14 day treatment period has been noted in prior studies independently of the LBT values. Our data showed that higher LBT values were related to longer treatment durations, and an adequate treatment period was also effective in improving abdominal symptoms in all groups, as assessed using ROME III classifications, while subjective symptoms showed an improvement earlier than objective results.
Up to 25% of individuals suffer from IBS symptoms, and the prevalence rate is increasing worldwide (14). Patients with functional colonic symptoms showed improved responses to rifaximin with relief of their chronic gas-related symptoms and improvement in LBT values, which had been reported in a previous study (8). Rifaximin is a rifamycin derivative with antibacterial activity caused by the inhibition of bacterial RNA synthesis. Physicians use rifaximin for SIBO patients since it is effective against both gram-positive and -negative bacteria, including aerobes and anaerobes, and acts topically in the gut lumen against bacterial overgrowth (15, 16).
In this study, rifaximin was given to IBS patients for up to 12 weeks. This is a remarkable treatment period in that it is difficult for researchers to perform a long term antibiotic treatment in IBS subjects. The results indicated that even when treatment duration for a patient receiving rifaximin was long, the effectiveness was sustained. In addition, this study suggest that subjects do not develop clinical resistance because after 12 weeks of medication, even subjects with abnormal LBT results had a decrease in their LBT values and symptom scores relative to the initial values or scores. If resistance were developed, then it would be reasonable to presume that subsequent treatments would be less and less effective.
As previously stated, rifaximin is a critical treatment method for SIBO. The treatment duration as well as compliance by the patient are important factors that must be considered. We classified the treatment groups at 4 week intervals and compared the mean LBT value and global GI symptom between the groups. The present study found that higher LBT values were associated with longer treatment durations for LBT normalization. However, the gas levels assessed with the LBT were not associated with the subject's global abdominal symptoms although prior studies documented that methane levels were associated with the severe GI symptoms (17,18,19). In addition, the initial global symptom scores among the groups according to the treatment periods were not significantly different in our data. These discrepancies may be a result of variations in the inclusion criteria for the subjects, the sample size, and study design. Furthermore, an assessment of the patients' global symptoms, and not the individual symptoms (bloating, nausea, vomiting and pain, etc.) may have contributed to the lack of significant differences among the groups with different treatment periods. However, we did find that elevated LBT values were associated with a longer treatment duration.
The abdominal symptoms reported by the patients improved earlier than objective measurements. Although we cannot rule out a placebo effect for the improvement in early symptoms, if patients' abdominal symptoms are relatively subdued after taking medication, patients may not find it necessary to contact the doctor. When abdominal symptoms affect daily life or exceed the tolerance threshold of the individual, then the patients will seek medical attention again, which could contribute to the incidence of IBS relapse. In addition, when rifaximin treatment is administered in response to IBS symptoms, a premature termination of the treatment can happen while SIBO still exists. Indeed, two weeks of rifaximin leads to frequent relapse of symptoms, possibly due to an incomplete treatment for SIBO (2). Even when the current abdominal symptoms improve, the symptoms can recur at a later time. Thus, long-term tracking is required in order to further investigate such occurrences.
This study had several limitations. First, the study is retrospective, but this is balanced by some strengths of our study. This study represents actual clinical experience with rifaximin use during a long period of time, whereas previous similar studies only considered a short period of time. In addition, it would be difficult to conduct and retain study participants in a long-term antibiotic treatment protocol. Thus, this chart review represents rather, how rifaximin treatment worked in a clinical practice over a long period of time. Second, we included subjects who had shown good compliance with medication and complete abdominal symptom relief, and a physician determined the treatment end-point. Another limitation was that some patients were lost during treatment before follow-up due to 1) symptoms significantly improving after medication use or 2) symptoms not improving as expected and the patient not feeling the need for hospital care. It was difficult to determine whether such interruptions where due to whether or not patients felt relief. However, our study had a high follow-up rate (71.3%), meaning that the influence of such cases was negligible. Third, even when LBT results had not normalized, rifaximin was not used for more than 12 weeks. This was due to prior studies that rifaximin resistance may potentially develop outside the gastrointestinal tract through the long term use of rifaximin (20, 21). A high prevalence of Mycobacterium tuberculosis has been continually reported in Korea, hence physicians should pay attention to drug resistance. A further limitation of our study is related to the ongoing controversy regarding the validity and interpretation of the LBT in SIBO. The problems inherent to LBT include difficulty in distinguishing SIBO from rapid intestinal transit where similar gas production patterns were observed (22). In fact, it has been suggested that LBT positivity in IBS patients may be related to rapid intestinal transit, and not SIBO (23).
In conclusion, we here evaluated the appropriate treatment period with rifaximin in nonconstipated IBS patients with SIBO in accordance with their LBT levels. The clinical chart review of subjects with rifaximin-mediated eradication of bacterial overgrowth in the gut demonstrated that different treatment periods with rifaximin, according to LBT levels, were needed to improve IBS and normalize LBT. These findings suggest that the adjustment of the treatment period might be considered to provide more efficient management of SIBO symptoms.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceived and designed the study: Kim KN, Bae SH. Data review and analysis: Lee KJ, Bae SH. Writing the first draft: Kim KN, Bae SH, Lee KJ, Kim YS. Agree with manuscript results: Kim YS. Reviewed and approved the final version of the manuscript: KN Kim, SH Bae, KJ Lee, YS Kim.
References
- 1.Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology. 1987;92:1282–1284. doi: 10.1016/s0016-5085(87)91099-7. [DOI] [PubMed] [Google Scholar]
- 2.Pimentel M, Morales W, Chua K, Barlow G, Weitsman S, Kim G, Amichai MM, Pokkunuri V, Rook E, Mathur R, et al. Effects of rifaximin treatment and retreatment in nonconstipated IBS subjects. Dig Dis Sci. 2011;56:2067–2072. doi: 10.1007/s10620-011-1728-5. [DOI] [PubMed] [Google Scholar]
- 3.Pyleris E, Giamarellos-Bourboulis E, Koussoulas B, Barbatzas C. Prevalence of small intestinal bacterial overgrowth in a Greek cohort: relationship with irritable bowel syndrome. Barcelona, Spain: United European Gastroenterology Week; 2010. pp. 23–27. [Google Scholar]
- 4.Van Citters GW, Lin HC. Management of small intestinal bacterial overgrowth. Curr Gastroenterol Rep. 2005;7:317–320. doi: 10.1007/s11894-005-0025-x. [DOI] [PubMed] [Google Scholar]
- 5.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hoover WW, Gerlach EH, Hoban DJ, Eliopoulos GM, Pfaller MA, Jones RN. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn Microbiol Infect Dis. 1993;16:111–118. doi: 10.1016/0732-8893(93)90004-q. [DOI] [PubMed] [Google Scholar]
- 8.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Lauritano EC, Gabrielli M, Lupascu A, Santoliquido A, Nucera G, Scarpellini E, Vincenti F, Cammarota G, Flore R, Pola P, et al. Rifaximin dose-finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;22:31–35. doi: 10.1111/j.1365-2036.2005.02516.x. [DOI] [PubMed] [Google Scholar]
- 10.Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014;39:312–321. doi: 10.1111/apt.12573. [DOI] [PubMed] [Google Scholar]
- 11.Bond JH, Jr, Levitt MD, Prentiss R. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J Lab Clin Med. 1975;85:546–555. [PubMed] [Google Scholar]
- 12.Joseph F, Jr, Rosenberg AJ. Breath hydrogen testing: diseased versus normal patients. J Pediatr Gastroenterol Nutr. 1988;7:787–788. doi: 10.1097/00005176-198809000-00031. [DOI] [PubMed] [Google Scholar]
- 13.Di Stefano M, Corazza GR. Treatment of small intestine bacterial over growth and related symptoms by rifaximin. Chemotherapy. 2005;51:103–109. doi: 10.1159/000081996. [DOI] [PubMed] [Google Scholar]
- 14.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion. 2006;73:13–27. doi: 10.1159/000089776. [DOI] [PubMed] [Google Scholar]
- 16.Scarpellini E, Gabrielli M, Lauritano CE, Lupascu A, Merra G, Cammarota G, Cazzato IA, Gasbarrini G, Gasbarrini A. High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2007;25:781–786. doi: 10.1111/j.1365-2036.2007.03259.x. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841. doi: 10.1111/j.1572-0241.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 18.Davidson GP, Robb TA, Kirubakaran CP. Bacterial contamination of the small intestine as an important cause of chronic diarrhea and abdominal pain: diagnosis by breath hydrogen test. Pediatrics. 1984;74:229–235. [PubMed] [Google Scholar]
- 19.Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86–92. doi: 10.1023/a:1021738515885. [DOI] [PubMed] [Google Scholar]
- 20.Calanni F, Renzulli C, Fogli MV, Barbanti M. Comment on: Rifaximin in the treatment of irritable bowel syndrome. Is there a high risk for development of antimicrobial resistance? J Clin Gastroenterol. 2013;47:814. doi: 10.1097/MCG.0b013e3182951b6c. [DOI] [PubMed] [Google Scholar]
- 21.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. quiz 6. Am J Gastroenterol. 2012;107:28–35. doi: 10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 22.Ghoshal UC. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–317. doi: 10.5056/jnm.2011.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340. doi: 10.1136/gut.2009.205476. [DOI] [PubMed] [Google Scholar]