Abstract
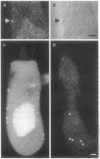
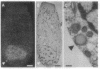
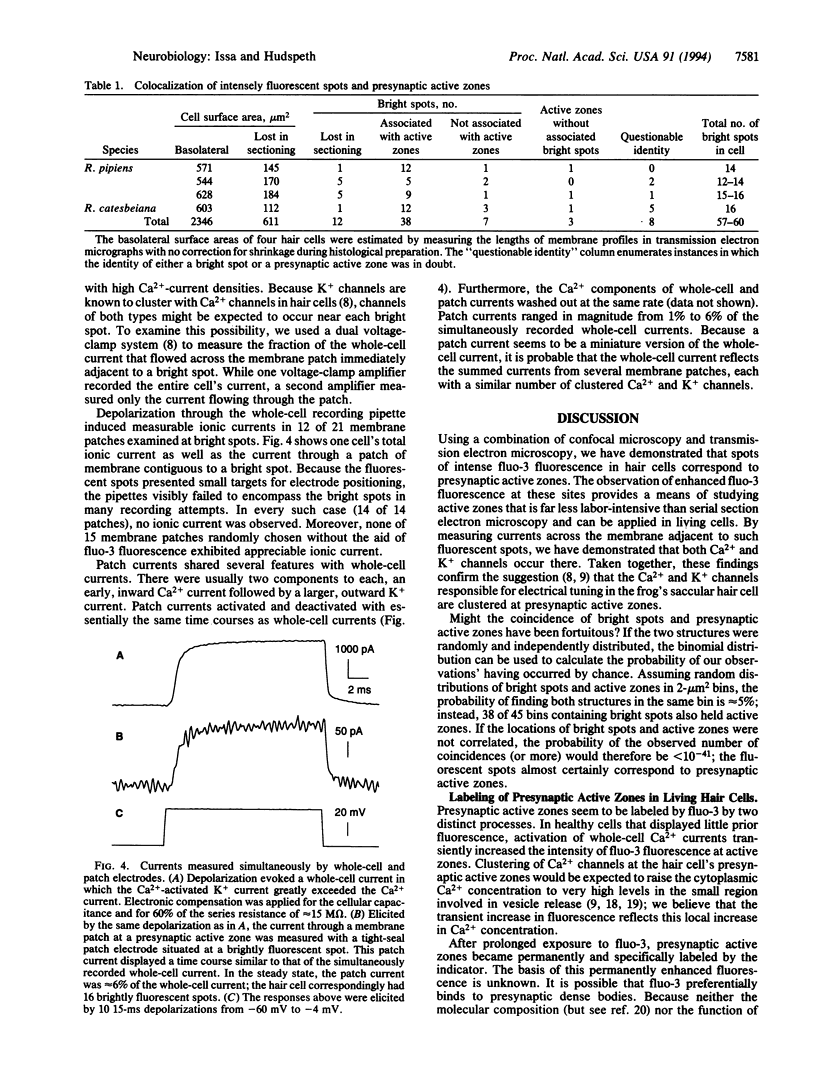
Electrical resonance, which in some hair cells provides a mechanism for frequency tuning, is mediated by clusters of Ca2+ channels and Ca(2+)-activated K+ channels that have been proposed to occur at presynaptic active zones. To localize Ca2+ channels on the cellular surface, we loaded hair cells from the frog's sacculus with the Ca2+ indicator fluo-3 and imaged them by fluorescence confocal microscopy. When a cell was depolarized, we observed on its basolateral surface several foci of transiently enhanced fluorescence due to local Ca2+ influx. After protracted recording, each cell displayed on average 18 brightly and permanently fluorescent spots at the same positions. We mapped these spots in four hair cells and compared their locations with those of presynaptic active zones, as determined from transmission electron micrographs of serial sections through the same cells. The results demonstrated that enhanced fluo-3 fluorescence marks active zones. Measurement of currents through membrane patches at fluorescently labeled active zones demonstrated that both voltage-activated Ca2+ channels and Ca(2+)-activated K+ channels occur there. These results confirm that the ion channels involved in electrical tuning and synaptic transmission by hair cells cluster together at presynaptic active zones.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Art J. J., Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987 Apr;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol. 1990 Dec;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Steinberg T. H., Silverstein S. C. Inhibition of Fura-2 sequestration and secretion with organic anion transport blockers. Cell Calcium. 1990 Feb-Mar;11(2-3):57–62. doi: 10.1016/0143-4160(90)90059-4. [DOI] [PubMed] [Google Scholar]
- Eatock R. A., Saeki M., Hutzler M. J. Electrical resonance of isolated hair cells does not account for acoustic tuning in the free-standing region of the alligator lizard's cochlea. J Neurosci. 1993 Apr;13(4):1767–1783. doi: 10.1523/JNEUROSCI.13-04-01767.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleisner L., Flock A., Wersäll J. The ultrastructure of the afferent synapse on hair cells in the frog labyrinth. Acta Otolaryngol. 1973 Aug-Sep;76(2):199–207. doi: 10.3109/00016487309121500. [DOI] [PubMed] [Google Scholar]
- Gola M., Crest M. Colocalization of active KCa channels and Ca2+ channels within Ca2+ domains in helix neurons. Neuron. 1993 Apr;10(4):689–699. doi: 10.1016/0896-6273(93)90170-v. [DOI] [PubMed] [Google Scholar]
- Gulley R. L., Reese T. S. Freeze-fracture studies on the synapses in the organ of Corti. J Comp Neurol. 1977 Feb 15;171(4):517–543. doi: 10.1002/cne.901710407. [DOI] [PubMed] [Google Scholar]
- Hama K. Fine structure of the afferent synapse and gap junctions on the sensory hair cell in the saccular macula of goldfish: a freeze-fracture study. J Neurocytol. 1980 Dec;9(6):845–860. doi: 10.1007/BF01205023. [DOI] [PubMed] [Google Scholar]
- Hama K., Saito K. Fine structure of the afferent synapse of the hair cells in the saccular macula of the goldfish, with special reference to the anastomosing tubules. J Neurocytol. 1977 Aug;6(4):361–373. doi: 10.1007/BF01178223. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:275–297. doi: 10.1113/jphysiol.1988.sp017120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. A., Hudspeth A. J. Ultrastructural correlates of mechanoelectrical transduction in hair cells of the bullfrog's internal ear. Cold Spring Harb Symp Quant Biol. 1990;55:547–561. doi: 10.1101/sqb.1990.055.01.053. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. M., Flock A. The ultrastructure of lateral line sense organs in the adult salamander Ambystoma mexicanum. J Neurocytol. 1973 Jun;2(2):133–142. doi: 10.1007/BF01474715. [DOI] [PubMed] [Google Scholar]
- Kirsch J., Wolters I., Triller A., Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993 Dec 23;366(6457):745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kros C. J., Crawford A. C. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol. 1990 Feb;421:263–291. doi: 10.1113/jphysiol.1990.sp017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Ophir I., Angel I. ATP-stimulated Ca2+ transport into cholinergic Torpedo synaptic vesicles. J Neurochem. 1980 Jul;35(1):116–124. doi: 10.1111/j.1471-4159.1980.tb12496.x. [DOI] [PubMed] [Google Scholar]
- Miller M. R., Beck J. Auditory hair cell innervational patterns in lizards. J Comp Neurol. 1988 May 22;271(4):604–628. doi: 10.1002/cne.902710410. [DOI] [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Roberts W. M., Howard J., Hudspeth A. J. Hair cells: transduction, tuning, and transmission in the inner ear. Annu Rev Cell Biol. 1988;4:63–92. doi: 10.1146/annurev.cb.04.110188.000431. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. The hair cell as a presynaptic terminal. Ann N Y Acad Sci. 1991;635:221–233. doi: 10.1111/j.1749-6632.1991.tb36494.x. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994 May;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. M. Spatial calcium buffering in saccular hair cells. Nature. 1993 May 6;363(6424):74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Charlton M. P. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992 Jan;12(1):297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R., Garcia M. L., Kaczorowski G. J., Charlton M. P. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993 Oct;11(4):645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- Sneary M. G. Auditory receptor of the red-eared turtle: II. Afferent and efferent synapses and innervation patterns. J Comp Neurol. 1988 Oct 22;276(4):588–606. doi: 10.1002/cne.902760411. [DOI] [PubMed] [Google Scholar]
- Srinivasan Y., Elmer L., Davis J., Bennett V., Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988 May 12;333(6169):177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]