Abstract
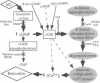
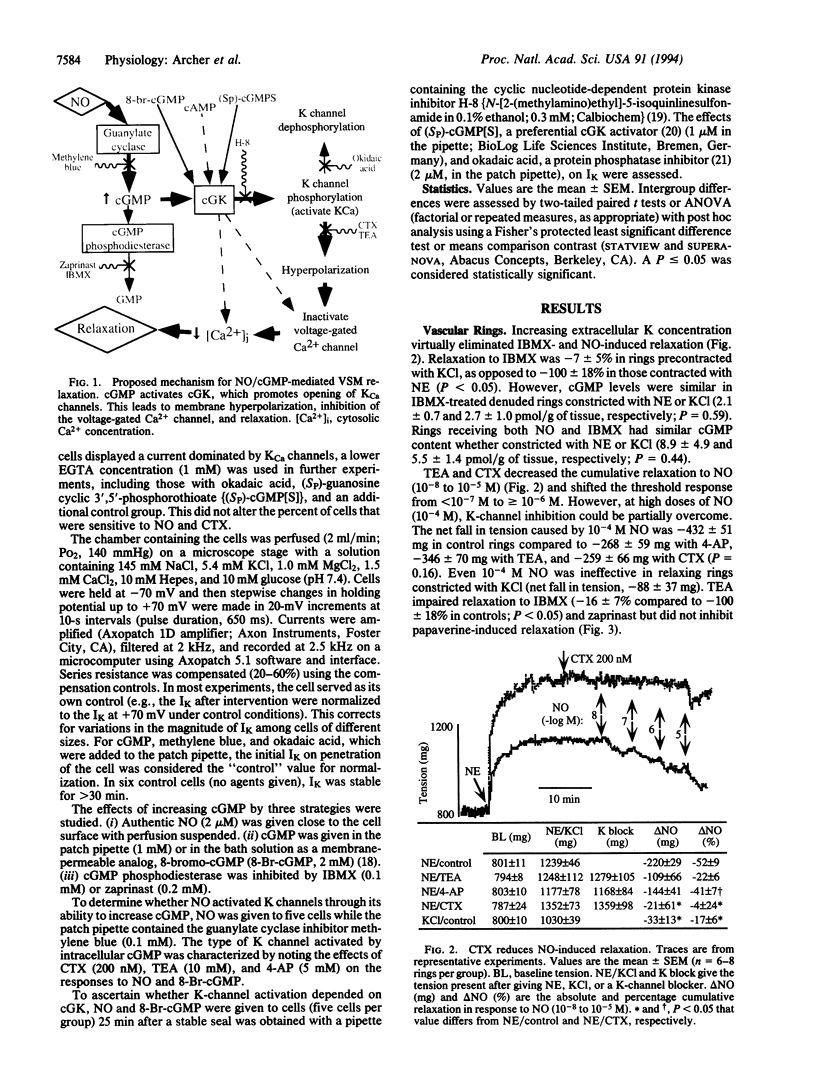
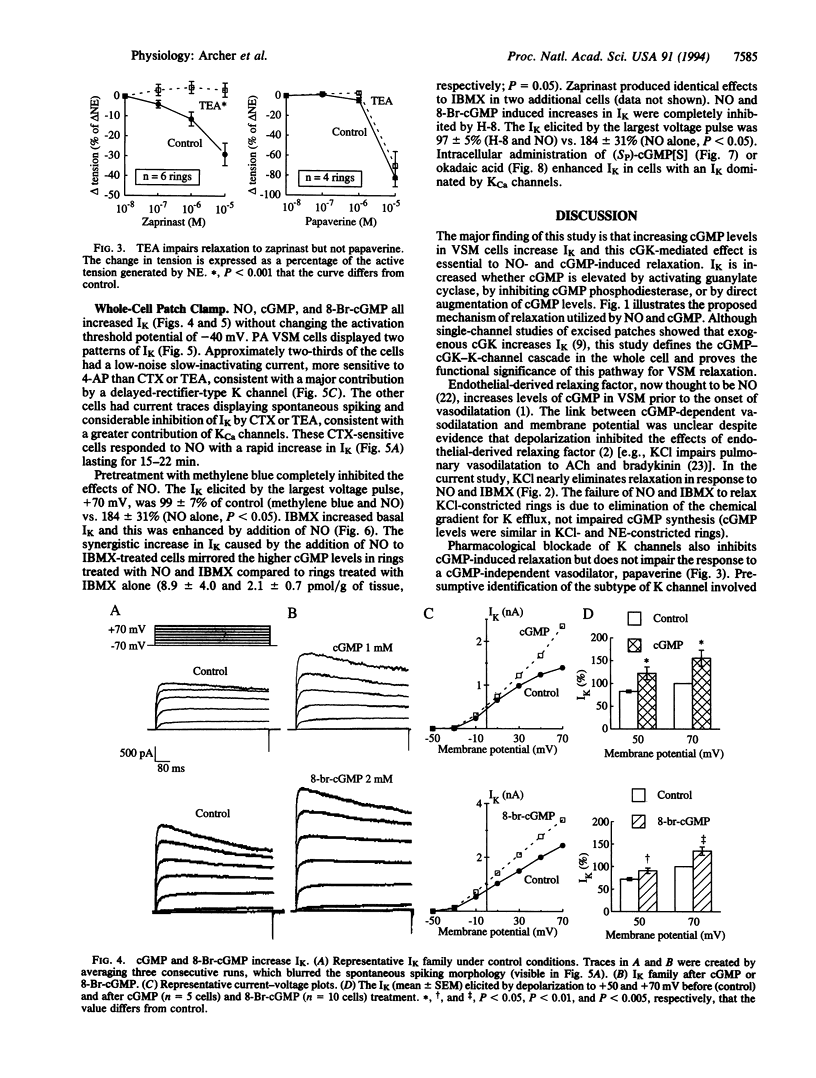
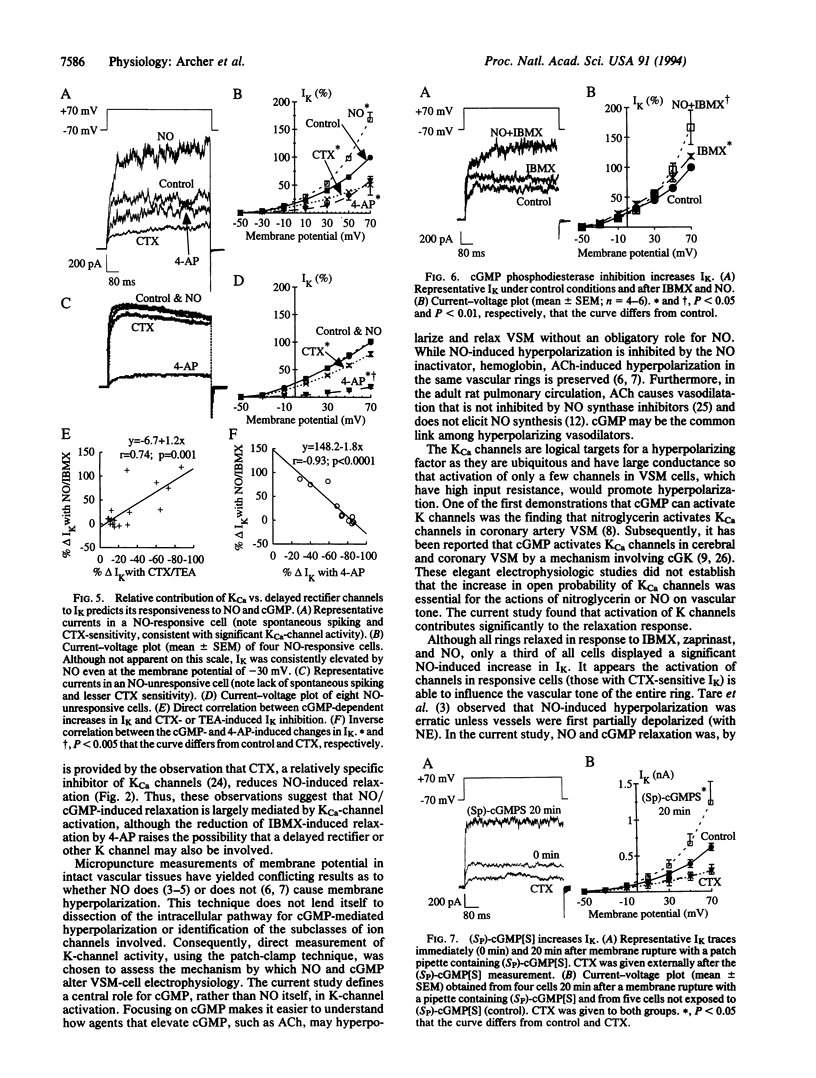
Nitric oxide (NO)-induced relaxation is associated with increased levels of cGMP in vascular smooth muscle cells. However, the mechanism by which cGMP causes relaxation is unknown. This study tested the hypothesis that activation of Ca-sensitive K (KCa) channels, mediated by a cGMP-dependent protein kinase, is responsible for the relaxation occurring in response to cGMP. In rat pulmonary artery rings, cGMP-dependent, but not cGMP-independent, relaxation was inhibited by tetraethylammonium, a classical K-channel blocker, and charybdotoxin, an inhibitor of KCa channels. Increasing extracellular K concentration also inhibited cGMP-dependent relaxation, without reducing vascular smooth muscle cGMP levels. In whole-cell patch-clamp experiments, NO and cGMP increased whole-cell K current by activating KCa channels. This effect was mimicked by intracellular administration of (Sp)-guanosine cyclic 3',5'-phosphorothioate, a preferential cGMP-dependent protein kinase activator. Okadaic acid, a phosphatase inhibitor, enhanced whole-cell K current, consistent with an important role for channel phosphorylation in the activation of NO-responsive KCa channels. Thus NO and cGMP relax vascular smooth muscle by a cGMP-dependent protein kinase-dependent activation of K channels. This suggests that the final common pathway shared by NO and the nitrovasodilators is cGMP-dependent K-channel activation.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. L., Cowan N. J. Measurement of endothelial cytosolic calcium concentration and nitric oxide production reveals discrete mechanisms of endothelium-dependent pulmonary vasodilatation. Circ Res. 1991 Jun;68(6):1569–1581. doi: 10.1161/01.res.68.6.1569. [DOI] [PubMed] [Google Scholar]
- Archer S. L., Tolins J. P., Raij L., Weir E. K. Hypoxic pulmonary vasoconstriction is enhanced by inhibition of the synthesis of an endothelium derived relaxing factor. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1198–1205. doi: 10.1016/0006-291x(89)91796-8. [DOI] [PubMed] [Google Scholar]
- Archer S. Measurement of nitric oxide in biological models. FASEB J. 1993 Feb 1;7(2):349–360. doi: 10.1096/fasebj.7.2.8440411. [DOI] [PubMed] [Google Scholar]
- Ashizawa N., Kobayashi F., Tanaka Y., Nakayama K. Relaxing action of okadaic acid, a black sponge toxin on the arterial smooth muscle. Biochem Biophys Res Commun. 1989 Aug 15;162(3):971–976. doi: 10.1016/0006-291x(89)90768-7. [DOI] [PubMed] [Google Scholar]
- Brayden J. E. Membrane hyperpolarization is a mechanism of endothelium-dependent cerebral vasodilation. Am J Physiol. 1990 Sep;259(3 Pt 2):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Mishima A., Hagiwara M., Sano M., Hayashi K., Inoue T., Naito K., Toshioka T., Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990 Mar 25;265(9):5267–5272. [PubMed] [Google Scholar]
- Fujino K., Nakaya S., Wakatsuki T., Miyoshi Y., Nakaya Y., Mori H., Inoue I. Effects of nitroglycerin on ATP-induced Ca(++)-mobilization, Ca(++)-activated K channels and contraction of cultured smooth muscle cells of porcine coronary artery. J Pharmacol Exp Ther. 1991 Jan;256(1):371–377. [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Iguchi M., Nakajima T., Hisada T., Sugimoto T., Kurachi Y. On the mechanism of papaverine inhibition of the voltage-dependent Ca++ current in isolated smooth muscle cells from the guinea pig trachea. J Pharmacol Exp Ther. 1992 Oct;263(1):194–200. [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Krippeit-Drews P., Morel N., Godfraind T. Effect of nitric oxide on membrane potential and contraction of rat aorta. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S72–S75. doi: 10.1097/00005344-199204002-00022. [DOI] [PubMed] [Google Scholar]
- Lau K., Bourdeau J. E. Evidence for cAMP-dependent protein kinase in mediating the parathyroid hormone-stimulated rise in cytosolic free calcium in rabbit connecting tubules. J Biol Chem. 1989 Mar 5;264(7):4028–4032. [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Minami K., Fukuzawa K., Nakaya Y., Zeng X. R., Inoue I. Mechanism of activation of the Ca(2+)-activated K+ channel by cyclic AMP in cultured porcine coronary artery smooth muscle cells. Life Sci. 1993;53(14):1129–1135. doi: 10.1016/0024-3205(93)90549-i. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Post J. M., Hume J. R., Archer S. L., Weir E. K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992 Apr;262(4 Pt 1):C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Robertson B. E., Schubert R., Hescheler J., Nelson M. T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993 Jul;265(1 Pt 1):C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Shultz P. J., Tayeh M. A., Marletta M. A., Raij L. Synthesis and action of nitric oxide in rat glomerular mesangial cells. Am J Physiol. 1991 Oct;261(4 Pt 2):F600–F606. doi: 10.1152/ajprenal.1991.261.4.F600. [DOI] [PubMed] [Google Scholar]
- Taniguchi J., Furukawa K. I., Shigekawa M. Maxi K+ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflugers Arch. 1993 May;423(3-4):167–172. doi: 10.1007/BF00374390. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. K., Francis S. H., Corbin J. D. Characterization of a purified bovine lung cGMP-binding cGMP phosphodiesterase. J Biol Chem. 1990 Sep 5;265(25):14964–14970. [PubMed] [Google Scholar]
- Twort C. H., van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988 May;62(5):961–964. doi: 10.1161/01.res.62.5.961. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Jr, Katz G. M., Roy-Contancin L., Reuben J. P. Guanosine 5'-monophosphate modulates gating of high-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9360–9364. doi: 10.1073/pnas.85.23.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]