Abstract
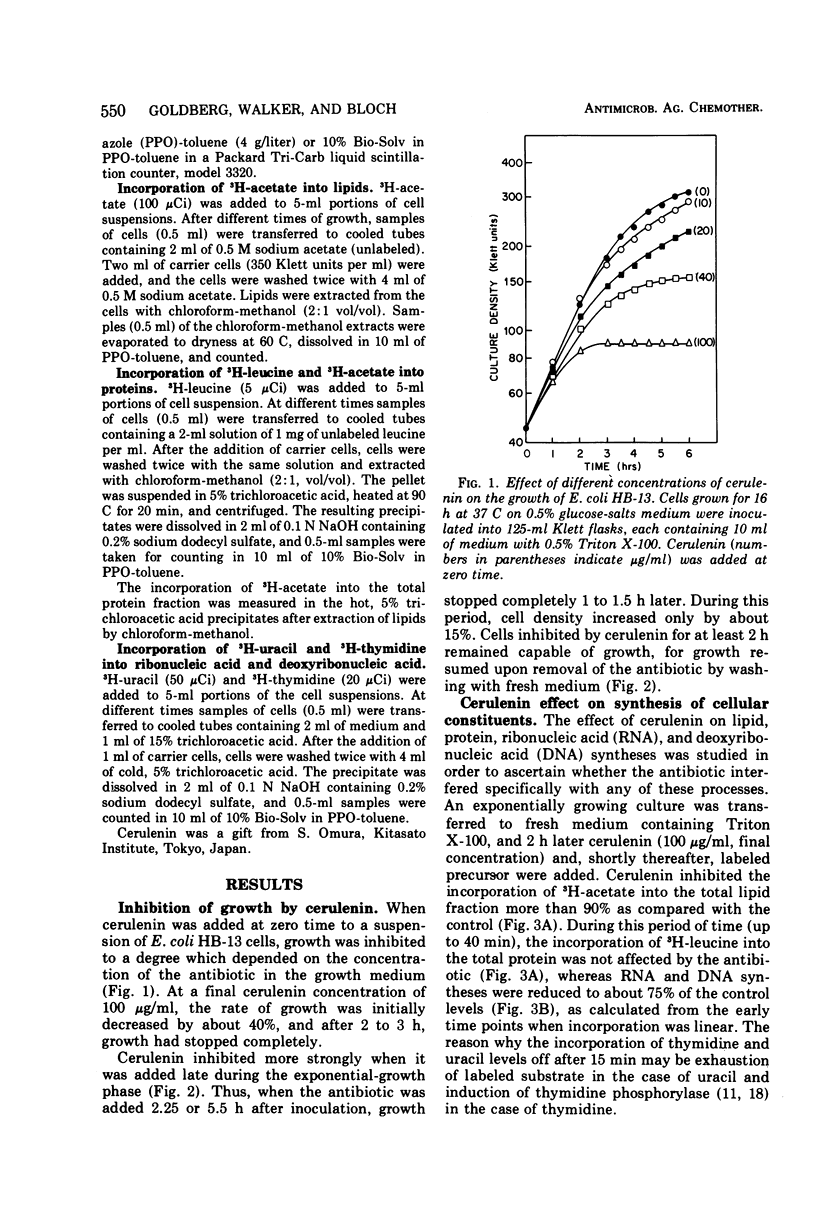
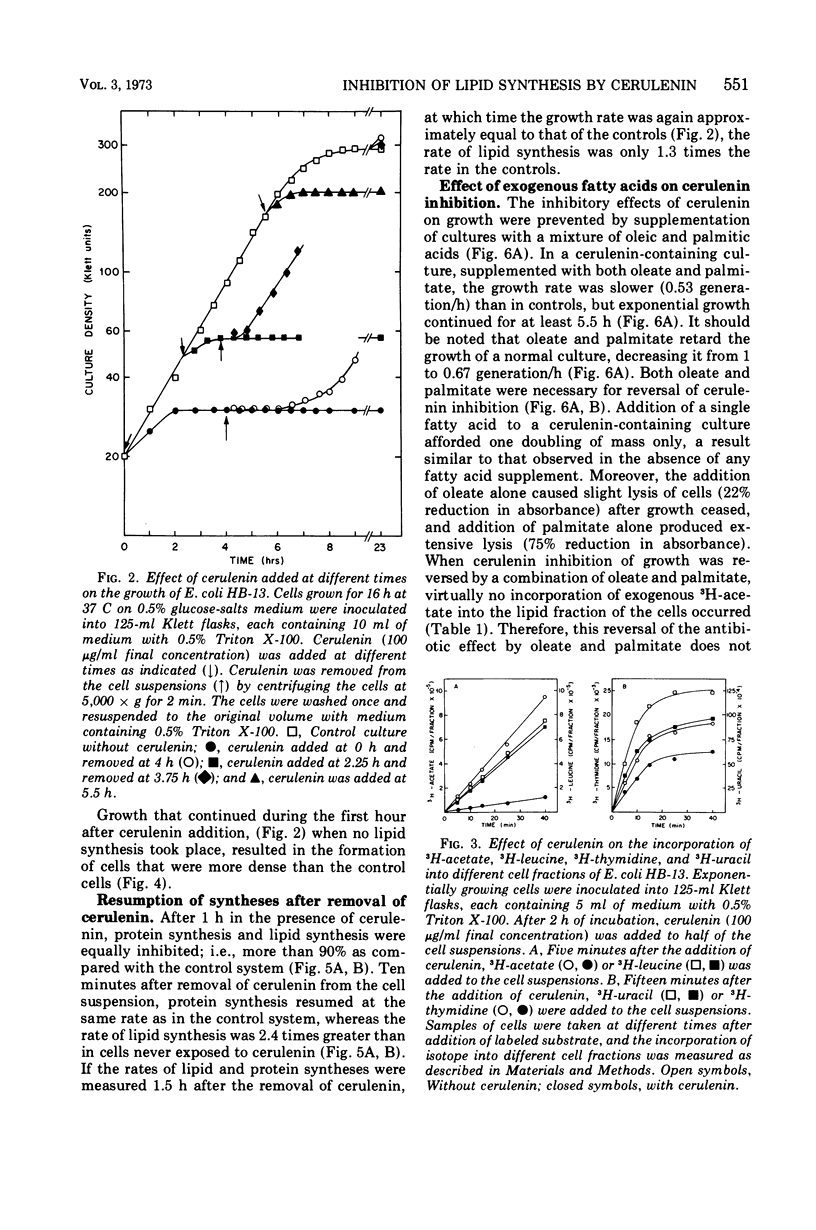
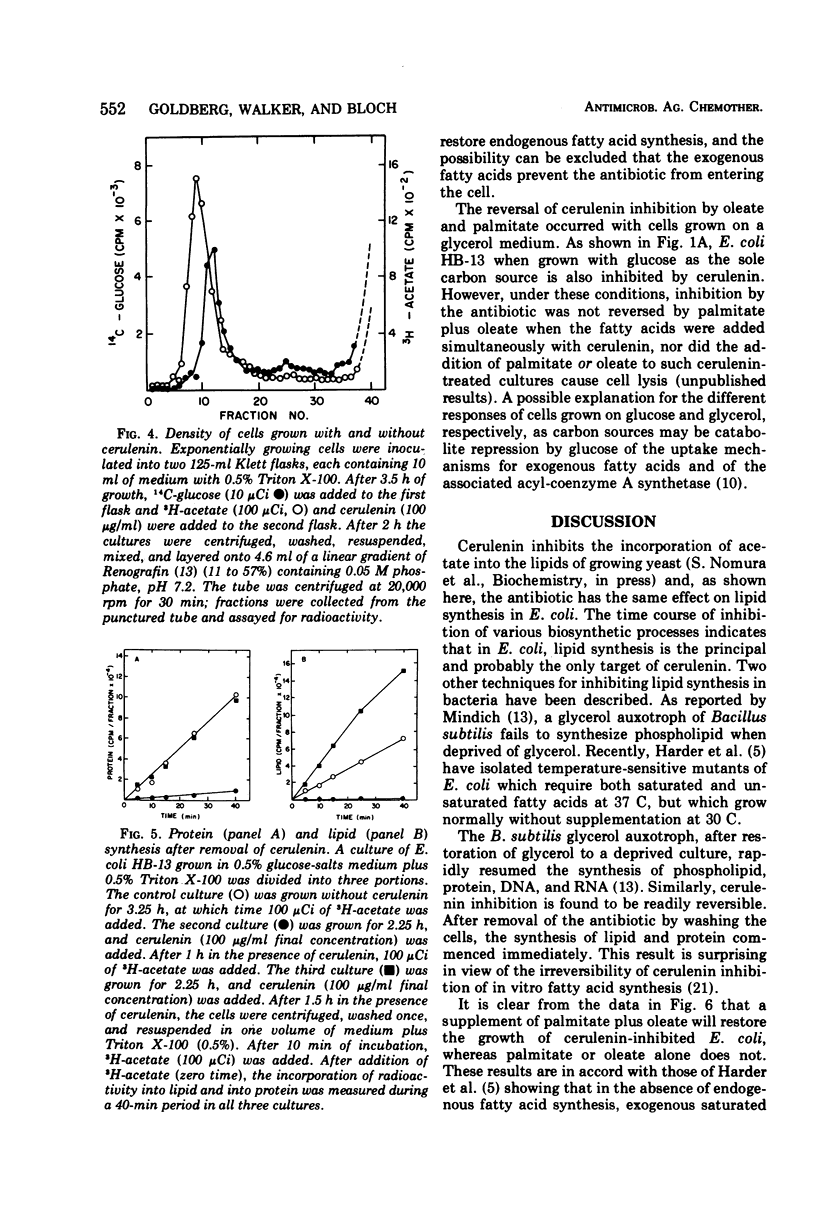
The antibiotic cerulenin markedly inhibits the growth of Escherichia coli. The effects of the antibiotic on cellular syntheses were studied by measuring the incorporation of labeled precursors into lipids and macromolecules. During the first 40 min after the addition of cerulenin to a culture of growing cells, lipid synthesis was inhibited more than 90% and ribonucleic acid and deoxyribonucleic acid synthesis about 25%, whereas protein synthesis was not affected. At later periods after cerulenin addition (1 to 2 h), the inhibition of cell growth and of lipid and protein synthesis was complete. Upon removal of cerulenin from the culture, growth was restored and lipid synthesis resumed more rapidly than did the synthesis of protein.
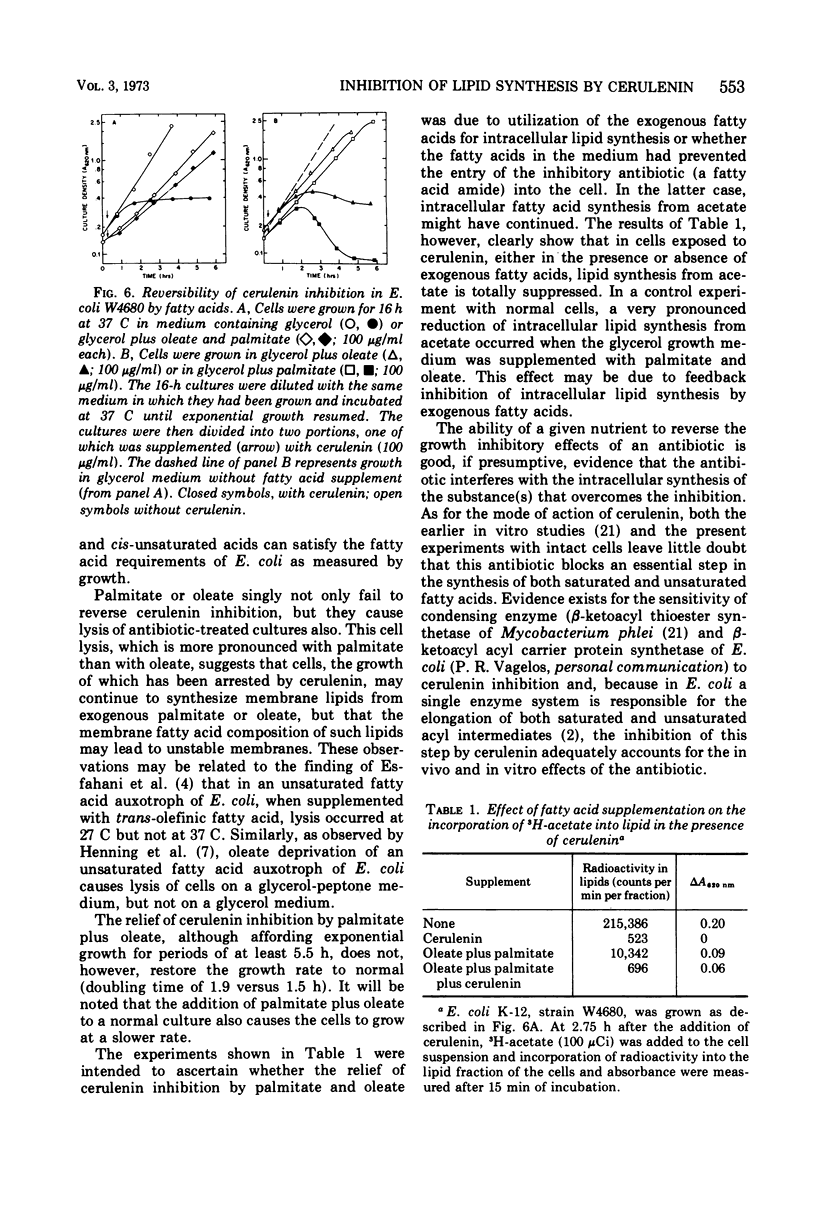
Addition of both palmitate and oleate, but not of either fatty acid alone, reversed the inhibition of growth by cerulenin. These findings support the conclusion that the antibiotic effects of cerulenin are due to a specific inhibition of fatty acid synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behme R. J., Fitz-James P. C. Temperature-sensitive mutant of Bacillus subtilis that accumulates membrane-associated protein inclusions. J Bacteriol. 1972 Feb;109(2):906–915. doi: 10.1128/jb.109.2.906-915.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge C. H., Vagelos P. R. Acyl carrier protein. XVII. Purification and properties of -hydroxyacyl acyl carrier protein dehydrase. J Biol Chem. 1972 Aug 25;247(16):4930–4938. [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani M., Barnes E. M., Jr, Wakil S. J. Control of fatty acid composition in phospholipids of Escherichia coli: response to fatty acid supplements in a fatty acid auxotroph. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1057–1064. doi: 10.1073/pnas.64.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder M. E., Beacham I. R., Cronan J. E., Jr, Beacham K., Honegger J. L., Silbert D. F. Temperature-sensitive mutants of Escherichia coli requiring saturated and unsaturated fatty acids for growth: isolation and properties. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3105–3109. doi: 10.1073/pnas.69.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechemy K., Goldfine H. Isolation and characterization of a temperature-sensitive mutant of Escherichia coli with a lesion in the acylation of lysophosphatidic acid. Biochem Biophys Res Commun. 1971 Jan 22;42(2):245–251. doi: 10.1016/0006-291x(71)90094-5. [DOI] [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- MANSON L. A., LAMPEN J. O. The metabolism of desoxyribose nucleosides in Escherichia coli. J Biol Chem. 1951 Dec;193(2):539–547. [PubMed] [Google Scholar]
- MATSUMAE A., NOMURA S., HATA T. STUDIES ON CERULENIN. IV. BIOLOGICAL CHARACTERISTICS OF CERULENIN. J Antibiot (Tokyo) 1964 Jan;17:1–7. [PubMed] [Google Scholar]
- Mindich L. Induction of Staphylococcus aureus Lactose Permease in the Absence of Glycerolipid Synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):420–424. doi: 10.1073/pnas.68.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Omura S., Katagiri M., Nakagawa A., Sano Y., Nomura S. Studies on cerulenin. V. Structure of cerulenin. J Antibiot (Tokyo) 1967 Nov;20(6):349–354. [PubMed] [Google Scholar]
- Omura S., Nakagawa A., Sekikawa K., Otani M., Hata T. Studies on cerulenin. VI. Some spectroscopic features of cerulenin. Chem Pharm Bull (Tokyo) 1969 Nov;17(11):2361–2363. doi: 10.1248/cpb.17.2361. [DOI] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]



