Abstract
Budding yeast can undergo filamentous growth in response to nutrient limitation. Filament formation can be examined under glucose-limiting conditions by the single-cell invasive growth assay, or under nitrogen-limiting conditions with the pseudohyphal growth assay, both described here. The single-cell assay allows robust quantitation of changes in budding pattern and cell length, and most cells in the population show the response. The pseudohyphal growth assay reveals filamentous patterns in larger microcolonies and adjoining subpopulations of cells. Historically, the single-cell assay has been used to study filamentous growth in haploid cells, and the pseudohyphal growth assay in diploid cells. However, both assays can be used in either cell type.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPES: Please see the end of this protocol for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Distilled H2O (dH2O), sterile
Glucose (50%) (optional; see Step 5)
-
Plates for single-cell invasive growth assay or pseudohyphal growth assay (see Step 5)
Isobutanol agar plates <R>
S-GLU agar plates <R>
-
SLAHD agar plates <R>
Prepare plates within 24 h of the experiment, as the level of moisture in the plates is critical for optimal filamentous growth.
SD agar plates <R>
SD liquid medium <R>
-
Yeast strains of interest
The Σ1278b background undergoes filamentous growth (Gimeno et al. 1992). Commonly used laboratory strains have lost the ability to undergo filamentous growth (Liu et al. 1996). A MAPK pathway mutant (e.g., ste11Δ) should be included in the assay as a negative control. Maintain stocks on SD plates (not YEPD).
Equipment
These assays require the limitation of glucose or nitrogen. Use clean dishware and glass spreaders, as trace glucose or nitrogen contamination suppresses the filamentous morphology.
Coverslips
Flat toothpicks, sterile
Glass plate spreader, sterile
Glass tubes with metal tops, sterile
Incubator set at 30°C
Innoculation loop or long wooden toothpick, sterile
Light microscope with 100× objective
Manual dissection microscope (e.g., SPOREPLAY, Singer Instruments) (optional; see Step 5)
Microcentrifuge
Microcentrifuge tubes, sterile
Microscope oil (optional; see Step 7)
Parafilm
Pipette tips, sterile
Shaking incubator or a shaker in a 30°C room
METHOD
-
Using a long wooden toothpick or inoculation loop, inoculate yeast from freshly patched SD plates (from freezer stocks or a plate stored at 4°C) into 5 mL of SD medium in a glass tube with a metal top and grow until saturation is reached.
We typically grow cultures for 16 h (overnight) at 30°C with shaking at 225 rpm. Synthetic (SD) medium reduces clumpiness, and clumps of cells are a poor starting material for the single-cell assay.
Remove 1 mL of cells from the overnight culture. Pellet the cells by centrifugation at 16,000g for 1 min at 20°C.
Wash the cells twice in 1 mL of sterile dH2O. Resuspend the cells in 1 mL of sterile dH2O.
Dilute 50 μL of cells in 1 mL dH2O.
-
Spread the cells on plates as follows. Alternatively, place individual cells onto the appropriate plates under a dissection microscope.
-
For the single-cell invasive growth assay, spread 50 μL of cells from the dilution onto S-GLU agar plates using a sterile glass spreader. Allow the plates to dry for 5 min, and then proceed to Step 6.
Isobutanol can been used to stimulate filamentous growth (Lorenz et al. 2000; Chen and Fink 2006). Grow cells on isobutanol agar plates prepared with and without glucose.
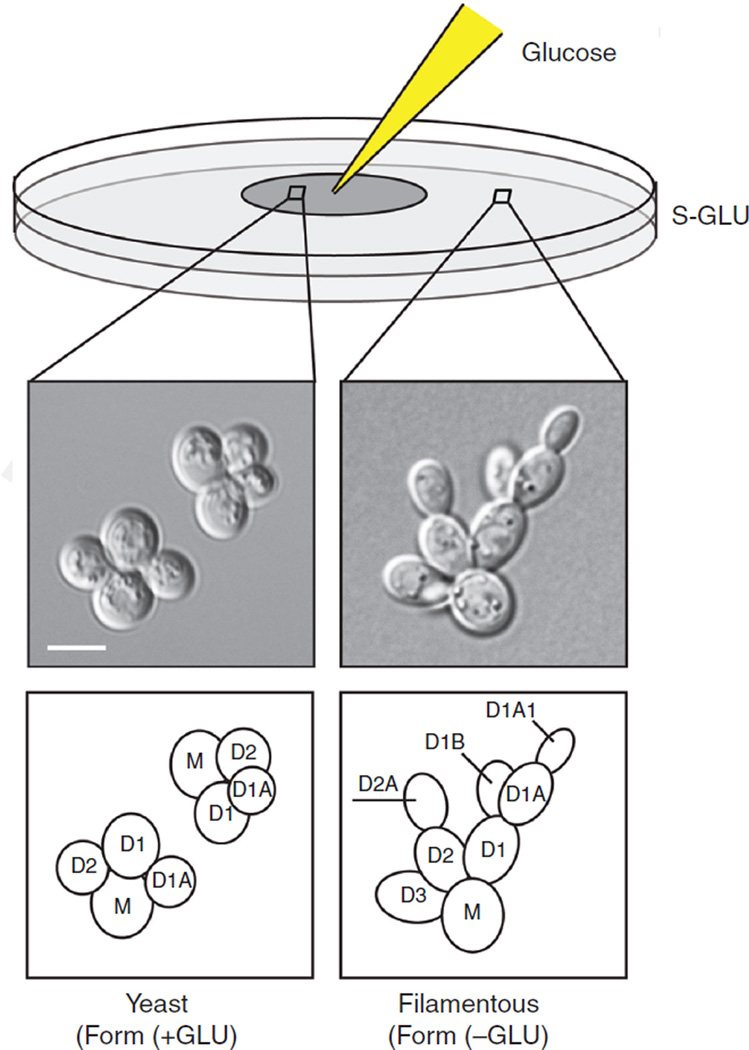
To visualize yeast-form morphology (within the region of diffused glucose) and filamentous-form morphology (outside the region of diffused glucose) (Fig. 1), spot 10 μL of 50%glucose at the center of the plate after the cells are spread.
For the pseudohyphal growth assay, spread 50 μL of cells from the dilution onto SLAHD agar plates using a glass spreader. Allow the plates to dry for 5 min, and then proceed to Step 6.
-
Wrap agar plates with lids in Parafilm and incubate them without inversion at 30°C for 16 h for the single-cell invasive growth assay or for 48 h for the pseudohyphal growth assay.
-
Remove the plates from the incubator and allow them to equilibrate to room temperature. Place the plates on the microscope stage and examine at 20×. Place a coverslip on the plates, directly over the cells, and observe at 40× or at 100× using oil immersion.
Be careful when manipulating plates on a microscope stage. Microscope presets are designed for slides. Objectives can be damaged if the objective runs into the plate.
FIGURE 1.
A single-cell invasive growth assay. Cells grown on synthetic media lacking glucose undergo filamentous growth within the first few cell divisions. Round mother cells (M) produce elongated progeny (D1, D2, and D3) that propagate away from the mother (D2A, D1A, D1B, and D1A1). Bar, 5 μm. Spotting glucose (GLU) at the center of the plate rescues the round cell shape and axial budding pattern.
DISCUSSION
In the single-cell invasive growth assay (Fig. 1; Cullen and Sprague 2000), cells within the region of diffused glucose should be in the yeast form, appear round, and show an axial budding pattern. Cells outside the region of diffused glucose should be in the filamentous form, appear elongated, and show a distal-unipolar budding pattern (e.g., D1A and D1A1). If desired, microcolony development can be visualized by photographing the cells over a time series starting immediately after they have been spread.
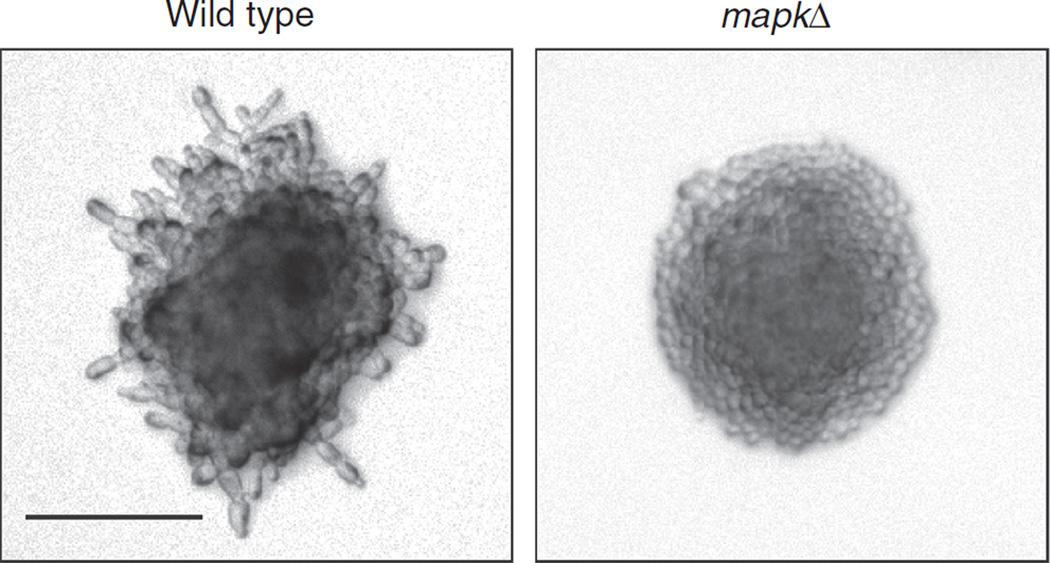
In the pseudohyphal growth assay (Fig. 2; Gimeno et al. 1992), wild-type cells should produce filaments, whereas MAPK pathway mutant cells should not.
FIGURE 2.
Results from a pseudohyphal growth assay. Wild-type cells grown on limiting nitrogen produce filaments (left), whereas a MAPK mutant (right) does not. Bar, 100 μm.
RECIPES
Isobutanol Agar Plates
2% glucose (prepare plates with and without glucose)
6.7 g/L yeast nitrogen base without amino acids
-
Amino acids
20 mg/L histidine
120 mg/L leucine
60 mg/L lysine
20 mg/L arginine
20 mg/L tryptophan
20 mg/L tyrosine
40 mg/L threonine
20 mg/L methionine
50 mg/L phenylalanine
20 mg/L uracil
20 mg/L adenine
20 g/L agar
Include any additional amino acids that may be required for the growth of an auxotrophic strain. Prepare in H2O and sterilize by autoclaving. Add 1% isobutanol or isoamyl alcohol. Fill sterile Petri dishes with ~25 mL of autoclaved medium.
SD Agar Plates
2% Glucose
6.7 g/L yeast nitrogen base without amino acids
-
Amino acids
20 mg/L histidine
120 mg/L leucine
60 mg/L lysine
20 mg/L arginine
20 mg/L tryptophan
20 mg/L tyrosine
40 mg/L threonine
20 mg/L methionine
50 mg/L phenylalanine
20 mg/L uracil
20 mg/L adenine
20 g/L agar
Include any additional amino acids that may be required for the growth of an auxotrophic strain. Prepare in H2O and sterilize by autoclaving. Fill sterile Petri dishes with ~25 mL of autoclaved medium.
SD Liquid Medium
2% glucose
6.7 g/L yeast nitrogen base without amino acids
-
Amino acids:
20 mg/L histidine
120 mg/L leucine
60 mg/L lysine
20 mg/L arginine
20 mg/L tryptophan
20 mg/L tyrosine
40 mg/L threonine
20 mg/L methionine
50 mg/L phenylalanine
20 mg/L uracil
20 mg/L adenine
Include any additional amino acids that may be required for the growth of an auxotrophic strain. Prepare in H2O and sterilize by autoclaving.
S-GLU Agar Plates
6.7 g/L yeast nitrogen base without amino acids
-
Amino acids
20 mg/L histidine
120 mg/L leucine
60 mg/L lysine
20 mg/L arginine
20 mg/L tryptophan
20 mg/L tyrosine
40 mg/L threonine
20 mg/L methionine
50 mg/L phenylalanine
20 mg/L uracil
20 mg/L adenine
20 g/L agar
Include any additional amino acids that may be required for the growth of an auxotrophic strain. Prepare in H2O and sterilize by autoclaving. Fill sterile Petri dishes with ~25 mL of autoclaved medium.
SLAHD Agar Plates
2% glucose
6.7 g/L yeast nitrogen base without amino acids and ammonium sulfate
20 mg/L uracil
20 mg/L histidine or 500 mg/L proline
20 g/L agar
Histidine or proline can be used interchangeably, depending on the genotype of the yeast strain used. Include any additional amino acids that may be required for the growth of an auxotrophic strain. Prepare in H2O and sterilize by autoclaving. Fill sterile Petri dishes with ~25 mL of autoclaved medium.
ACKNOWLEDGMENTS
P.J.C. is supported by a U.S. Public Health Service grant (GM098629).
REFERENCES
- Chen H, Fink GR. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006;20:1150–1161. doi: 10.1101/gad.1411806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Sprague GF., Jr Glucose depletion causes haploid invasive growth in yeast. Proc Natl Acad Sci. 2000;97:13619–13624. doi: 10.1073/pnas.240345197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: Regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MC, Cutler NS, Heitman J. Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:183–199. doi: 10.1091/mbc.11.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]