Abstract
Carcinosarcomas of the uterine cervix are extremely rare. Cervical carcinosarcoma can be characterized by having two different origins: the Müllerian ducts and the mesonephric duct remnants. A 53-year-old Korean woman was admitted to the hospital because of pelvic mass detected on computed tomography scan done at private clinic. A Radical hysterectomy with bilateral salpingooophorectomy and pelvic lymphadenectomy was carried out upon a diagnosis of stage IB2 cervical sarcoma. Immunohistochemically, the epithelial component was positive for pancytokeratin and estrogen receptor, but negative for CD 10 and carletinin. The mesenchymal component was positive for vimentin. The histopathologic diagnosis was a carcinosarcoma of the uterine cervix arising from Müllerian ducts. She underwent chemotherapy. She developed systemic recurrence seven months after operation and died of disease. The origin of cervical carcinosarcoma needs to be verified and immunohistochemical studies using mesonephric marker (CD 10, carletinin, and estrogen receptor) is helpful.
Keywords: Breast neoplasms, Carcinosarcoma, Cervix uteri, Müllerian duct, Müllerian tumor mixed
Introduction
Carcinosarcomas account for less than 5% of uterine malignancies [1], most of which arise from the uterine corpus. Among these, cervical carcinosarcomas are extremely rare. The mesenchymal component of carcinosarcoma has recently been recognized as a metaplastic change of carcinoma [2]. Although not much attention has been paid to it, cervical carcinosarcoma can be characterized by origins: the Müllerian ducts and the mesonephric duct remnants [3,4,5,6]. Around 62 cases of cervical carcinosarcoma have been reported in the English literature, including the current case. In Korea, only two cases of cervical carcinosarcoma have been reported, but their origin of carcinoma was not stated [7,8]. Owing to the relative infrequency of the disease, most of the available data on the natural history of cervical carcinosarcomas are derived from case reports and small case series. Due to the lack of information regarding these neoplasms, there is as yet no consensus regarding their prognosis and treatment.
We describe here a case of an individual with carcinosarcoma of the uterine cervix arising from Müllerian ducts coincident with breast cancer, which was diagnosed by immunohistochemical studies. We also present a review of the literature in order to better understand the disease entity and its origin.
Case report
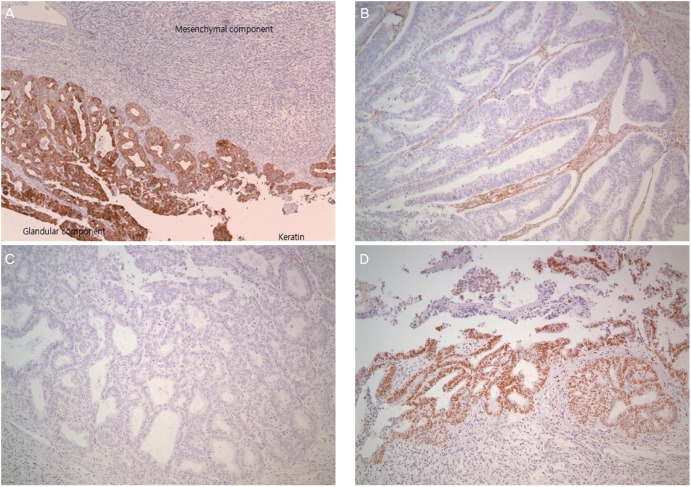
A 53-year-old woman was referred to our hospital because of a pelvic mass. Pelvic mass was detected on computed tomography scan which was done due to intractable cystitis at private clinic. Magnetic resonance imaging (M)showed an 8-cm-sized round and well-defined mass in her uterine cervix (Fig. 1). Low signal intensity on T1-weighted images, high signal intensity on T2-weighted images, partial heterogeneous enhancement on Gadolinium-Enhanced image was appeared in the cervical mass. Preoperative diagnosis based on magnetic resonance imaging findings is cystic degeneration of cervical myoma or cervical malignancy. There was no obvious metastasis to pelvic or paraaortic lymph nodes in imaging studies. Cytology of the uterine cervix showed no abnormal cells. The cervical biopsy was reported to be a sarcoma or undifferentiated carcinoma. The tumor markers were as follows: CA-125, 63.1 U/mL; CEA, 0.5 ng/mL; SCC Ag, 1.26 ng/mL. Radical hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy was performed upon diagnosis of a stage IB2 cervical sarcoma. Grossly, there was a pedunculated solid mass originating from the uterine cervix measuring 9×7×6.5 cm. The mass was a yellowish white color with partial hemorrhage and necrosis. Microscopically, the cervical mass was revealed to be a malignant tumor with epithelial and mesenchymal components. Most of the tumor consisted of the epithelial component, which showed poorly to moderately differentiated adenocarcinoma. The tumor was confined to the uterine cervix and did not reach the parametrium. Lymphatic and vascular invasion of the tumor cells were present, but the regional lymph nodes were negative for tumor cells. The surgical margins of the vagina and parametrium were free of the malignancy. The epithelial component was composed of adenocarcinoma and tested positive for pancytokeratin and estrogen receptor in immunohistochemical studies but was negative for CD 10 and carletinin. The mesenchymal component was positive for vimentin (Fig. 2). The postoperative histopathologic diagnosis was of a carcinosarcoma of the uterine cervix arising from Müllerian ducts. As an additional postoperative treatment, the patient was scheduled to undergo chemotherapy consisting of six courses of ifosfamide and cisplatin. She complained of hot flashes after surgery. Mammography and breast sonography, which were done for hormone therapy as a baseline work-up, showed a 1.3-cm-sized irregular shaped low echoic nodule in her left breast which was revealed by fine needle biopsy to be a ductal carcinoma in situ. Breast conserving surgery and sentinel lymph node dissection were carried out after the first course of chemotherapy. Final histopathologic diagnosis was of a 0.4-cm-sized invasive ductal carcinoma with negative sentinel lymph node. The remaining five courses of chemotherapy were administered and the patient had no evidence of disease after three months of follow up. Seven months after the operation, the patient developed systemic recurrence of cervical carcinoma confirmed by colonoscopic biopsy and imaging study (rectal, cutaneous, and hepatic). She underwent two additional courses of chemotherapy consisting of paclitaxel and carboplatin. The chemotherapy had no effect and she died of disease two months after starting the 2nd line chemotherapy.
Fig. 1. Magnetic resonance imaging. An 8-cm-sized round and well-defined mass in the uterine cervix.
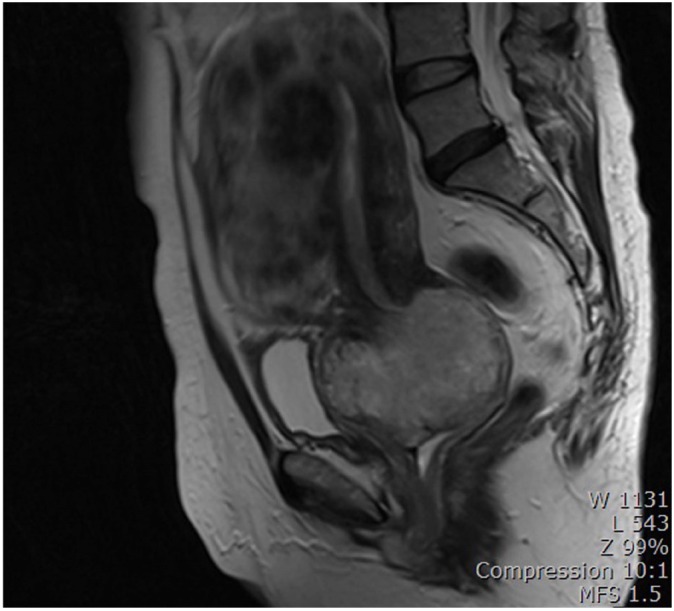
Fig. 2. Immunohistochemical staining. (A) Positive for pancytokeratin in epithelial component (×40), (B) negative for CD 10 (×100), (C) negative for carletinin (×100), and (D) positive for esgrogen receptor (×100).
Discussion
Carcinosarcoma (previously called malignant mixed Müllerian tumors) is the neoplasm composed of malignant epithelial and mesenchymal components. Carcinosarcoma arises less frequently in the uterine cervix than in the uterine corpus.
According to data from the National Cancer Institute's SEER (Surveillance, Epidemiology, and End Results) database, 128 cases of cervical carcinosarcoma have been documented [9]. Cervical carcinosarcoma mainly occurs in postmenopausal women, with a mean age of 64 years at diagnosis and a range of 25 to 93 years [9]. Almost all patients complained of vaginal bleeding, and usually had a detectable mass in the uterine cervix. Most cervical carcinosarcomas including our present case, for which staging information is available, are found to be stage IB [9,10]. Patients have been commonly treated by total abdominal hysterectomy, salpingo-oophorectomy, and pelvic lymph node dissection. Treatment is often combined with radiation alone or both radiation and chemotherapy. Patients in advanced stage are usually treated by radiation alone.
Laterza et al. [11] reported the treatment and outcome of patients with cervical carcinosarcoma. Sixteen patient with stage IB. three patients with stage II, two patients with stage III, 12 patients whose stage was not available were included. Six of sixteen patients with stage IB were treated by surgery alone, 5 of 16 patients with stage IB were treated by surgery and radiation, 4 of 16 patients with stage IB were treated by surgery and chemotherapy with or without radiation, 1 of 16 patients with stage IB were treated by radiation alone. During median 16-month (6-156) follow-up, 6 were no evidence of disease, 1 were alive with disease, 5 died of disease, and 2 were lost to follow up. Two of three patients with stage II were treated by surgery and radiation. One of three patients with stage II were treated by radiation alone. Two were no evidence of disease and 1 died of disease. All 2 patients with stage III were treated by radiation alone, 2 of 2 died of disease. Fifty percent of the patients with early stage tumors were free of disease at last follow up, regardless of treatment method. Median time to death were 10.5 months (2-42). In Korea, Kang et al. [7] treated a patient with stage IB cervical carcinosarcoma by radical hysterectomy and adjuvant chemotherapy. No evidence of disease was shown at last follow up, but duration of follow up was not stated. Sunwoo et al. [8] performed hysterectomy with bilateral salpingoophorectomy only on a patient with stage IB cervical carcinosarcoma and the patient were free of disease during 14-month follow-up. In this case, our patient with stage IB2 underwent radical hysterectomy and chemotherapy and died of disease seven months later.
Cases of extracervical neoplastic disease are associated with a worse prognosis. Cervical carcinoma usually presents at an earlier stage than carcinosarcoma of the uterine corpus and is therefore associated with an earlier diagnosis and better prognosis [10].
Unlike carcinosarcoma of the uterine corpus, cervical carcinosarcoma derives from two different origins: the Müllerian ducts and mesonephric duct remnants [3,4,5,6]. Mesonephric adenocarcinoma can arise from the uterine cervix and 42 subjects with mesonephric adenocarcinoma of the uterine cervix have been reported in the English literature. Mesonephric adenocarcinomas are often accompanied by sarcomatous components, as observed in 10 out of the 42 cases (24%) [3,4,5,6]. To explain the diverse histological components of gynaecologic carcinosarcoma, some hypotheses have been proposed. It was recently discovered that carcinosarcomas are dedifferentiated (metaplastic) carcinomas comprised of carcinomatous and sarcomatous elements arising from a single malignant clone. It is necessary to consider sarcomatoid squamous cell carcinoma as a possible differential diagnosis. Before epithelial-mesenchymal transition theory, i.e., the theory that carcinoma transforms into sarcoma, was widely accepted, there was some confusion about differentiating between the two diseases [12]. The traditional view was that carcinosarcoma does not exhibit merging of its carcinomatous and sarcomatous components. Therefore sarcomatoid squamous cell carcinoma could be differentiated from carcinosarcoma of the cervix [12]. It is now known that carcinosarcoma does indeed exhibit merging of its carcinomatous and sarcomatous components, i.e., the component that is positive for both cytokeratin and vimentin can be observed. Though some authors classify tumors with both squamous cell carcinoma and sarcoma as carcinosarcoma [13], the carcinomatous component of carcinosarcoma is usually of the adenocarcinomatous or undifferentiated type. It is reasonable to consider sarcomatoid squamous cell carcinoma and carcinosarcoma as having common pathogenesis of epithelial-mesenchymal transition but their carcinomatous components contain different cell types.
Mesonephric adenocarcinoma with a sarcomatous component is also a subtype of cervical carcinosarcoma [2]. Meguro et al. [2] reported that 16% of cervical carcinosarcoma is of mesonephric duct origin. Subsequently, if a sarcomatous component is recognised in the biopsy specimen, it may be suspected of being of mesonephric duct origin. In our present case, the cervical punch biopsy was reported to be a sarcoma or undifferentiated carcinoma. To assess the possibility of origination from mesonephric duct remnants, immunohistochemical studies including mesonephric marker (CD 10, carletinin, estrogen receptor) were performed to determine its origin. The epithelial component was composed of adenocarcinoma, positive for pancytokeratin and estrogen receptor, but negative for CD 10 and carletinin in immunohistochemical studies (Fig. 2). The mesenchymal component was positive for vimentin. None of the components were positive for both cytokeratin and vimentin. Unlike the usual endocervical-type adenocarcinoma, it is known that mesonephric adenocarcinoma is not related to human papillomavirus infection. P16 regarded as a surrogate marker of the presence of human papillomavirus was positive in this case. Our case is of Müllerian duct origin, although the clinical significance of differentiating the origin of cervical carcinosarcoma is unknown so far.
In conclusion, cervical carcinosarcoma is extremely rare, especially when coincident with breast cancer. Although evidence-based recommendations for treatment guidelines are unavailable due to rarity of the disease, surgery is the mainstay of treatment. Radiotherapy and adjuvant chemotherapy may also be used. Cervical carcinosarcoma can be characterized by their origin which can be either the Müllerian ducts or mesonephric duct remnants. The origin of cervical carcinosarcoma needs to be verified and immunohistochemical studies using mesonephric markers can be invaluable for verification.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Arend R, Doneza JA, Wright JD. Uterine carcinosarcoma. Curr Opin Oncol. 2011;23:531–536. doi: 10.1097/CCO.0b013e328349a45b. [DOI] [PubMed] [Google Scholar]
- 2.Meguro S, Yasuda M, Shimizu M, Kurosaki A, Fujiwara K. Mesonephric adenocarcinoma with a sarcomatous component, a notable subtype of cervical carcinosarcoma: a case report and review of the literature. Diagn Pathol. 2013;8:74. doi: 10.1186/1746-1596-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clement PB, Young RH, Keh P, Ostor AG, Scully RE. Malignant mesonephric neoplasms of the uterine cervix. A report of eight cases, including four with a malignant spindle cell component. Am J Surg Pathol. 1995;19:1158–1171. doi: 10.1097/00000478-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bague S, Rodriguez IM, Prat J. Malignant mesonephric tumors of the female genital tract: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2004;28:601–607. doi: 10.1097/00000478-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, Tavassoli FA. Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol. 2001;25:379–387. doi: 10.1097/00000478-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Chardi L, Gonzalez-Bosquet E, Rovira Zurriaga C, Lailla Vicens JM. Mesonephric carcinosarcoma of the uterine cervix: a case report. Eur J Gynaecol Oncol. 2013;34:336–338. [PubMed] [Google Scholar]
- 7.Kang JS, Kim T, Lee HK, Lim NI, Park MH. Primary carcinosarcoma of the uterine cervix. Obstet Gynecol Sci. 1994;37:1872–1879. [Google Scholar]
- 8.Sunwoo JG, Cho IS, Jeon S, Bae DH, Shin YW, Kim CJ, et al. A case of malignant mixed Müllerian Tumor (MMMT) of the uterine cervix. Obstet Gynecol Sci. 2008;51:350–354. [Google Scholar]
- 9.Bansal S, Lewin SN, Burke WM, Deutsch I, Sun X, Herzog TJ, et al. Sarcoma of the cervix: natural history and outcomes. Gynecol Oncol. 2010;118:134–138. doi: 10.1016/j.ygyno.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Clement PB, Zubovits JT, Young RH, Scully RE. Malignant mullerian mixed tumors of the uterine cervix: a report of nine cases of a neoplasm with morphology often different from its counterpart in the corpus. Int J Gynecol Pathol. 1998;17:211–222. [PubMed] [Google Scholar]
- 11.Laterza R, Seveso A, Zefiro F, Formenti G, Mellana L, Donadello N, et al. Carcinosarcoma of the uterine cervix: case report and discussion. Gynecol Oncol. 2007;107:S98–S100. doi: 10.1016/j.ygyno.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Kumar M, Bahl A, Sharma DN, Agarwal S, Halanaik D, Kumar R, et al. Sarcomatoid squamous cell carcinoma of uterine cervix: pathology, imaging, and treatment. J Cancer Res Ther. 2008;4:39–41. doi: 10.4103/0973-1482.39604. [DOI] [PubMed] [Google Scholar]
- 13.Kadota K, Haba R, Ishikawa M, Kushida Y, Katsuki N, Hayashi T, et al. Uterine cervical carcinosarcoma with heterologous mesenchymal component: a case report and review of the literature. Arch Gynecol Obstet. 2009;280:839–843. doi: 10.1007/s00404-009-1017-0. [DOI] [PubMed] [Google Scholar]