Abstract
A 90-day oral toxicity test in rats was performed to evaluate the toxicity of 2-tetradecylcyclobutanone (2-tDCB), a unique radiolytic product of stearic acid. Six-week-old male and female F344 rats (n=15/group) were given 2-tDCB at concentrations of 0, 12, 60 and 300 ppm in a powder diet for 13 weeks. Slight dose-dependent increases in serum total protein and albumin in male rats were found, but these changes were not considered to be a toxic effect. The fasting, but not non-fasting, blood glucose levels of the male rats in the 300 ppm group and female rats in the 60 and 300 ppm groups were lower than those of the controls. Gas chromatography-mass spectrometry analysis showed dose-dependent accumulation of 2-tDCB in adipose tissue, notably in males. Next, we performed an azoxymethane (AOM)-induced two-stage carcinogenesis study. After injection of 6-week-old male F344 rats (n=30/group) once a week for 3 weeks, the animals received 2-tDCB at concentrations of 0, 10, 50 and 250 ppm in a powder diet for 25 weeks. The incidences of colon tumors for the 2-tDCB dosages were 34%, 45%, 40% and 37%, respectively, and were not statistically significant. These data suggest that 2-tDCB shows no toxic or tumor-modifying effects under the present conditions, and that the no-observed-adverse-effect level for 2-tDCB is 300 ppm in both sexes, equivalent to 15.5 mg/kg b.w./day in males and 16.5 mg/kg b.w./day in females.
Keywords: food irradiation, 2-tetradecylcyclobutanone, 90-day oral toxicity, colon carcinogenesis
Introduction:
Worldwide, food irradiation has been practiced as an effective method of reducing microbial and insect contamination, and for inhibiting sprouting1. Irradiation of food is known to induce lipid peroxidation by reactive oxygen species and other radiolytic products2,3,4. Known indicators of food irradiation include the 2-alkylcyclobutanones (2-ACBs), which are unique radiolytic products formed from the fatty acids of fat-containing food5, 6. Although it was thought that these products were found exclusively in irradiated foods, a recent study has reported finding 2-ACBs in nonirradiated cashew nut and nutmeg samples7. Since the late 1990s, both the cytotoxicity and genotoxicity of 2-ACBs have been investigated in Europe and the United States8,9,10,11,12. While 2-ACBs have been shown to be negative for mutagenicity in tests using E. coli or Salmonella strains11,12,13,14, a comet assay of 2-dodecylcyclobutanone (2-dDCB), a radiolytic product of palmitic acid, was found to induce DNA strand breaks in normal human colon cells and adenoma cells15. However, the validity of the test methods utilized in these previous investigations as well as the genotoxicity of 2-ACBs remains controversial. 2-Tetradecylcyclobutanone (2-tDCB) is a radiolytic compound originating from stearic acid and has the same number of carbon atoms as stearic acid with the alkyl group in ring position 2. While an investigation of azoxymethane (AOM)-induced colon carcinogenesis in Wistar rats demonstrated that both 2-tDCB and 2-(tetradec-5’-enyl)-cyclobutanone, a radiolytic product of oleic acid, had tumor-promoting activity, the number of rats examined in the study was small, and statistical significance was only shown for the average number of colonic tumors; it was not shown for the tumor incidence10. Therefore, further investigations have been recommended16. At the present time, there have been no oral toxicity tests of 2-ACBs that have analyzed either biochemical or histological findings. In the present study, we performed a 90-day oral toxicity test and an AOM-induced two-stage carcinogenesis study of 2-tDCB using a significant number of rats. In addition, 28-day oral toxicity test for high-doses of 2-tDCB and stearic acid were performed.
Materials and Methods
Animals
Four-week-old male F344/DuCrlCrlj rats were obtained from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). The rats were housed three to a plastic cage with sterilized woodchips for bedding in an air-conditioned room maintained at 23 ± 2°C and 55 ± 10% humidity with a 12 h light/dark cycle. All rats received a pellet diet until the start of the experiment (Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum. The study was performed using an experimental protocol approved by the Animal Committee of the University of Tokushima and conducted according to the Guidelines for the Care and Use of Laboratory Animals of the same institution.
Chemicals
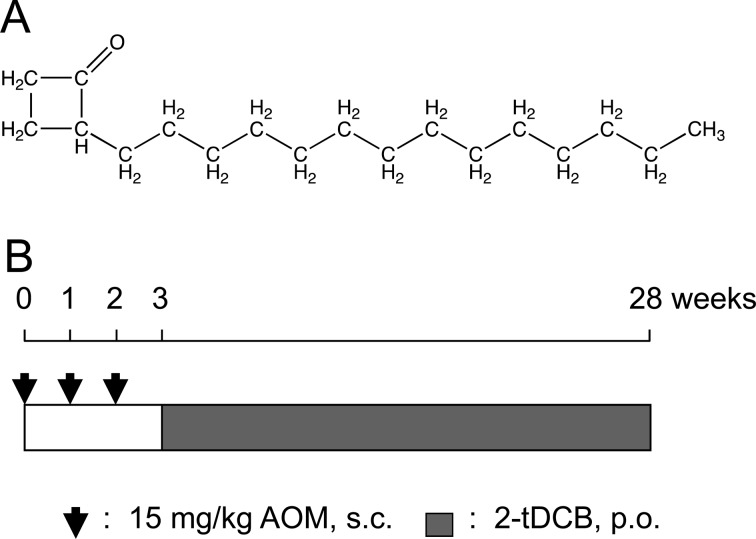
2-tDCB (purity: >99.3%) was synthesized at Hayashi Pure Chemical Ind., Ltd., Osaka, Japan (Fig. 1A). The concentration of 2-tDCB in the powder diet (n=3/group) was determined by gas chromatography-mass spectrometry (GC-MS), and the stability of 2-tDCB after 72 h of storage at room temperature was confirmed. AOM and stearic acid were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Fig. 1.
Structure of 2-tDCB and experimental design for AOM-induced carcinogenesis. A: 2-ACBs have a cyclobutanone ring and an alkyl chain. The alkyl chain length differs depending on the precursor fatty acid. B: For the two-stage carcinogenesis study, AOM was dissolved in 0.9% NaCl and administered subcutaneously in 6-, 7- and 8-week-old rats. At 9 weeks of age, administration of the powder diet containing 2-tDCB was begun.
90-Day oral toxicity test
After a 2-week acclimation period, 6-week-old male and female F344 rats (n=15/group) were given 2-tDCB at concentrations of 0, 12, 60 and 300 ppm in a powder diet for 13 weeks. The diet was prepared every 2 to 3 days. Body weight was monitored once a week. At the end of 13 weeks, fresh urine was collected and analyzed using urinalysis test strips (Multistix PRO 10 LS, Siemens Healthcare Diagnostics). Non-fasting glucose levels in tail blood determined by the glucose oxidase method (Sanwa Kagaku Kenkyusho, Nagoya, Japan) were measured at 10 a.m. 1 day before euthanization. After fasting overnight, body weight and fasting blood glucose levels were measured, after which the rats were euthanized by carbon dioxide overdose. Blood samples for hematological and serological tests were collected via the inferior vena cava, followed by measurements of the organ weights. Blood samples of 6 rats in each group were analyzed hematologically (Shikoku Chuken, Inc., Tokushima, Japan), and serum of 7 to 9 rats in each group was frozen in liquid nitrogen, stored −80°C and then analyzed by SRL (Tokyo, Japan). For histological examinations, the organs were fixed in 10% buffered formalin, embedded in paraffin, cut into 4-μm sections and then stained with hematoxylin and eosin.
Measurement of 2-tDCB concentrations by GC-MS
At the end of the 90-day oral toxicity test, retroperitoneal adipose tissue was removed from 6 males and 3 females during autopsy, weighed and then stored at −80°C until use. Briefly, adipose tissue was homogenized with anhydrous sodium sulfate and extracted with n-hexane in a Soxhlet apparatus for 6 h. Concentrations of 2-tDCB in hexane extracts were analyzed using a GC-MS system (QP2010 Plus, Shimadzu, Kyoto, Japan) as per a previously described method17.
28-Day oral toxicity test for high doses of 2-tDCB and stearic acid
Six-week-old male F344 rats (n=9/group) were given a basal powder diet, or a powder diet that contained 300 ppm 2-tDCB or 300 ppm stearic acid for 5 weeks. At the end of 4 weeks, an oral 2 g/kg glucose tolerance test (OGTT) was performed after 16 h of fasting. Fasting glucose and 30-min post-glucose loading blood glucose were measured. At the end of 5 weeks, white blood cell (WBC) counts were measured using a disposable hemocytometer (Digital Bio Technology Co., Seoul, South Korea).
AOM-induced two-stage carcinogenesis
Six-week-old male F344 rats (n=30/group) were treated with subcutaneous injections of 15 mg/kg AOM once weekly for 3 weeks (Fig. 1B). AOM was dissolved in 0.9% NaCl immediately before use. Starting from week 3, a powder diet containing 0, 10, 50 or 250 ppm 2-tDCB was given for 25 weeks. All animals were euthanized at week 28. The stomach and intestines were opened, the size and location of tumors larger than 2 mm in diameter were recorded, and the tissue was fixed in 10% buffered formalin on a cork board. All neoplastic lesions and major organs were examined histologically.
Statistical analyses
Statistical processing was conducted using statistical software (Social Survey Research Information Co., Ltd., Tokyo, Japan). The dose-dependency of results of the toxicity test was analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s test. Concentrations of 2-tDCB were analyzed by Student t-test. Tumor incidence was analyzed by Fisher’s exact probability test. P-values of <0.05 were considered statistically significant.
Results
90-day oral toxicity test
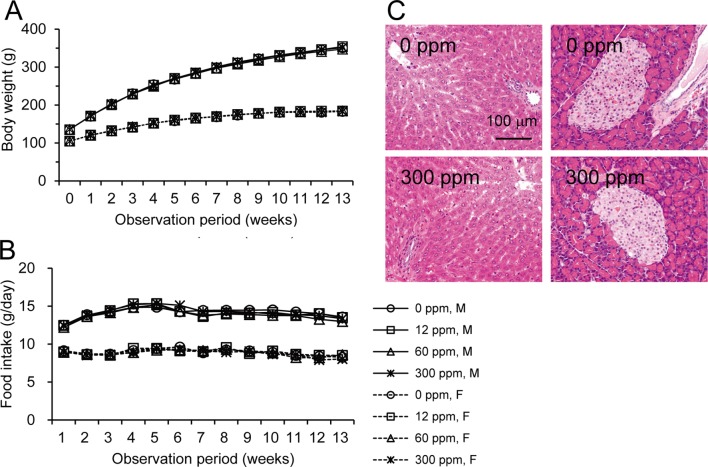
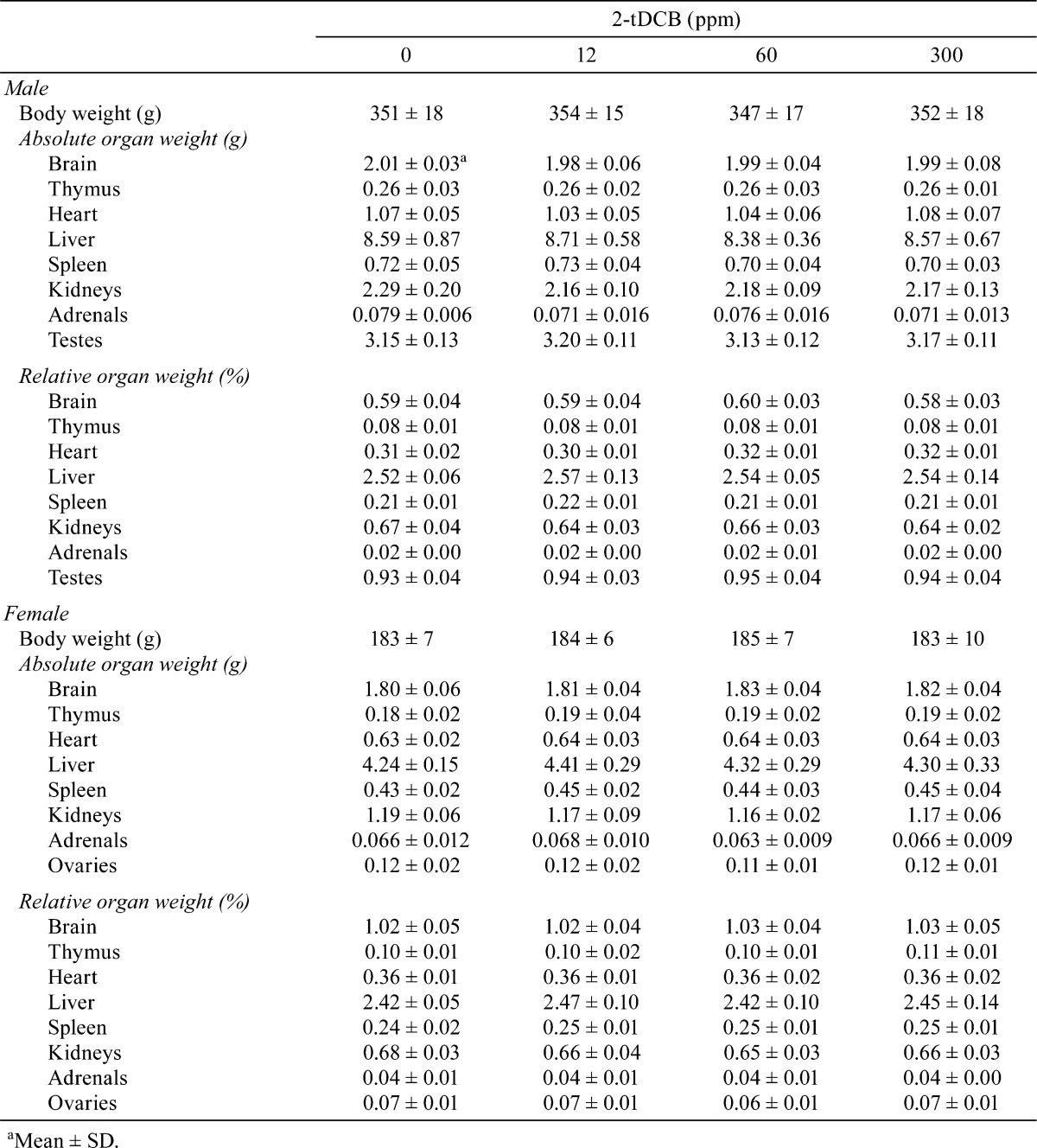
Body weight and food intake in each group were similar throughout the experimental period (Fig. 2A and B). Urinalysis found no treatment-related changes for urobilinogen, protein, pH, occult blood, ketone, bilirubin or glucose. Although both sexes exhibited no significant dose-dependent changes in the hematological data, a mild increase in WBC count was found in the male and female rats in the 300 ppm group (Table 1). However, the low WBC count in female control rats was thought to be an outlier (historical control data for WBC count in female F344 rat: about 40 ×102/μl, Charles River Laboratories Japan, Inc.). Non-fasting blood glucose levels showed no treatment-related changes in both sexes, but the fasting blood glucose levels of the males in the 300 ppm group and females in the 60 and 300 ppm groups were significantly lower than those of the controls (Table 2). Serum biochemical analysis indicated there were dose-dependent increases in total protein (P=0.018, one-way ANOVA) and albumin (P=0.013) in males (Table 3). Serum total protein in the 60 and 300 ppm groups, and albumin in the 300 ppm group were significantly higher in males as compared with the controls. In both sexes, there were no differences in absolute and relative organ weights of all organs (Table 4), and histologically, no toxicity was noted in any of the organs (Fig. 2C).
Fig. 2.
Body weight, food intake and the histological appearances found during the 90-day oral toxicity test. Similar average body weights (A) and food intakes (B) were found in each group throughout the experimental period. None of the groups exhibited any histological differences in the liver (C, left) or pancreas (C, right).
Table 1. Hematological Data for the Male and Female Rats Given 2-tDCB for 13 Weeks.
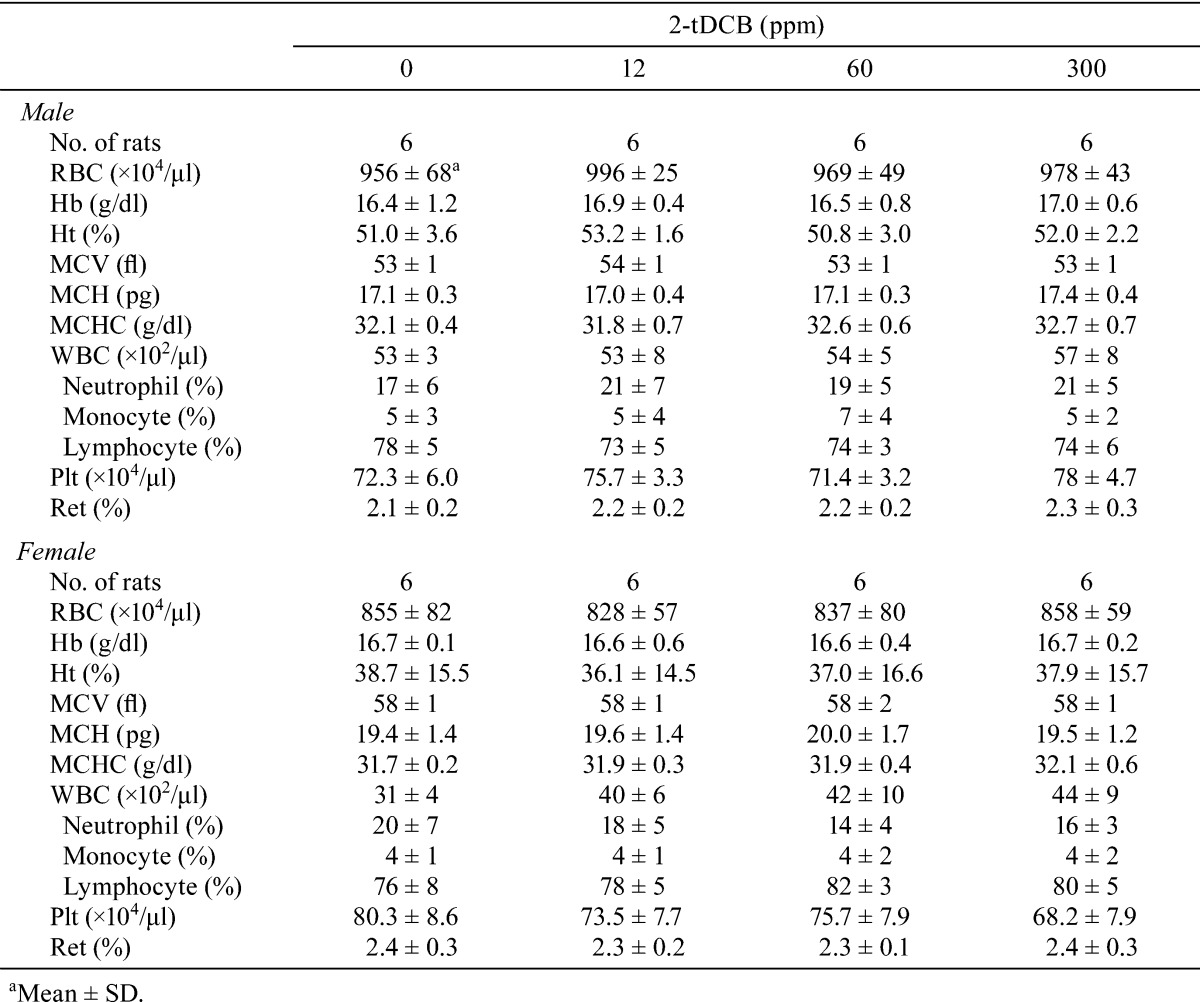
Table 2. Blood Glucose for the Male and Female Rats Given 2-tDCB for 13 Weeks.
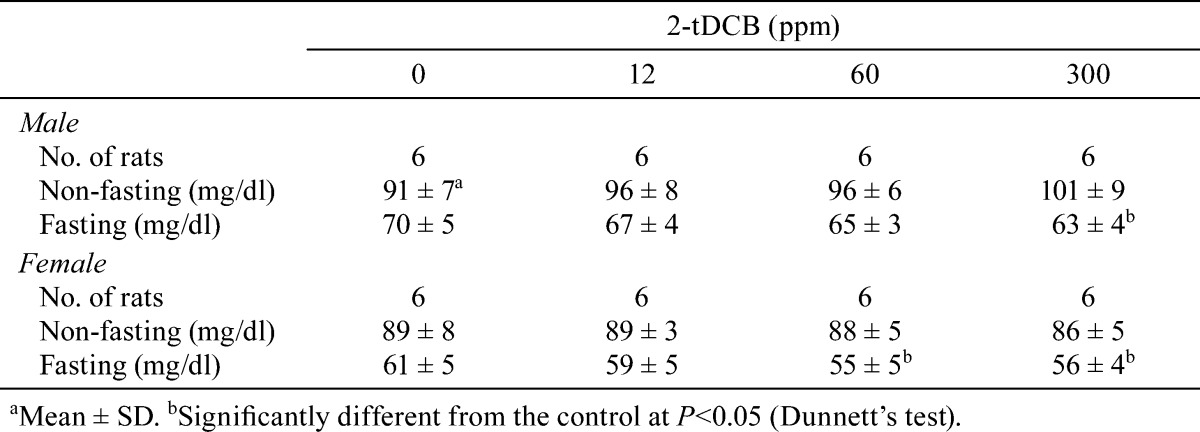
Table 3. Serum Biochemical Data for the Male and Female Rats Given 2-tDCB for 13 Weeks.
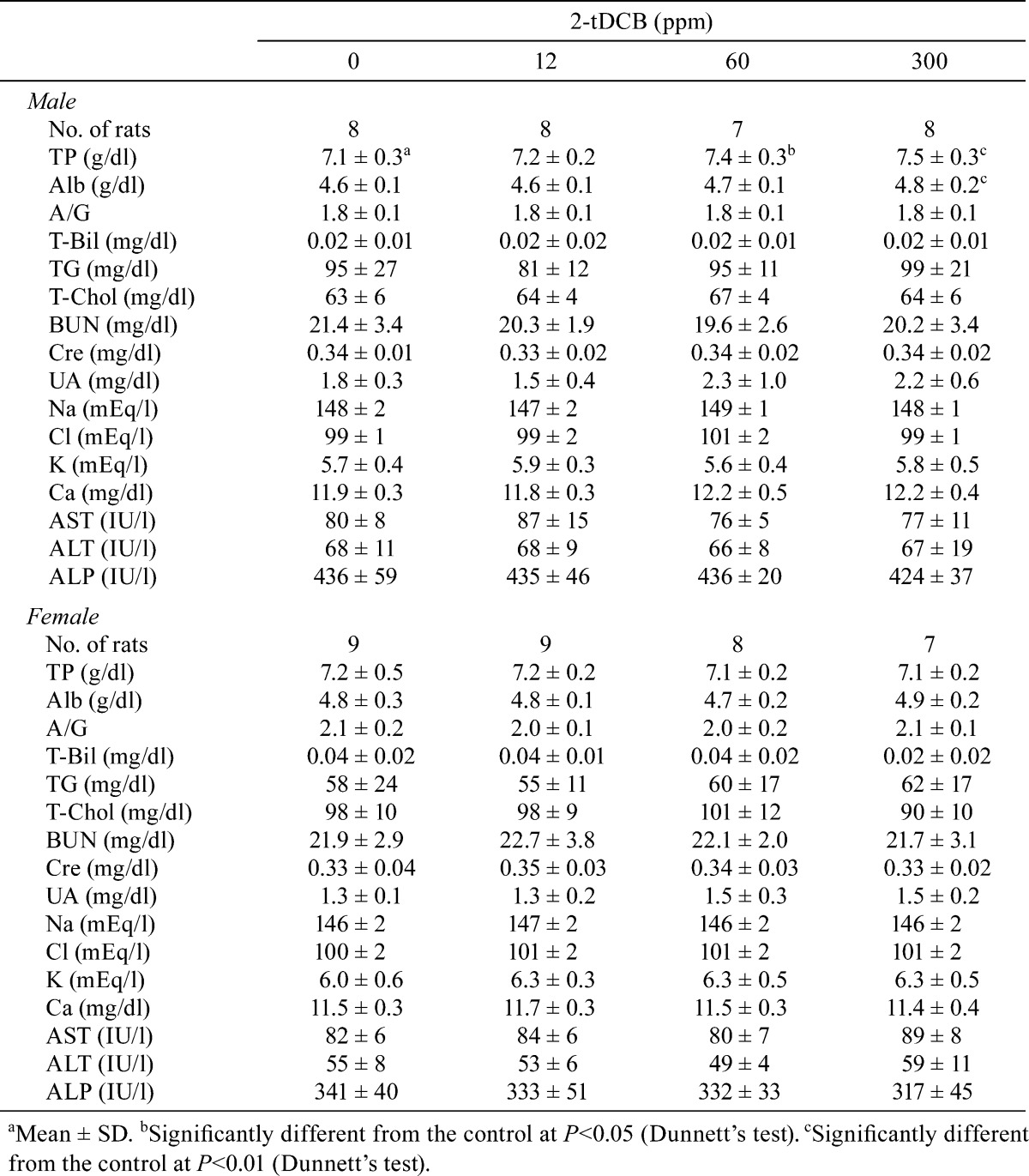
Table 4. Absolute and Relative Organ Weights for the Male and Female Rats Given 2-tDCB for 13 Weeks (n=12/group).
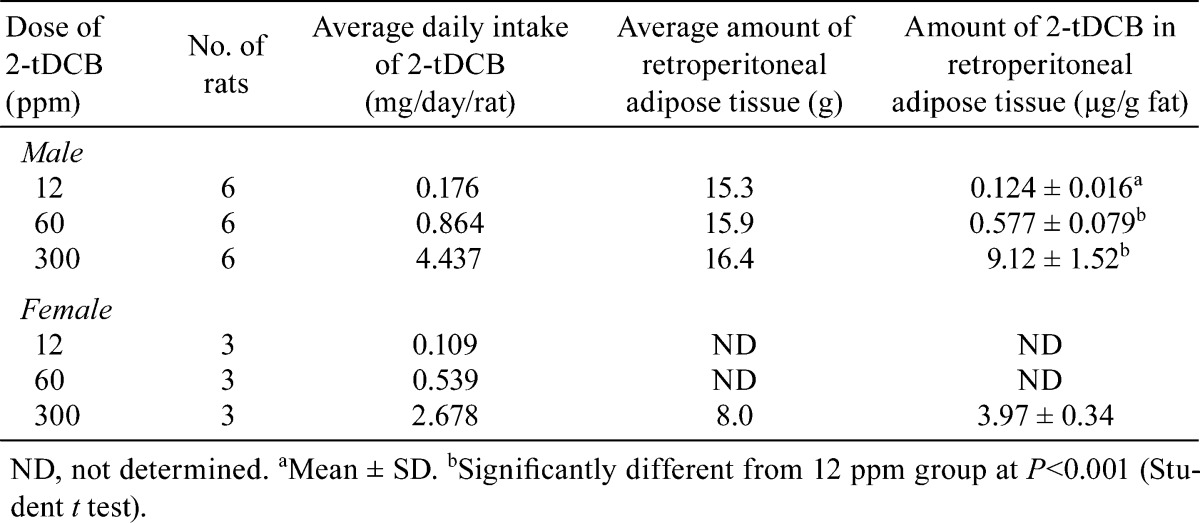
Concentrations of 2-tDCB in retroperitoneal adipose tissue
In male rats, the concentrations of 2-tDCB in adipose tissue increased dose dependently, with an extremely high level observed in the 300 ppm group (Table 5). For females, the value in the 300 ppm group was 44.5% of that found for the males.
Table 5. Daily Intake of 2-tDCB and the Amount of the Compound Found in Retroperitoneal Adipose Tissue During the 90-day Oral Toxicity Test.
OGTT and WBC count during the 28-day oral toxicity test
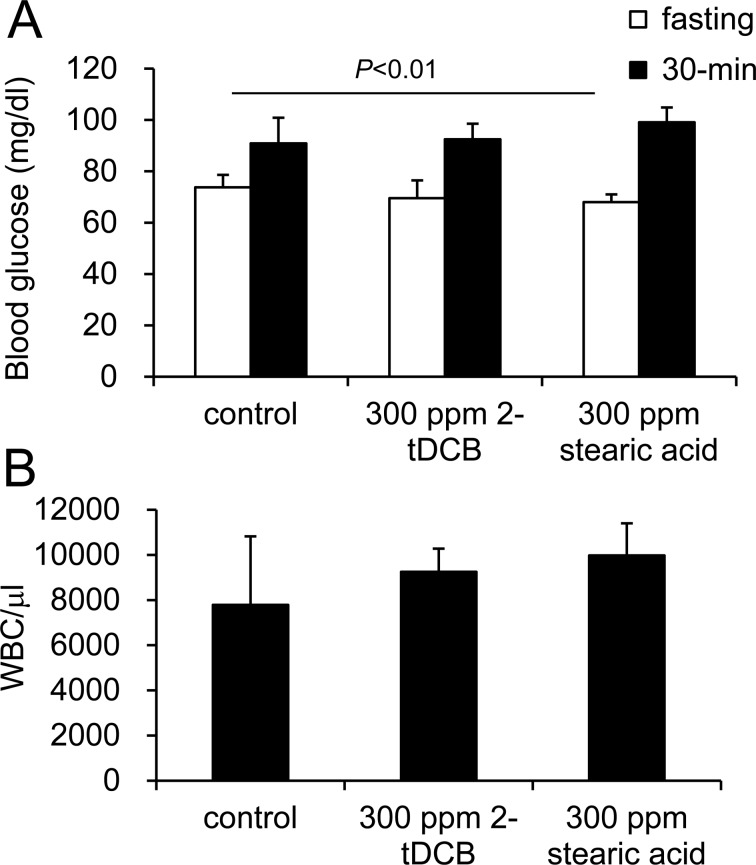
Administration of 300 ppm 2-tDCB for 4 weeks had no effect on either the fasting or 30-min post-glucose loading blood glucose levels, although the fasting glucose was decreased by the 300 ppm stearic acid treatment (Fig. 3A). A significant increase in WBC count was not found for 300 ppm 2-tDCB (Fig. 3B).
Fig. 3.
Blood glucose and WBC counts in rats treated with 300 ppm 2-tDCB and 300 ppm stearic acid during the 28-day oral toxicity study. After the 300 ppm stearic acid treatment, fasting glucose decreased. No significant increase in WBC count was shown after administration of 300 ppm 2-tDCB. Data are shown as the mean ± SD.
AOM-induced two-stage carcinogenesis
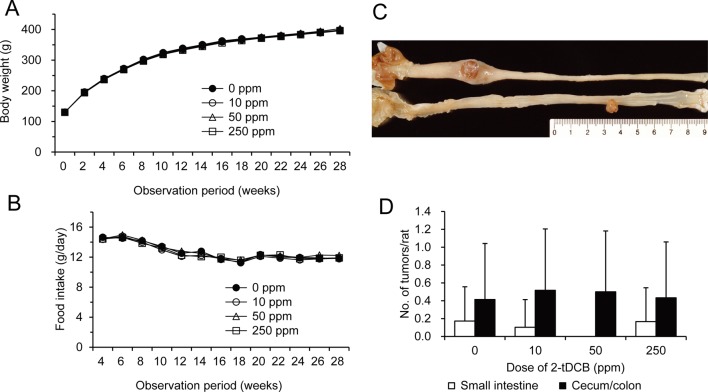
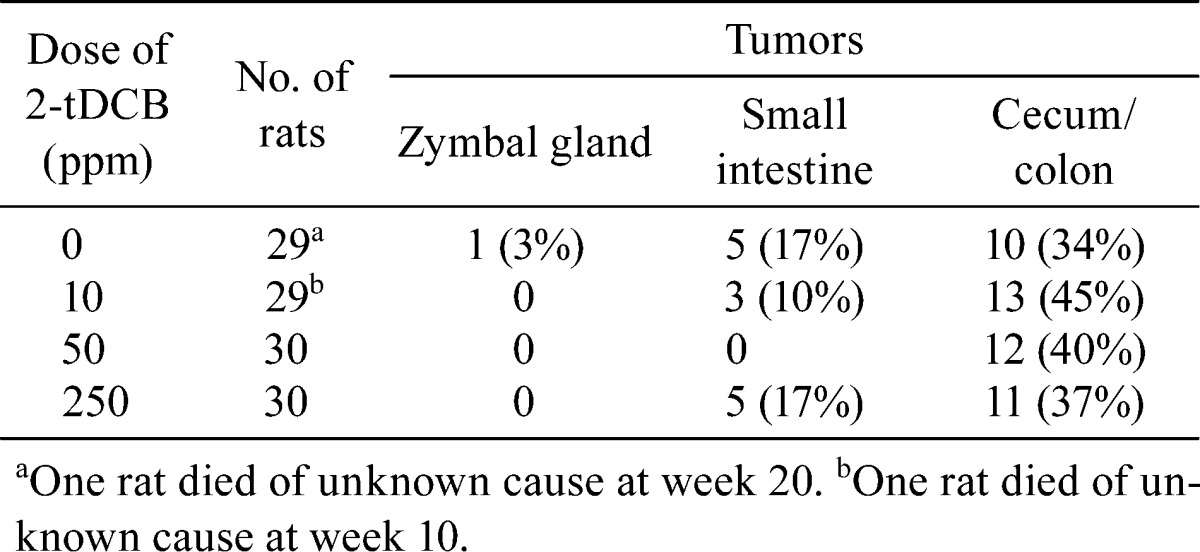
The average values for body weight and food intake were similar in each group throughout the experimental period (Fig. 4A and B). The absolute (data not shown) and relative organ weights of the liver, kidneys and spleen were also similar (Table 6). Although in general the tumor incidence in the small intestine and cecum/colon did not vary in any of the groups, it should be noted that no small intestinal tumors developed in the 50 ppm 2-tDCB group (Table 7). Fig. 4C shows polypoid tumors of the small intestine and colon in the 250 ppm 2-tDCB group. Colonic tumors developed equally in the proximal and distal colon, while all of the small intestinal tumors developed in the proximal half portion, including in the duodenum. Histologically, all of the intestinal neoplasms were adenocarcinomas. Signet-ring cell and mucinous carcinomas developed at the rates of 3, 10, 7 and 3% in the 0, 10, 50 and 250 ppm 2-tDCB groups, respectively, and all were located in the cecum and proximal colon. The number of small intestine and colon tumors per rat in each group did not differ (Fig. 4D).
Fig. 4.
AOM-induced carcinogenesis. The average values for body weight (A) and food intake (B) were similar. Figure 4C shows the gross appearance of the intestinal tumors in a case from the 250 ppm group. The average number of small intestine and cecum/colon tumors per rat (D) did not differ between the groups. Data are shown as the mean ± SD.
Table 6. Relative Organ Weights in AOM-induced Carcinogenesis.
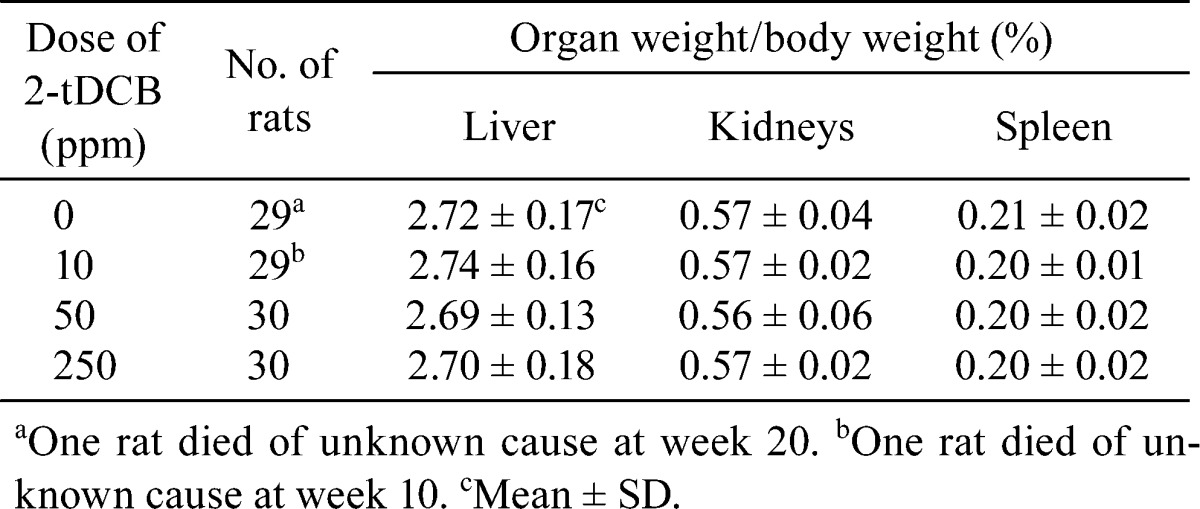
Table 7. Tumor Incidence in AOM-induced Carcinogenesis.
Discussion
Until recently, there have been no positive results reported for toxicity studies that examined animals fed diets containing irradiated food3,4,18. In 2009, it was first reported that experimental induction of leukoencephalopathy occurred in cats given gamma-irradiated diets19. The authors of that study further suggested that elevated peroxide and reduced vitamin A concentrations in the γ-irradiated diet may have been the cause of the disease. However, there have been no toxicity tests that have determined whether ACBs can induce biochemical changes. In our present 90-day oral toxicity test, we demonstrated that oral administration of a high dose of 2-tDCB induced mild hyperalbuminemia in male rats, but these changes were not considered to be a toxic effect. Mild hypoglycemia (fasting but not non-fasting glucose levels) and possible leukocytosis were also observed, although these were not demonstrated by the glucose tolerance test and WBC counts in the 28-day oral toxicity study. Accordingly, these changes were also not considered to be toxic effects. Further investigations will be necessary to elucidate the mechanism of these biochemical and hematological changes.
In males, the average amounts of 2-tDCB in retroperitoneal adipose tissue in the 12, 60 and 300 ppm groups were 1.3, 1.2 and 3.9% of the average daily intake of 2-tDCB, respectively (Table 6). In contrast, the ratio was 1.4% in females in the 300 ppm group. These data suggest that 2-tDCB tends to accumulate more in males than in females. Since it has been shown that 2-ACBs accumulate in adipose tissue20, further clarification of the significance of adipose tissue accumulation of 2-tDCB will need to be performed.
ACBs are produced dose dependently by ionizing radiation, and the concentrations of 2-dDCB and 2-tDCB are correlated with the palmitic acid and stearic acid concentrations, respectively21. The daily intake of ACBs in humans has been estimated to be between 5 to 10 µg/kg body weight maximally10. In the present 90-day oral toxicity test, the values of daily intake of 2-tDCB correspond to more than 1,550 to 3,100 times the estimated daily intake of ACBs in human males and more than 1,650 to 3,300 times the estimated daily intake of ACBs in human females. Thus the doses used in our animal experiment can satisfactorily evaluate the toxicity and carcinogenic potential of 2-tDCB in humans.
In our two-stage carcinogenesis study, we used 30 rats in each group and selected 250 ppm as the highest dose of 2-tDCB, as this dose was 5-fold higher than that reported in the study by Raul et al.10. In our study, the average daily intake of 2-tDCB in the 250 ppm group was 9.6 mg/kg/day. However, we found no toxic or tumor-promoting effect of 2-tDCB on AOM-induced carcinogenesis. The purity of the 2-tDCB used in the present study was >99.3%, which was higher than that reported in another previous study9. Moreover, the strain of rats and the vehicle for 2-tDCB used in our study also differed from the previous study, as the previous researchers dissolved the compound in 1% ethanol and administered it in drinking water. To avoid the modifying effect of the vehicle in our study, we decided to use a powder diet.
It is well recognized that saturated fatty acids such as palmitic acid (C16:0) and stearic acid (C18:0) are cytotoxic to β-cells, and can induce apoptosis in vitro22,23. We found that 2-dDCB as well as its precursor to palmitic acid induced apoptosis in human lymphoma cells24. However, in vivo toxicity of dietary stearic acid has not been clearly demonstrated25,26,27. In the present study, histological changes were not observed in pancreatic islets after 2-tDCB administration.
Although we tested 2-tDCB in the present study, additional experiments using other ACBs with shorter side chains than 2-tDCB might be necessary, as it has been reported that compounds with shorter side chains are more cytotoxic13. To our knowledge, there have been no other reports of long-term carcinogenicity tests using 2-ACBs in rodents. In order to evaluate the risk of 2-ACBs in humans and to determine the acceptable daily intake, 2-year carcinogenicity tests in rodents may be necessary.
In conclusion, 2-tDCB accumulated in adipose tissue, notably in males, and induced no dose-dependent hematological, biochemical or morphological changes in both sexes in a 90-day oral toxicity test, with the no-observed-adverse-effect level for 2-tDCB determined to be a dietary dose of 300 ppm in both sexes, which is equivalent to 15.5 mg/kg b.w./day in males and 16.5 mg/kg b.w./day in females. In addition, 2-tDCB exerted no tumor-promoting effects on AOM-induced intestinal tract carcinogenesis in the rats.
Acknowledgments
We are grateful to Miss Hitomi Umemoto and Mrs. Megumi Sato for laboratory assistance. This work was supported in part by the Food Safety Commission, Cabinet Office, Japan (#0906). The authors declare that they have no conflicts of interest.
References
- 1.Kume T, Furuta M, Todoriki S, Uenoyama N, and Kobayashi Y. Status of food irradiation in the world. Radiat Phys Chem. 78: 222–226. 2009. [Google Scholar]
- 2.Schubert J. Mutagenicity and cytotoxicity of irradiated foods and food components. Bull World Health Organ. 41: 873–904. 1969. [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. High-dose irradiation: wholesomeness of food irradiated with doses above 10 kGy. WHO Tech Rep Ser. No. 890. 1999. [PubMed]
- 4.EFSA. Scientific opinion on the chemical safety of irradiation of food. EFSA Journal. 9: 1930 [57 pp.]. 2011.
- 5.Letellier PR, and Nawar WW. 2-Alkylcyclobutanones from the radiolysis of triglycerides. Lipids. 7: 75–76. 1972. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson MH, Crone AVJ, and Hamilton JT. Irradiation detection. Nature. 344: 202–203. 1990. [DOI] [PubMed] [Google Scholar]
- 7.Variyar PS, Chatterjee S, Sajilata MG, Singhal RS, and Sharma A. Natural existence of 2-alkylcyclobutanones. J Agric Food Chem. 56: 11817–11823. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Ashley BC, Birchfield PT, Chamberlain BV, Kotwal RS, McClellan SF, Moynihan S, Patni SB, Salmon SA, and Au WW. Health concerns regarding consumption of irradiated food. Int J Hyg Environ Health. 207: 493–504. 2004. [DOI] [PubMed] [Google Scholar]
- 9.Delincée H, Soika C, Horvatovich P, Rechkemmer G, and Marchioni E. Genotoxicity of 2-alkylcyclobutanones, markers for an irradiation treatment in fat-containing food-Part I: cyto- and genotoxic potential of 2-tetradecylcyclobutanone. Radiat Phys Chem. 63: 431–435. 2002. [Google Scholar]
- 10.Raul F, Gosse F, Delincée H, Hartwig A, Marchioni E, Miesch M, Werner D, and Burnouf D. Food-borne radiolytic compounds (2-alkylcyclobutanones)may promote experimental colon carcinogenesis. Nutr Cancer. 44: 189–191. 2002. [DOI] [PubMed] [Google Scholar]
- 11.Sommers CH. 2-Dodecylcyclobutanone does not induce mutations in the Escherichia coli tryptophan reverse mutation assay. J Agric Food Chem. 51: 6367–6370. 2003. [DOI] [PubMed] [Google Scholar]
- 12.Sommers CH, and Schiestl RH. 2-Dodecylcyclobutanone does not induce mutations in the Salmonella mutagenicity test or intrachromosomal recombination in Saccharomyces cerevisiae. J Food Prot. 67: 1293–1298. 2004. [DOI] [PubMed] [Google Scholar]
- 13.Hartwig A, Pelzer A, Burnouf D, Titéca H, Delincée H, Briviba K, Soika C, Hodapp C, Raul F, Miesch M, Werner D, Horvatovich P, and Marchioni E. Toxicological potential of 2-alkylcyclobutanones—specific radiolytic products in irradiated fat-containing food—in bacteria and human cell lines. Food Chem Toxicol. 45: 2581–2591. 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gadgil P, and Smith JS. Mutagenicity and acute toxicity evaluation of 2-dodecylcyclobutanone. J Food Sci. 69: C713–C716. 2004. [Google Scholar]
- 15.Knoll N, Weise A, Claussen U, Sendt W, Marian B, Glei M, and Pool-Zobel BL. 2-Dodecylcyclobutanone, a radiolytic product of palmitic acid, is genotoxic in primary human colon cells and in cells from preneoplastic lesions. Mutat Res. 594: 10–19. 2006. [DOI] [PubMed] [Google Scholar]
- 16.Rao CV. Do irradiated foods cause or promote colon cancer? Nutr Cancer. 46: 107–109. 2003. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Morita Y, Saito K, Kameya H, Nakajima M, and Todoriki S. Identification of irradiated prawn (Penaeus monodon ) using thermoluminescence and 2-alkylcyclobutanone analyses. J Agric Food Chem. 59: 78–84. 2011. [DOI] [PubMed] [Google Scholar]
- 18.Hagiwara A, Yoshino H, Sano M, Kawabe M, Tamano S, Sakaue K, Nakamura M, Tada M, Imaida K. and Shirai T. Thirteen-week feeding study of thaumatin (a natural proteinaceous sweetener), sterilized by electron beam irradiation, in Sprague-Dawley rats. Food Chem Toxicol. 43: 1297–1302. 2005. [DOI] [PubMed]
- 19.Caulfield CD, Kelly JP, Jones BR, Worrall S, Conlon L, Palmer AC, and Cassidy JP. The experimental induction of leukoencephalomyelopathy in cats. Vet Pathol. 46: 1258–1269. 2009. [DOI] [PubMed] [Google Scholar]
- 20.Horvatovich P, Raul F, Miesch M, Burnouf D, Delincee H, Hartwig A, Werner D, and Marchioni E. Detection of 2-alkylcyclobutanones, markers for irradiated foods, in adipose tissues of animals fed with these substances. J Food Prot. 65: 1610–1613. 2002. [DOI] [PubMed] [Google Scholar]
- 21.Obana H, Furuta M, and Tanaka Y. Analysis of 2-alkylcyclobutanones with accelerated solvent extraction to detect irradiated meat and fish. J Agric Food Chem. 53: 6603–6608. 2005. [DOI] [PubMed] [Google Scholar]
- 22.Shimabukuro M, Zhou YT, Levi M, and Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 95: 2498–2502. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eitel K, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Häring HU, and Kellerer M. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochem Biophys Res Commun. 299: 853–856. 2002. [DOI] [PubMed] [Google Scholar]
- 24.Yu DY, Zhao QL, Furuta M, Todoriki S, Izumi K, Yamakage K, Matsumoto K, Nomura T. and Kondo T. Molecular mechanisms of apoptosis induction by 2-dodecylcyclobutanone, a radiolytic product of palmitic acid, in human lymphoma U937 cells. Apoptosis. 17: 636–645.2012. [DOI] [PubMed] [Google Scholar]
- 25.Ebbesson SO, Tejero ME, López-Alvarenga JC, Harris WS, Ebbesson LO, Devereux RB, MacCluer JW, Wenger C, Laston S, Fabsitz RR, Howard BV, and Comuzzie AG. Individual saturated fatty acids are associated with different components of insulin resistance and glucose metabolism: the GOCADAN study. Int J Circumpolar Health. 69: 344–351. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louheranta AM, Turpeinen AK, Schwab US, Vidgren HM, Parviainen MT, and Uusitupa MI. A high-stearic acid diet does not impair glucose tolerance and insulin sensitivity in healthy women. Metabolism. 47: 529–534. 1998. [DOI] [PubMed] [Google Scholar]
- 27.Evaluation of certain food additives and contaminants. WHO Tech Rep Ser. No. 960. 2011. [PubMed]