Abstract
Medical ultrasound imaging with Doppler plays an essential role in the diagnosis of vascular disease. This study intended to review the clinical use of “to-and-fro” waveform at duplex Doppler ultrasonography (DDU) in the diagnosis of pseudoaneurysms in the arterial vessels of upper and lower extremities, abdominal aorta, carotid and vertebral arteries as well as to review our personal experiences of “to-and-fro” waveform at DDU also. After receiving institutional review board approval, an inclusive literature review was carried out in order to review the scientific foundation of “to-and-fro” waveform at DDU and its clinical use in the diagnosis of pseudoaneurysms in various arterial vessels. Articles published in the English language between 2000 and 2013 were evaluated in this review study. Pseudoaneurysms in arterial vessels of the upper and lower extremities, abdominal aorta, carotid and vertebral arteries characterized by an extraluminal pattern of blood flow, which shows variable echogenicity, interval complexity, and “to-and-fro” flow pattern on color Doppler ultrasonography. In these arterial vessels, Duplex ultrasonography can demonstrate the degree of clotting, pseudoaneurysm communication, the blood flow patterns and velocities. Spectral Doppler applied to pseudoaneurysms lumen revealed systolic and diastolic turbulent blood flow with traditional “to-and-fro” waveform in the communicating channel. Accurate diagnosis of pseudoaneurysm by spectral Doppler is based on the documentation of the “to-and-fro” waveform. The size of pseudoaneurysm determines the appropriate treatment approach as surgical or conservative.
Keywords: Pseudoaneurysm, To-and-fro waveform, Ultrasonography, Percutaneous thrombin injection, Yin-Yang sign
Core tip: A review of the clinical use of “to-and-fro” waveform at duplex Doppler ultrasonography (DDU) in the diagnosis of pseudoaneurysms in the arterial vessels of upper and lower extremities, abdominal aorta, carotid and vertebral arteries as well as to review our personal experiences of “to-and-fro” waveform at DDU also.
INTRODUCTION
A pseudoaneurysm is defined as an arterial wall deficiency, which leads to accumulation of oxygenated blood in the nearby extra-luminal region. Therefore arterial blood spread out of the vessel, forming a sac surrounding by soft tissue and compressed thrombus[1]. Consequently, a pseudoaneurysm is formed as a result of fibrin wall formation nearby the swelling[2]. The basic difference of arterial aneurysm and pseudoaneurysm is that the three-layers of the arterial wall don’t bind the later one[3]. Pseudoaneurysms which are the most common in the femoral and radial arteries, often noticed in the groin and forearm after cardiac catheterization. Furthermore, it may observe also after arterial punctures of blood gas analysis, after the placement of indwelling catheter or after direct arterial trauma[2-5].
Ultrasonography (US) has been widely utilized as a noninvasive imaging modality for the investigation of vascular diseases[6-9]. US which is a valuable tool for diagnosis of pseudoaneurysms has been widely utilized as a noninvasive imaging modality for investigation of vascular disease. The main advantage of US imaging is no use of ionizing radiation, cheap, and availability[10,11]. It has been reported that US has 94% and 97% of sensitivity and specificity, respectively in the diagnosis of postcatheterization pseudoaneurysms, but this sensitivity is not enough to diagnose the pseudoaneurysms of the deep visceral arteries[12,13]. The major limitation of US it is an operator dependent imaging technique, has low sensitivity in the evaluation of deep visceral artery pseudoaneurysm, and evaluation of vessels in trauma patient accompanied with hematoma or fracture[14].
In pseudoaneurysms, Analog US images (grayscale) usually illustrate hypoechoic cystic structures nearby a supplying artery[15,16]. Grayscale can be used to evaluate many pseudoaneurysmal findings such as the size, the number of pseudoaneurysm, and its relation to the artery[17]. However, grayscale is not a conclusive evidence in diagnosis pseudoaneurysm because its findings are accompanied by other clinical conditions such as hematomas and cystic masses either simple or complex[1].
Therefore, Doppler US can be used to confirm the diagnosis. In addition to that, blood flow in a cystic structure distinguished by swirling motion pattern “yin-yang sign”. Also, this type of flow can be detected in saccular aneurysm. Therefore, differential diagnosis is essential for pseudoaneurysm. The cornerstone of pseudoaneurysm diagnosis is dependent upon the appearance of the communicating neck between the arterial vessel and pseudoaneurysmal sac with “to-and-fro” waveform at duplex Doppler ultrasonography (DDU). The “to” represents the arterial blood going into the pseudoaneurysmal sac in systolic cycle, while “fro” illustrate blood exiting the sac in diastolic cycle[18].
In this article authors review the clinical use of “to-and-fro” waveform at DDU in the diagnosis of pseudoaneurysms in various arterial vessels, as well as our personal experiences of “to-and-fro” waveform at DDU in the Radiology Department of King Fahad Medical City (KFMC) at Riyadh, Saudi Arabia.
LITERATURE SEARCH
After receiving institutional review board approval, an inclusive literature review was carried out in order to review the scientific foundation of “to-and-fro” waveform at DDU and its clinical use in the diagnosis of pseudoaneurysms in various arterial vessels of the upper and lower extremities, abdominal Aorta, carotid arteries and vertebral arteries.
The ScienceDirect, PubMed, MEDLINE, NCBI and SAGE database were searched in April 2014 for publications containing information about “to-and-fro” sign in the diagnosis of pseudoaneurysms in various arterial vessels in the title of the report. Abstracts resulting from this search were reviewed for relevance to the clinical outcomes from the procedure. Full manuscripts were retrieved and reviewed if they contained information regarding the evaluation of the evidence on the role of “to-and-fro” sign in the diagnosis of pseudoaneurysms and the published clinical literature in this field.
Only those papers published between 2000 and 2013 were included in the outcomes analysis, and this was due to the tremendous development in this medical diagnostic specialty at the beginning of the new millennium so far. Regarding place of the study or journal type in order to include all available sources of experience.
GENERAL SONOGRAPHIC FEATURES REGARDING ARTERIAL PSEUDOANEURYSMS
US is a readily available imaging modality, which does not expose the patient to ionizing radiation. Grayscale and color Doppler techniques are utilized, and standardized protocols in an accredited ultrasound laboratory will increase the likelihood of detection of pseudoaneurysm. Grayscale is often initially performed. Linear (high frequency) US probe has acceptable depth penetration and visualization should be used. After a general overview of the area of concern, attention should be given to any anechoic collections or regions of hematoma[19].
Color Doppler is placed in any anechoic collection to detect flow. The flow can be characterized if the scale is properly set to avoid aliasing due to under sampling. Spectral Doppler is then performed if flow is detected to help characterize arterial vs venous flow. Spectral waveforms may be diagnostic of the pseudoaneurysm and help to exclude arteriovenous fistula (AVF). A low wall filter may be initially used to detect slow flow, and the waveform should fill at least two-thirds of the spectral window. In grayscale, a patent pseudoaneurysm appears as an anechoic rounded or ovoid structure. Because other fluid collections, including cysts, seromas, or hematomas can have this appearance, color Doppler imaging is used to confirm the presence of blood flow within the pseudoaneurysm. When present, thrombus in the pseudoaneurysm appears mildly echogenic or hypoechoic without flow; it may be mural or centrally fill a portion of the pseudoaneurysm lumen[1]. Turbulent blood flow is illustrated by interchangeable coloring appearance, either in red or blue color. If large areas of color aliasing are identified in the adjacent tissues, grayscale may help to differentiate pseudoaneurysm from tissue reverberation associated with AVF[19].
The scientific foundation of “to-and-fro” waveform at DDU and its clinical use in the diagnosis of pseudoaneurysms in various arterial vessels, could be discussed on the basis that, the DDU monitoring will present the conventional “to-and-fro” waveform with blood flow of the bidirectional pattern at the neck of pseudoaneurysm. Occasionally, the neck is the only patent portion of the pseudoaneurysm if partial thrombosis has occurred[19].
INCIDENCE OF PSEUDOANEURYSM IN THE PERIPHERAL ARTERIES, ABDOMINAL AORTA AND NECK ARTERIES
The incidence of pseudoaneurysms has increased in hospital based practice, due to the large number of invasive procedures performed[20]. Their incidence varies in the literature due to different definitions, methods of interrogation and presence of certain complications[21]. According to medical literature, the incidence of pseudoaneurysms ranges from 0.1% to 6% and up to 0.5% to 9%, depending on the diagnostic or therapeutic procedure performed[22,23].
The frequency of peripheral arteries pseudoaneurysms is much less in the upper extremities than in the lower extremities (less than 2% of all lesions)[24,25]. Aortic pseudoaneurysms are rare, life-threatening sequelae of cardiac surgery[26]. The incidence, risk factors, and natural history of aortic pseudoaneurysm are unknown, because so few cases have been reported[27]. Pseudoaneurysms of the abdominal aorta are rare, especially those found to be mycotic. Abdominal aorta pseudoaneurysms following trauma have been reported fairly often[28]. Common carotid artery pseudoaneurysms are rare and potentially lethal, and adequate treatment is warranted in order to prevent rupture or neurologic sequelae[29]. Vertebral arteries pseudoaneurysm formation after central line placement has been well documented in the literature, with an incidence rate of 0.05% to 2%[30,31].
AETIOLOGY OF PSEUDOANEURYSM IN THE PERIPHERAL ARTERIES, ABDOMINAL AORTA AND NECK ARTERIES
Iatrogenic complication
Unintentional pseudoaneurysm due to surgical intervention for numerous medical procedure (e.g., pseudoaneurysm can be induced in femoral artery during cardiac catheterization). It accounts up to 70%-80% of the incidence of pseudoaneurysms[32].
Trauma
It had been estimated that 79% of pseudoaneurysms are traumatic in origin of the internal solid organs such as liver, kidneys, pancreas, and gastrointestinal tract of the digestive system[33].
Injury by tumor
Pseudoaneurysm can be initiated due to blood vessel erosion by an erosive tumor, either benign or malignant. This is most commonly seen in osteochondroma, neurofibromatosis, leukemia, and lymphoma[34]. Yang et al[35] reported that 25% of pseudoaneurysms are caused by neoplastic aneurysms as choriocarcinoma. Kim et al[36] also reported leukemia and lymphoma as a cause of pseudoaneurysm by damaging the arterial vessel wall.
Infection
Pseudoaneurysm can be initiated by primary (wall infection) or secondary (adjacent focus) infection of blood vessels. It has been reported that pseudoaneurysms are more frequent in incidence than true aneurysms, this is because the infection can disturb blood vessels wall more easily[37].
Vasculitis and inflammation
Formation of pseudoaneurysm in blood vessels is caused by destroying the elastic fibers of the media, induced by inflammation. The majority of pseudoaneurysms is caused by Behcet’s syndrome, while pseudoaneurysms caused by primary vasculitis are not common in incidence[38].
Atherosclerosis
Aortic pseudoaneurysms are caused by atherosclerotic ulcer due to disturbance of internal elastic lamina, which can lead to aortic rupture or aortic dissection[39].
Infarction
Another cause of pseudoaneurysm is infarction of the left ventricle. It occurs due to separation of the left ventricle free wall enclosed by superimposing adherent pericardium, generated what has been named “pseudoaneurysm of the left ventricle”[40].
MANAGEMENT OF PSEUDOANEURYSM IN THE PERIPHERAL ARTERIES, ABDOMINAL AORTA AND NECK ARTERIES
Surgical approach
The gold stander of pseudoaneurysm treatment in general is surgical intervention. The intervention includes arterial ligation, organectomy either partially or totally, and resection using bypass techniques. Surgical treatment is associated with increased morbidity and mortality as compared with minimally invasive treatment options. The complications associated with surgery include bleeding, infection, lymphocele formation, radiculopathy, perioperative myocardial infarction, and death[41].
Endovascular approaches
Endovascular approaches to therapy offers distinct advantage to conventional surgical repair in patients with visceral pseudoaneurysms[42]. Several endovascular techniques have been described to treat pseudoaneurysms. These techniques include catheter-guided embolization with use of coils or detachable balloons[43,44]. Similar management principles are applied to management of aortic pseudoaneurysms[45]. Compared to other techniques, endovascular procedures have lower morbidity and mortality rate in the management of pseudoaneurysm compared to surgical intervention[19].
Percutaneous approach
Percutaneous US-guided thrombin injection is an important treatment option for the treatment of pseudoaneurysms. This approach appears to be a safe and expeditious method for treating postcatheterization femoral pseudoaneurysms. It has significant advantages with respect to ultrasound guided compression repair or surgical repair[19]. Recently, the percutaneous thrombin injection was introduced for the treatment of iatrogenic pseudoaneurysm of femoral artery[46,47].
In addition to that, this procedure can be used to treat arteries above the inguinal ligament and is considered as an alternative technique to US-guided compression in order to avoid arterial rupture[48]. Complications of thrombin injection are uncommon, occurring in 0%-4% of cases[12]. Most reported complications involve the escape of thrombin into the native circulation, causing distal embolization. This occurs in as many as 2% of all patients treated[41].
US-guided compression
US-guided compression of pseudoaneurysms is a safe and cost-effective method for achieving pseudoaneurysm thrombosis. However, it has been demonstrated that the success rate is higher and procedure time is much shorter for thrombin injection compared with US compression[48]. Furthermore, compression of pseudoaneurysm is painful to the patient and time-consuming for the practitioner. US-guided compression is more likely to fail in a patient with anticoagulation, large pseudoaneurysm size, chronic pseudoaneurysm, and longer procedure time. The incidence of complications is small but they occasionally do occur[19].
TO-AND-FRO WAVEFORM IN PSEUDOANEURYSMS OF UPPER EXTREMITY ARTERIAL VESSELS
The characteristic appearance of pseudoaneurysm in upper extremities arterial vessels is the extraluminal pattern of blood flow, which shows variable echogenicity, interval complexity, and “to-and-fro” flow pattern on color Doppler ultrasonography (CDUS)[49,50]. It has been estimated that 2% to 3% of pseudoaneurysm in Subclavian artery occur due to blunt trauma, or injuries after clavicle fracture[51].
Pseudoaneurysm of radial artery could be caused also as a result of bacterial infection at cannulation site[8]. It has been considered that radial artery pseudoaneurysm is a rare pathological condition accounting and incidence of 0.048%[52].
Rozen et al[53] reported that pseudoaneurysm of radial artery are a common finding in patients with anticoagulated or patient under antiplatelet treatment. It’s crucial to deliberate pseudoaneurysm diagnosis in any swelling that may presents swelling in order to avoid puncture or incision of the vessel because this swelling could be tender and warm[54].
Several imaging modalities may be used to detect pseudoaneurysms in upper extremity arterial vessels, including conventional arteriography, computed tomography (CT) angiography, radionuclide angiography, and CDUS. US imaging is a diagnostic method of choice required to access pseudoaneurysm before US guided intervention is established for pseudoaneurysm of radial artery[55,56]. CDUS is accurate, noninvasive imaging technique, and widely available. Therefore, it can be used to diagnose pseudoaneurysm of radial artery without even a side effect[51,57,58]. US imaging procedure has the ability to differentiate between solid and cystic lesions adjacent to the radial artery in the wrist area[53]. The sonographic appearance of the radial artery characterized by a feature of sonolucent pulsatile tube[59]. Spectral Doppler of the radial artery pseudoaneurysm is usually shown both “yin-yang” sign and “to-and-fro” waveform[51,57,60].
A recent report describes the attempted repair of a brachial artery pseudoaneurysm in an infant that resulted in the thrombosis of the underlying brachial artery and an emergent thrombectomy[61]. The light of the fact that neonates brachial artery injuries are uncommon, but induced by a brachial artery puncture. Therefore, this intervention is not recommended in neonates[62]. In the literature induction of brachial artery pseudoaneurysm due to venipuncture was documented in two instances[63]. Arterial injuries can be diagnosed promptly by using Duplex US imaging technique (Figure 1), without any further need for angiography[64,65]. Also DDU can be up to 95% to 100% sensitive for diagnosing vascular injuries in the hands of highly qualified personnel with a high index of suspicion[5].
Figure 1.
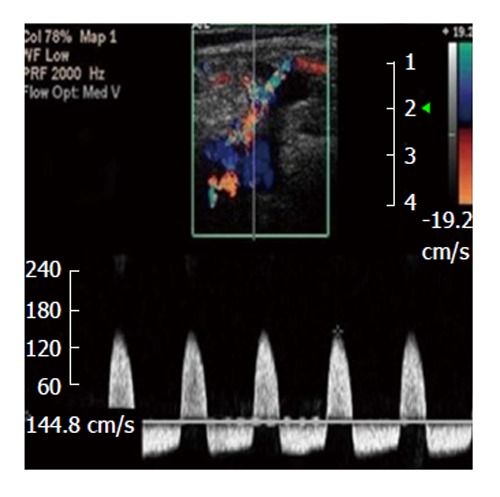
To-and-fro spectral waveform of a pseudoaneurysm; neck wasn't depicted[65].
TO-AND-FRO WAVEFORM IN PSEUDOANEURYSMS OF LOWER EXTREMITY ARTERIAL VESSELS
The incidence of pseudoaneurysm in lower extremity arterial vessels was estimated to be ranged from 3.5%-5.5% and 0.1%-0.2% of the Interventional examination and diagnostic radiography, in that order[66,67]. Femoral artery pseudoaneurysms are usually accompanied with a certain features of an audible “to-and-fro” pulsatile mass and touchable thrill (Figure 2). Duplex US of femoral artery (Figure 3) is the diagnostic method of choice for the diagnosis of Pseudoaneurysm[67]. This imaging technique can reveal the blood flow waveform, blood clotting, and the relation with the femoral artery[67].
Figure 2.
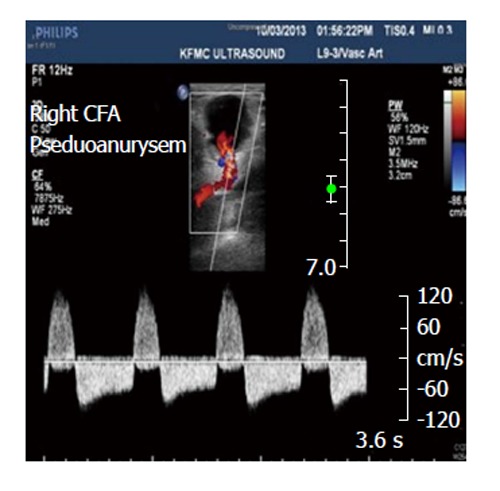
Right common femoral artery pseudoaneurysms associated with the characteristic findings of a pulsatile mass, a palpable thrill, and an audible “to-and-fro” murmur. CFA: Common femoral artery.
Figure 3.
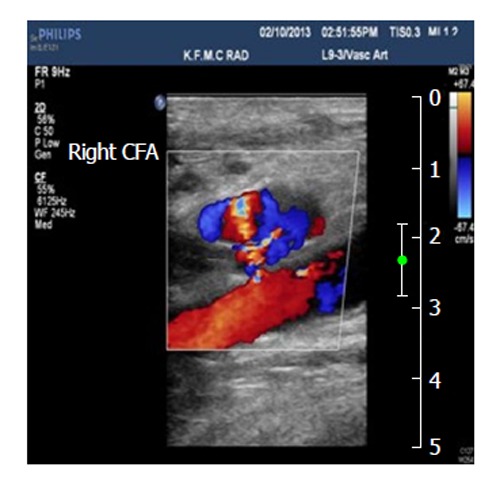
Pseudoaneurysm communicated with the right common femoral artery, and the blood flow patterns and velocities in the affected area. CFA: Common femoral artery.
The common femoral artery is the most frequent site of pseudoaneurysm in the lower extremities (Figure 4). This can be attributed to the localization of the common femoral artery inside the neurovascular sheet and it’s supported by the head of the femur. Also the common femoral artery site is the place of choice to introduce cardiac catheterization. The incidence of pseudoaneurysm in the superficial femoral artery is less frequent in occurrence when compare with the common femoral artery because this artery is usually not selected for cardiac catheterization as a result of insufficient supportive tissue around it[68,69].
Figure 4.
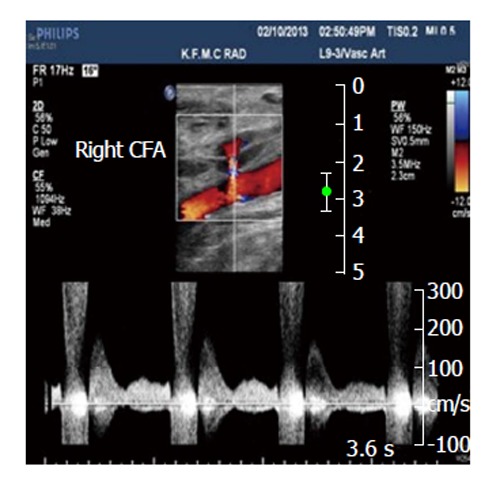
Spectral analysis of the right common femoral artery showing prominent flow with a component of reversed flow, in the region of the neck of the pseudoaneurysm. CFA: Common femoral artery.
Also popliteal artery is the most frequent region for pseudoaneurysm incidence because this artery is not supported by muscular tissue to shield it from dilatation and bending, compared to superficial and deep femoral arteries[70]. Enlarging and pulsatile mass located in the popliteal artery are the common features of aneurysmal lesion[70,71]. There are a similarity in diagnostic findings between popliteal artery mycotic pseudoaneurysm and other pseudoaneurysms on the basis of CDUS finding[2,72].
Pseudoaneurysms of the anterior tibial artery and tibioperoneal trunk are exceedingly rare[73,74]. Owen et al[75] reported that pseudoaneurysms of the tibial arteries can be treated using percutaneous injection of thrombin and tissue adhesive. To prevent sudden incidence of a thrombosis in the native vessel, occlusion balloon can be used. An important study reported by Davis et al[76] showed that pseudoaneurysm can be treated with percutaneous infection of thrombin at the posterior tibial and distal superficial femoral arteries. Pseudoaneurysm can be formed during surgical replacement of the knee joint. This can occur either direct (intra-operative) or indirect (intimal plaque disruption)[77].
Some studies reported that the incidence of pseudoaneurysms or aneurysms are rare in the dorsalis pedis artery and usually accompanied with trauma[78-80]. Surgical intervention is preferred to reduce the risk of complication, such as ischemia, arterial rupture, and thrombosis[80,81].
To differentiate between a hematoma and pseudoaneurysm in lower extremities arterial vessels, DDU can be used to establish the accurate diagnosis by demonstrating the relation between the injured artery and aneurysmal neck[82]. In addition, triplex Doppler US can be used for diagnosis of pseudoaneurysm, by presenting “yin-yang” pattern. Bearing in mind that this pattern don’t usually differentiate between pseudoaneurysm and pulsating hematoma[83].
TO-AND-FRO WAVEFORM IN PSEUDOANEURYSMS OF ABDOMINAL AORTA
The incidence of abdominal aneurysms has been established by Ertürk et al[84] to be 1% of the overall abdominal aneurysms, concluding that pseudoaneurysms of abdominal aorta has a very low incidence. Pseudoaneurysms of the abdominal aorta are often diagnosed late or after catastrophic complications[85]. Pseudoaneurysms of abdominal aorta caused by medical interventions, these interventions are abdominal surgery, Interventional guided by X-ray imaging of the abdomen, as a complication of abdominal aortic aneurysm, vasculitis, external abdominal trauma, and mycotic aneurysms. Pseudoaneurysms due to external abdominal trauma showed a high incidence in patients treated with anticoagulant or antiplatelet[86].
Shanley et al[87] reported that pseudoaneurysms could be developed in the majority of the visceral artery. A different incidence rate was noted in the splenic artery (46%), renal artery (22%), hepatic artery (16.2%), and pancreaticoduodenal artery (1.3%)[88-90]. Those involving the gastroduodenal artery constitute just 1.5% of all published visceral artery aneurysms[90].
Pseudoaneurysms also took place as a result of a combination of an artery impeded with the wall of pseudocysts[91]. Gastroduodenal and splenic artery pseudoaneurysms are silent in the majority of cases, but in some cases, patients may experience upper abdominal pain or anemia due to bleeding in the gastrointestinal tract or peritoneal cavity[92].
Pseudoaneurysms of splenic artery in different patients are caused by pancreatitis, either chronic or acute pancreatitis. The majority of these patients is characterized by a history of excessive alcohol consumption. The main cause of pseudoaneurysms formation by the aforementioned method is due to the digestion of splenic artery by pancreatic enzymes[93]. Pseudoaneurysm development in the splenic artery due to blunt abdominal trauma had been reported by Sugg et al[94]. Splenic artery slow blood flow is a predisposing factor of pseudoaneurysm as reported by Norotsky et al[95]. In recent year’s noninvasive procedure, therefore the incidence of pseudoaneurysm of splenic artery is increasing in incidence among patients[96]. It has been reported that pseudoaneurysm may develop rarely due to peptic ulcer or as a result of iatrogenic causes. An a tiny number of patients developed pseudoaneurysm in the splenic artery without specific reasons[10].
False aneurysms of the gastroduodenal artery can arise from an impairment in the integrity of the arterial wall, by direct injury via a biopsy needle, enzymatic digestion, as a result of pancreatitis, surgery, or trauma[97]. This defect can lead to the formation of an open communication between the lumen of the artery and its surroundings, which can have two fates. If no soft tissues surround the site of injury, hemorrhage into the peritoneal cavity can occur. The presence of surrounding soft tissue, conversely, can result in containment of the hematoma, which can be followed by fibrosis and enlargement[98]. Pseudoaneurysms have been reported to spontaneous thrombosis, but this is a rare event occurring only under certain conditions[99]. More often, the hematomas become unstable and rupture, being associated with a mortality rate of around 50%[100].
Diagnosis of gastroduodenal and splenic pseudoaneurysm can be made with a number of imaging methods. Contrast-enhanced CT and Doppler sonography are widely used as noninvasive techniques in the diagnosis and monitoring of the lesion[101,102]. On contrast-enhanced CT, a pseudoaneurysm appears as an eccentric mass with a well-defined region of central enhancement in the arterial phase. Doppler sonography shows a mass that generally has a well-defined, solid peripheral component composed of a thrombus and a central anechoic area of varying size. This cavity fills on color Doppler imaging and produces the typical “yin-yang” pattern of pseudoaneurysms anywhere in the body. A “to-and-fro” pattern at the neck of the lesion is confirmatory of a pseudoaneurysm.
Angiography remains the definitive modality used to diagnose, locate, and evaluate the presence of a gastroduodenal and splenic pseudoaneurysm[101,102]. The advantage of this method is that it can be used in the treatment of the lesion as well. Angiography is useful in establishing confirmation of the diagnosis and in cases of an acute rupture or major gastrointestinal bleeding requiring immediate care[93]. The sonographic appearance of abdominal aortic pseudoaneurysm is anechoic blood accumulation in a sac nearby within the artery. This accumulation can be detected by using color Doppler[84]. Sonographic examination of patient using color duplex Doppler revealed a pattern of turbulent flow within pseudoaneurysm illustrated in (Figure 5). Whirlwind flow and “to-and-fro” waveform are seen in the neck of pseudoaneurysm also by using pulsed Doppler[103].
Figure 5.
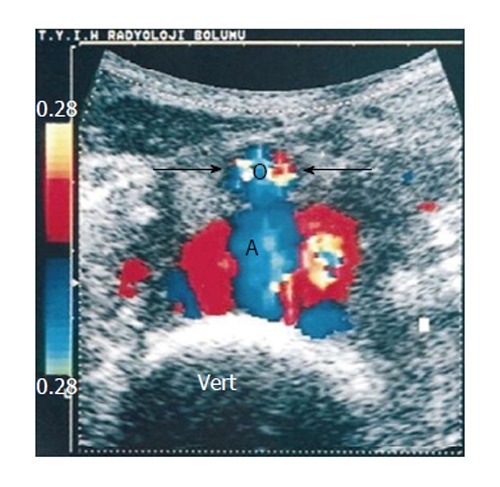
Transverse color Doppler sonogram shows turbulent flow in the pseudoaneurysm. Note the anterior displacement of the normal-sized aorta (arrows and AO) and the drape of the posterior wall of the pseudoaneurysm over the anterior aspect of the spine (vert)[84].
The limitations of color duplex Doppler in the diagnosis of pseudoaneurysms are obese patients and the presence of excessive gasses in the bowel. Neverless ultrasound should be used to establish preliminary diagnosis, especially for patient with pulsatile abdominal masses[84,104].
TO-AND-FRO WAVEFORM IN PSEUDOANEURYSMS OF CAROTID AND VERTEBRAL ARTERIES
Carotid and vertebral artery pseudoaneurysms are uncommon lesions that may occur as sequelae of blunt trauma, cancer or radiation necrosis, and mycotic infection[104]. Although Doppler ultrasound is a noninvasive imaging procedure, more accurate imaging modalities have been developed such as magnetic resonance angiogram or angiography. However US is the imaging method of choice (Figure 6) to study the midcervical portion of the carotid or vertebral arteries[105-107].
Figure 6.
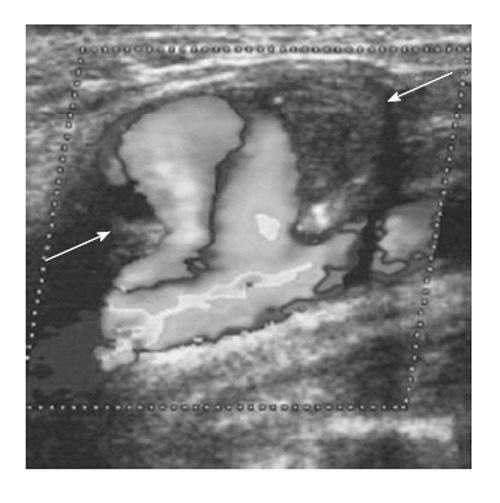
Color Doppler sonogram showing the blood flow of the right common carotid artery, and the haematoma with the rotatory flow within its cavity (arrows). Note the large neck connecting the carotid to the pseudoaneurysm[90].
The degree of confidence is high in detection carotid (mid cervical region) and vertebral artery pseudoaneurysms. While the degree of confidence is low in the detection of an intrathoracic segment of the carotid and vertebral arteries[108].
Duplex ultrasound is used on a routine basis to evaluate atherosclerotic lesions. The main findings include dissection, occlusion, pseudoaneurysms, and intimal flaps. Nemours studies used DDU reported that around 92%-100% sensitivity in detection of arterial lesions due to neck trauma[109-111]. The contour of pseudoaneurysms affecting carotid arteries showed variable color flow, depending on the presence of thrombosis[112], while swirling blood flow and “to-and-fro” pattern is shown by spectral Doppler[113]. In common carotid arteries, ultrasound is an effective means of diagnosing a pseudoaneurysm. It may also be used for serial follow the progression of these occurrences once they are diagnosed, as well as to aid in treatment in certain cases[114]. When investigated internal carotid artery pseudoaneurysm by color Doppler it shows swirling of blood flow within the pseudoaneurysm with a communicating channel of the parent artery (yin-yang phenomenon), while pulse Doppler shows “to-and-fro” waveforms[115].
Vertebral artery pseudoaneurysms typically present over the course of several days as a pulsatile mass. Duplex US is used to define the size of the pseudoaneurysms. Adequate visualization of the pseudoaneurysms neck of lesions arising vertebral arteries is limited owing to the overlying clavicle. Angiography is often indicated in order to precisely define the site of injury[30]. However, US examination of vertebral artery pseudoaneurysms is necessary in uncertain or difficult case from the beginning because it is convenient and sensitive in follow-up evaluation[116].
CONCLUSION
In conclusion, this review study showed that gray scale and Doppler ultrasound play an essential role in the diagnosis of pseudoaneurysms. The use of spectral Doppler in the diagnosis of pseudoaneurysms depends upon the presence of “to-and-fro” waveform. Incidence of arterial pseudoaneurysms are varied in the different body vasculature. Also the choice of pseudoaneurysms treatment is size dependent.
ACKNOWLEDGMENTS
The authors extend their appreciation to the College of Applied Medical Sciences Research Center and the Deanship of Scientific Research at King Saud University for funding this research. In addition, the authors would like to thank the staff of the Radiology Department at KFMC at Riyadh, Saudi Arabia for their cooperation and support during data collection for this article.
Footnotes
P- Reviewer: Aydogdu O, Seicean A S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
Supported by College of Applied Medical Sciences Research Center and the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia.
Conflict-of-interest: The authors declare that there is no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 20, 2014
First decision: December 12, 2014
Article in press: April 7, 2015
References
- 1.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25 Suppl 1:S173–S189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 2.Pellerito JS, Taylor KJ. Doppler color imaging. Peripheral arteries. Clin Diagn Ultrasound. 1992;27:97–112. [PubMed] [Google Scholar]
- 3.Zierler RE, Zierler BK. Duplex sonography of lower extremity arteries. Semin Ultrasound CT MR. 1997;18:39–56. doi: 10.1016/s0887-2171(97)90037-8. [DOI] [PubMed] [Google Scholar]
- 4.Pozniak MA, Mitchell C, Ledwidge M. Radial artery pseudoaneurysm: a maneuver to decrease the risk of thrombin therapy. J Ultrasound Med. 2005;24:119–122. doi: 10.7863/jum.2005.24.1.119. [DOI] [PubMed] [Google Scholar]
- 5.Levis JT, Garmel GM. Radial artery pseudoaneurysm formation after cat bite to the wrist. Ann Emerg Med. 2008;51:668–670. doi: 10.1016/j.annemergmed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Pellerito JS. Current approach to peripheral arterial sonography. Radiol Clin North Am. 2001;39:553–567. doi: 10.1016/s0033-8389(05)70297-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang HK, Chou YH, Chiou HJ, Chio SY, Chang CY. B-flow ultrasonography of peripheral vascular diseases. J Med Ultrasound. 2005;13:186–195. [Google Scholar]
- 8.Gudena R, Khetan N. Swelling of volar aspect of the wrist. Postgrad Med J. 2005;81:e9–e11. doi: 10.1136/pgmj.2004.028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zitsman JL. Pseudoaneurysm after penetrating trauma in children and adolescents. J Pediatr Surg. 1998;33:1574–1577. doi: 10.1016/s0022-3468(98)90504-8. [DOI] [PubMed] [Google Scholar]
- 10.Tessier DJ, Stone WM, Fowl RJ, Abbas MA, Andrews JC, Bower TC, Gloviczki P. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg. 2003;38:969–974. doi: 10.1016/s0741-5214(03)00710-9. [DOI] [PubMed] [Google Scholar]
- 11.Katz DS, Hon M. CT angiography of the lower extremities and aortoiliac system with a multi-detector row helical CT scanner: promise of new opportunities fulfilled. Radiology. 2001;221:7–10. doi: 10.1148/radiol.2211011087. [DOI] [PubMed] [Google Scholar]
- 12.Morgan R, Belli AM. Current treatment methods for postcatheterization pseudoaneurysms. J Vasc Intervent Radiol. 2003;14:697–710. doi: 10.1097/01.rvi.0000071089.76348.6a. [DOI] [PubMed] [Google Scholar]
- 13.Katyal S, Oliver JH, Buck DG, Federle MP. Detection of vascular complications after liver transplantation: early experience in multislice CT angiography with volume rendering. AJR Am J Roentgenol. 2000;175:1735–1739. doi: 10.2214/ajr.175.6.1751735. [DOI] [PubMed] [Google Scholar]
- 14.Soto JA, Múnera F, Morales C, Lopera JE, Holguín D, Guarín O, Castrillón G, Sanabria A, García G. Focal arterial injuries of the proximal extremities: helical CT arteriography as the initial method of diagnosis. Radiology. 2001;218:188–194. doi: 10.1148/radiology.218.1.r01ja13188. [DOI] [PubMed] [Google Scholar]
- 15.Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093–1114. doi: 10.1148/rg.235035031. [DOI] [PubMed] [Google Scholar]
- 16.Kwon JH, Kim GS. Obstetric iatrogenic arterial injuries of the uterus: diagnosis with US and treatment with transcatheter arterial embolization. Radiographics. 2002;22:35–46. doi: 10.1148/radiographics.22.1.g02ja0735. [DOI] [PubMed] [Google Scholar]
- 17.Krüger K, Zähringer M, Söhngen FD, Gossmann A, Schulte O, Feldmann C, Strohe D, Lackner K. Femoral pseudoaneurysms: management with percutaneous thrombin injections--success rates and effects on systemic coagulation. Radiology. 2003;226:452–458. doi: 10.1148/radiol.2262012107. [DOI] [PubMed] [Google Scholar]
- 18.Polak JF. The peripheral arteries. In: Rumack CM, Wilson SR, Charboneau JW, editors. Diagnostic ultrasound. St Louis: Mosby; 1998. pp. 921–941. [Google Scholar]
- 19.Kapoor BS, Haddad HL, Saddekni S, Lockhart ME. Diagnosis and management of pseudoaneurysms: an update. Curr Probl Diagn Radiol. 2009;38:170–188. doi: 10.1067/j.cpradiol.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Fransson SG, Nylander E. Vascular injury following cardiac catheterization, coronary angiography, and coronary angioplasty. Eur Heart J. 1994;15:232–235. doi: 10.1093/oxfordjournals.eurheartj.a060481. [DOI] [PubMed] [Google Scholar]
- 21.Kacila M, Vranic H, Hadzimehmedagic A, Sehovic S, Granov N. The frequency of complications of pseudoaneurysms after cardiac interventional diagnostic and therapeutic interventions. Med Arh. 2011;65:78–81. [PubMed] [Google Scholar]
- 22.Görge G, Kunz T, Kirstein M. [Non-surgical therapy of iatrogenic false aneurysms] Dtsch Med Wochenschr. 2003;128:36–40. doi: 10.1055/s-2003-36329. [DOI] [PubMed] [Google Scholar]
- 23.Righini M, Quéré I, Laroche JP. [Treatment of postcatheterization femoral false aneurysms] J Mal Vasc. 2004;29:63–72. doi: 10.1016/s0398-0499(04)96717-0. [DOI] [PubMed] [Google Scholar]
- 24.Wielenberg A, Borge MA, Demos TC, Lomasney L, Marra G. Traumatic pseudoaneurysm of the brachial artery. Orthopedics. 2000;23:1250, 1322–1324. doi: 10.3928/0147-7447-20001201-09. [DOI] [PubMed] [Google Scholar]
- 25.Szendro G, Golcman L, Klimov A, Yefim C, Johnatan B, Avrahami E, Yechieli B, Yurfest S. Arterial false aneurysms and their modern management. Isr Med Assoc J. 2001;3:5–8. [PubMed] [Google Scholar]
- 26.Dhadwal AK, Abrol S, Zisbrod Z, Cunningham JN. Pseudoaneurysms of the ascending aorta following coronary artery bypass surgery. J Card Surg. 2006;21:221–224. doi: 10.1111/j.1540-8191.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 27.Garisto JD, Medina A, Williams DB, Carrillo RG. Surgical management of a giant ascending aortic pseudoaneurysm. Tex Heart Inst J. 2010;37:710–713. [PMC free article] [PubMed] [Google Scholar]
- 28.Potts RG, Alguire PC. Pseudoaneurysm of the abdominal aorta: a case report and review of the literature. Am J Med Sci. 1991;301:265–268. doi: 10.1097/00000441-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kim HO, Ji YB, Lee SH, Jung C, Tae K. Cases of common carotid artery pseudoaneurysm treated by stent graft. Case Rep Otolaryngol. 2012;2012:674827. doi: 10.1155/2012/674827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernik TR, Friedman SG, Scher LA, Safa T. Pseudoaneurysm of the subclavian-vertebral artery junction--case report and review of the literature. Vasc Endovascular Surg. 2002;36:461–464. doi: 10.1177/153857440203600607. [DOI] [PubMed] [Google Scholar]
- 31.Cihangiroglu M, Rahman A, Yildirim H, Burma O, Uysal H. Iatrogenic vertebral artery pseudoaneurysm: US, CT and MRI findings. Eur J Radiol. 2002;43:14–18. doi: 10.1016/s0720-048x(01)00451-x. [DOI] [PubMed] [Google Scholar]
- 32.Franklin JA, Brigham D, Bogey WM, Powell CS. Treatment of iatrogenic false aneurysms. J Am Coll Surg. 2003;197:293–301. doi: 10.1016/S1072-7515(03)00375-2. [DOI] [PubMed] [Google Scholar]
- 33.Feliciano DV, Mattox KL. Traumatic aneurysms. In: Rutherford RB, editor. Vascular surgery. Philadelphia: Saunders; 1989. pp. 996–1003. [Google Scholar]
- 34.Smith BL, Munschauer CE, Diamond N, Rivera F. Ruptured internal carotid aneurysm resulting from neurofibromatosis: treatment with intraluminal stent graft. J Vasc Surg. 2000;32:824–828. doi: 10.1067/mva.2000.107769. [DOI] [PubMed] [Google Scholar]
- 35.Yang DM, Yoon MH, Kim HS, Kim HS, Shin DB. Intrarenal pseudoaneurysms complicating renal choriocarcinoma metastases: treatment with coil embolization. Clin Imaging. 2000;24:217–220. doi: 10.1016/s0899-7071(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 36.Kim MD, Kim H, Kang SW, Jeong BG. Nontraumatic hepatic artery pseudoaneurysm associated with acute leukemia: a possible complication of pyogenic liver abscess. Abdom Imaging. 2002;27:458–460. doi: 10.1007/s00261-001-0078-8. [DOI] [PubMed] [Google Scholar]
- 37.Gomes MN, Choyke PL. Infected aortic aneurysms: CT diagnosis. J Cardiovasc Surg (Torino) 1992;33:684–689. [PubMed] [Google Scholar]
- 38.Ko GY, Byun JY, Choi BG, Cho SH. The vascular manifestations of Behçet’s disease: angiographic and CT findings. Br J Radiol. 2000;73:1270–1274. doi: 10.1259/bjr.73.876.11205670. [DOI] [PubMed] [Google Scholar]
- 39.Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. 2003;181:309–316. doi: 10.2214/ajr.181.2.1810309. [DOI] [PubMed] [Google Scholar]
- 40.Brown SL, Gropler RJ, Harris KM. Distinguishing left ventricular aneurysm from pseudoaneurysm. A review of the literature. Chest. 1997;111:1403–1409. doi: 10.1378/chest.111.5.1403. [DOI] [PubMed] [Google Scholar]
- 41.La Perna L, Olin JW, Goines D, Childs MB, Ouriel K. Ultrasound-guided thrombin injection for the treatment of postcatheterization pseudoaneurysms. Circulation. 2000;102:2391–2395. doi: 10.1161/01.cir.102.19.2391. [DOI] [PubMed] [Google Scholar]
- 42.Tulsyan N, Kashyap VS, Greenberg RK, Sarac TP, Clair DG, Pierce G, Ouriel K. The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–283; discussion 283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson AA, Patel J, McPherson S, Shaw DR, Kessel D. Endovascular treatment of visceral aneurysms associated with pancreatitis and a suggested classification with therapeutic implications. J Vasc Interv Radiol. 2006;17:1279–1285. doi: 10.1097/01.RVI.0000231948.08617.04. [DOI] [PubMed] [Google Scholar]
- 44.Sharma RP, Shetty PC, Burke TH, Shepard AD, Khaja F. Treatment of false aneurysm by using a detachable balloon. AJR Am J Roentgenol. 1987;149:1279–1280. doi: 10.2214/ajr.149.6.1279. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell JH, Dougherty KG, Strickman NE, Mortazavi A, Krajcer Z. Endovascular repair of paraanastomotic aneurysms after aortic reconstruction. Tex Heart Inst J. 2007;34:148–153. [PMC free article] [PubMed] [Google Scholar]
- 46.Brophy DP, Sheiman RG, Amatulle P, Akbari CM. Iatrogenic femoral pseudoaneurysms: thrombin injection after failed US-guided compression. Radiology. 2000;214:278–282. doi: 10.1148/radiology.214.1.r00ja10278. [DOI] [PubMed] [Google Scholar]
- 47.Bydawell G. Percutaneous thrombin injection for pseudoaneurysm treatment. S Afr J Rad. 2013;17:41–42. [Google Scholar]
- 48.Paulson EK, Sheafor DH, Kliewer MA, Nelson RC, Eisenberg LB, Sebastian MW, Sketch MH. Treatment of iatrogenic femoral arterial pseudoaneurysms: comparison of US-guided thrombin injection with compression repair. Radiology. 2000;215:403–408. doi: 10.1148/radiology.215.2.r00ap35403. [DOI] [PubMed] [Google Scholar]
- 49.Eisenberg L, Paulson EK, Kliewer MA, Hudson MP, DeLong DM, Carroll BA. Sonographically guided compression repair of pseudoaneurysms: further experience from a single institution. AJR Am J Roentgenol. 1999;173:1567–1573. doi: 10.2214/ajr.173.6.10584803. [DOI] [PubMed] [Google Scholar]
- 50.Chiou HJ, Chou YH, Chiou SY, Wang HK. High-resolution ultrasonography in superficial soft tissue tumors. J Med Ultrasound. 2007;15:152–174. [Google Scholar]
- 51.Falk PS, Scuderi PE, Sherertz RJ, Motsinger SM. Infected radial artery pseudoaneurysms occurring after percutaneous cannulation. Chest. 1992;101:490–495. doi: 10.1378/chest.101.2.490. [DOI] [PubMed] [Google Scholar]
- 52.Cozzi DA, Morini F, Casati A, Pacilli M, Salvini V, Cozzi F. Radial artery pseudoaneurysm successfully treated by compression bandage. Arch Dis Child. 2003;88:165–166. doi: 10.1136/adc.88.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozen G, Samuels DR, Blank A. The to and fro sign: the hallmark of pseudoaneurysm. Isr Med Assoc J. 2001;3:781–782. [PubMed] [Google Scholar]
- 54.Barker C, Jefferson P, Ball DR. Portable ultrasound to diagnose true radial artery aneurysm. Anesth Analg. 2007;105:890–891. doi: 10.1213/01.ane.0000269691.81976.67. [DOI] [PubMed] [Google Scholar]
- 55.Gooding GA. Sonography of the radial artery at the wrist. AJR Am J Roentgenol. 1988;150:629–631. doi: 10.2214/ajr.150.3.629. [DOI] [PubMed] [Google Scholar]
- 56.Carrafiello G, Laganà D, Mangini M, Recaldini C, Mandas X, Fugazzola C. Post-traumatic pseudoaneurysm of radial artery: percutaneous treatment with thrombin injection. Injury Extra. 2006;37:78–81. [Google Scholar]
- 57.Lennox A, Griffin M, Nicolaides A, Mansfield A. Regarding “Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms”. J Vasc Surg. 1998;28:1120–1121. doi: 10.1016/s0741-5214(98)70041-2. [DOI] [PubMed] [Google Scholar]
- 58.Davison BD, Polak JF. Arterial injuries: a sonographic approach. Radiol Clin North Am. 2004;42:383–396. doi: 10.1016/j.rcl.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Truong AT, Thakar DR. Radial artery pseudoaneurysm: a rare complication with serious risk to life and limb. Anesthesiology. 2013;118:188. doi: 10.1097/aln.0b013e318279f925. [DOI] [PubMed] [Google Scholar]
- 60.Landau D, Schreiber R, Szendro G, Golcman L. Brachial artery pseudoaneurysm in a premature infant. Arch Dis Child Fetal Neonatal Ed. 2003;88:F152–F153. doi: 10.1136/fn.88.2.F152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demircin M, Peker O, Tok M, Ozen H. False aneurysm of the brachial artery in an infant following attempted venipuncture. Turk J Pediatr. 1996;38:389–391. [PubMed] [Google Scholar]
- 62.Verlato F, Zanon GF, Gamba PG, Verlato G, Rocco S, Orzali A, Camporese G, Signorini GP. Echo Doppler color flow (EDCF) evaluation of vascular pathology in pediatric age groups. Int Angiol. 1996;15:321–327. [PubMed] [Google Scholar]
- 63.Dzepina I, Unusic J, Mijatovic D, Bulic K. Pseudoaneurysms of the brachial artery following venipuncture in infants. Pediatr Surg Int. 2004;20:594–597. doi: 10.1007/s00383-004-1238-z. [DOI] [PubMed] [Google Scholar]
- 64.Gullo J, Singletary EM, Larese S. Emergency bedside sonographic diagnosis of subclavian artery pseudoaneurysm with brachial plexopathy after clavicle fracture. Ann Emerg Med. 2013;61:204–206. doi: 10.1016/j.annemergmed.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 65.Pero T, Herrick J. Pseudoaneurysm of the radial artery diagnosed by bedside ultrasound. West J Emerg Med. 2009;10:89–91. [PMC free article] [PubMed] [Google Scholar]
- 66.Goel PK, Modi N, Baijal SS, Kathuria M, Agrawal SK. Sonographically guided thrombin injection for the treatment of femoral artery pseudoaneurysm. Indian Heart J. 2003;55:365–367. [PubMed] [Google Scholar]
- 67.Kronzon I. Diagnosis and treatment of iatrogenic femoral artery pseudoaneurysm: a review. J Am Soc Echocardiogr. 1997;10:236–245. doi: 10.1016/s0894-7317(97)70061-0. [DOI] [PubMed] [Google Scholar]
- 68.Fellmeth BD, Roberts AC, Bookstein JJ, Freischlag JA, Forsythe JR, Buckner NK, Hye RJ. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology. 1991;178:671–675. doi: 10.1148/radiology.178.3.1994400. [DOI] [PubMed] [Google Scholar]
- 69.Demirbas O, Batyraliev T, Eksi Z, Pershukov I. Femoral pseudoaneurysm due to diagnostic or interventional angiographic procedures. Angiology. 2005;56:553–556. doi: 10.1177/000331970505600505. [DOI] [PubMed] [Google Scholar]
- 70.Sadler L, Bolden RO, Lenkey JL. Diagnosis of a ruptured deep femoral artery aneurysm--a case report. Angiology. 1989;40:678–681. doi: 10.1177/000331978904000711. [DOI] [PubMed] [Google Scholar]
- 71.Harman M, Irmak H, Arslan H, Arslan U, Kayan M. Popliteal artery pseudoaneurysm: a rare complication of brucellosis. J Clin Ultrasound. 2004;32:33–36. doi: 10.1002/jcu.10217. [DOI] [PubMed] [Google Scholar]
- 72.Murashita T, Yasuda K, Takigami T, Sakuma M, Matsui Y, Sasaki S, Shiiya N. Mycotic aneurysm of the bilateral tibioperoneal trunks associated with bacterial endocarditis: a case report. Int Angiol. 1997;16:176–179. [PubMed] [Google Scholar]
- 73.Cappendijk VC, Mouthaan PJ. A true aneurysm of the tibioperoneal trunk. Case report and literature review. Eur J Vasc Endovasc Surg. 1999;18:536–537. doi: 10.1053/ejvs.1999.0938. [DOI] [PubMed] [Google Scholar]
- 74.McKee MA, Ballard JL. Mycotic aneurysms of the tibioperoneal arteries. Ann Vasc Surg. 1999;13:188–190. doi: 10.1007/s100169900240. [DOI] [PubMed] [Google Scholar]
- 75.Owen RJ, Haslam PJ, Elliott ST, Rose JD, Loose HW. Percutaneous ablation of peripheral pseudoaneurysms using thrombin: a simple and effective solution. Cardiovasc Intervent Radiol. 2000;23:441–446. doi: 10.1007/s002700010101. [DOI] [PubMed] [Google Scholar]
- 76.Davis KA, Mansour MA, Kang SS, Labropoulos N, Esposito TJ, Silver GM, Reed RL. Pseudoaneurysms of the extremity without fracture: treatment with percutaneous ultrasound-guided thrombin injection. J Trauma. 2000;49:818–821. doi: 10.1097/00005373-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Law KY, Cheung KW, Chiu KH, Antonio GE. Pseudoaneurysm of the geniculate artery following total knee arthroplasty: a report of two cases. J Orthop Surg (Hong Kong) 2007;15:386–389. doi: 10.1177/230949900701500331. [DOI] [PubMed] [Google Scholar]
- 78.McKee TI, Fisher JB. Dorsalis pedis artery aneurysm: case report and literature review. J Vasc Surg. 2000;31:589–591. [PubMed] [Google Scholar]
- 79.Tempest HV, Wilson YG. Acute forefoot ischaemia: an unreported complication of dorsalis pedis artery aneurysm. Eur J Vasc Endovasc Surg. 2001;22:472–473. doi: 10.1053/ejvs.2001.1486. [DOI] [PubMed] [Google Scholar]
- 80.Taylor DT, Mansour MA, Bergin JT, Reyes CV, Stuck RM. Aneurysm of the dorsalis pedis artery -- a case report. Vasc Endovascular Surg. 2002;36:241–245. doi: 10.1177/153857440203600314. [DOI] [PubMed] [Google Scholar]
- 81.Ozdemir H, Mahmutyazicioğlu K, Ozkökeli M, Savranlar A, Ozer T, Demirel F. Pseudoaneurysm of the dorsalis pedis artery: color Doppler sonographic and angiographic findings. J Clin Ultrasound. 2003;31:283–287. doi: 10.1002/jcu.10164. [DOI] [PubMed] [Google Scholar]
- 82.Abu-Yousef MM, Wiese JA, Shamma AR. The „to-and-fro“ sign: duplex Doppler evidence of femoral artery pseudoaneurysm. AJR Am J Roentgenol. 1988;150:632–634. doi: 10.2214/ajr.150.3.632. [DOI] [PubMed] [Google Scholar]
- 83.Carroll BA, Graif M, Orron DE. Vascular ultrasound. In: Kim DS, Orron DE, editors. Peripheral vascular imaging and intervention. St. Louis: Mosby; 1992. pp. 211–225. [Google Scholar]
- 84.Ertürk H, Erden A, Yurdakul M, Calikoğlu U, Olçer T, Cumhur T. Pseudoaneurysm of the abdominal aorta diagnosed by color duplex Doppler sonography. J Clin Ultrasound. 1999;27:202–205. doi: 10.1002/(sici)1097-0096(199905)27:4<202::aid-jcu7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 85.van Herwaarden JA, Waasdorp EJ, Bendermacher BL, van den Berg JC, Teijink JA, Moll FL. Endovascular repair of paraanastomotic aneurysms after previous open aortic prosthetic reconstruction. Ann Vasc Surg. 2004;18:280–286. doi: 10.1007/s10016-004-0002-0. [DOI] [PubMed] [Google Scholar]
- 86.Siegel CL, Cohan RH. CT of abdominal aortic aneurysms. AJR Am J Roentgenol. 1994;163:17–29. doi: 10.2214/ajr.163.1.8010207. [DOI] [PubMed] [Google Scholar]
- 87.Shanley CJ, Shah NL, Messina LM. Uncommon splanchnic artery aneurysms: pancreaticoduodenal, gastroduodenal, superior mesenteric, inferior mesenteric, and colic. Ann Vasc Surg. 1996;10:506–515. doi: 10.1007/BF02000601. [DOI] [PubMed] [Google Scholar]
- 88.Deterling RA Jr. Aneurysm of the visceral arteries. J Cardiovasc Surg (Torino) 1997;12:309–322. [PubMed] [Google Scholar]
- 89.White AF, Baum S, Buranasiri S. Aneurysms secondary to pancreatitis. AJR Am J Roentgenol. 1976;127:393–396. doi: 10.2214/ajr.127.3.393. [DOI] [PubMed] [Google Scholar]
- 90.Walter JF, Chuang VP, Bookstein JJ, Reuter SR, Cho KJ, Pulmano CM. Angiography of massive hemorrhage secondary to pancreatic diseases. Radiology. 1977;124:337–342. doi: 10.1148/124.2.337. [DOI] [PubMed] [Google Scholar]
- 91.Friedman AC. Radiology of the liver, biliary tract, pancreas and spleen. Baltimore: Williams and Wilkins; 1987. p. 674. [Google Scholar]
- 92.Stanley JC, Zelenock GB. Splanchnic artery aneurysms. In: Rutherford RB, editor. Vascular surgery. Philadelphia: W.B. Saunders Company; 1995. pp. 1124–1128. [Google Scholar]
- 93.Golzarian J, Nicaise N, Devière J, Ghysels M, Wery D, Dussaussois L, Van Gansbeke D, Struyven J. Transcatheter embolization of pseudoaneurysms complicating pancreatitis. Cardiovasc Intervent Radiol. 1997;20:435–440. doi: 10.1007/s002709900189. [DOI] [PubMed] [Google Scholar]
- 94.Sugg SL, Gerndt SJ, Hamilton BJ, Francis IR, Taheri PA, Rodriguez JL. Pseudoaneurysms of the intraparenchymal splenic artery after blunt abdominal trauma: a complication of nonoperative therapy and its management. J Trauma. 1995;39:593–595. doi: 10.1097/00005373-199509000-00034. [DOI] [PubMed] [Google Scholar]
- 95.Norotsky MC, Rogers FB, Shackford SR. Delayed presentation of splenic artery pseudoaneurysms following blunt abdominal trauma: case reports. J Trauma. 1995;38:444–447. doi: 10.1097/00005373-199503000-00029. [DOI] [PubMed] [Google Scholar]
- 96.Davis KA, Fabian TC, Croce MA, Gavant ML, Flick PA, Minard G, Kudsk KA, Pritchard FE. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma. 1998;44:1008–1013; discussion 1013-1015. doi: 10.1097/00005373-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 97.Parildar M, Oran I, Memis A. Embolization of visceral pseudoaneurysms with platinum coils and N-butyl cyanoacrylate. Abdom Imaging. 2003;28:36–40. doi: 10.1007/s00261-002-0021-7. [DOI] [PubMed] [Google Scholar]
- 98.Bergan J, Yao J. Aneurysms: Diagnosis and Treatment. New York: Grune and Stratton; 1982. [Google Scholar]
- 99.Vanlangenhove P, Defreyne L, Kunnen M. Spontaneous thrombosis of a pseudoaneurysm complicating pancreatitis. Abdom Imaging. 1999;24:491–493. doi: 10.1007/s002619900546. [DOI] [PubMed] [Google Scholar]
- 100.Stanley JC, Wakefield TW, Graham LM, Whitehouse WM, Zelenock GB, Lindenauer SM. Clinical importance and management of splanchnic artery aneurysms. J Vasc Surg. 1986;3:836–840. [PubMed] [Google Scholar]
- 101.Gabelmann A, Görich J, Merkle EM. Endovascular treatment of visceral artery aneurysms. J Endovasc Ther. 2002;9:38–47. doi: 10.1177/152660280200900108. [DOI] [PubMed] [Google Scholar]
- 102.Pilleul F, Dugougeat F. Transcatheter embolization of splanchnic aneurysms/pseudoaneurysms: early imaging allows detection of incomplete procedure. J Comput Assist Tomogr. 2002;26:107–112. doi: 10.1097/00004728-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 103.González Llorente J, Gallego Gallego M, Martínez Arnaiz A. Chronic post-traumatic pseudoaneurysm of the abdominal aorta diagnosed by duplex Doppler ultrasonography. A case report. Acta Radiol. 1997;38:121–123. doi: 10.1080/02841859709171254. [DOI] [PubMed] [Google Scholar]
- 104.Chaikof EL, Shamberger RC, Brewster DC. Traumatic pseudoaneurysms of the abdominal aorta. J Trauma. 1985;25:169–173. doi: 10.1097/00005373-198502000-00018. [DOI] [PubMed] [Google Scholar]
- 105.Maras D, Lioupis C, Magoufis G, Tsamopoulos N, Moulakakis K, Andrikopoulos V. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol. 2006;29:958–968. doi: 10.1007/s00270-005-0367-7. [DOI] [PubMed] [Google Scholar]
- 106.Flor N, Sardanelli F, Ghilardi G, Tentori A, Franceschelli G, Felisati G, Cornalba GP. Common carotid artery pseudoaneurysm after neck dissection: colour Doppler ultrasound and multidetector computed tomography findings. J Laryngol Otol. 2007;121:497–500. doi: 10.1017/S0022215106005342. [DOI] [PubMed] [Google Scholar]
- 107.Nebelsieck J, Sengelhoff C, Nassenstein I, Maintz D, Kuhlenbäumer G, Nabavi DG, Ringelstein EB, Dittrich R. Sensitivity of neurovascular ultrasound for the detection of spontaneous cervical artery dissection. J Clin Neurosci. 2009;16:79–82. doi: 10.1016/j.jocn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Kochan JP, Kanamalla US. Imaging in carotid and vertebral artery dissection. Medscape serial online, 2013-07-24, cited 2014-04-10; 1(1): 7 screens. Available from: http://emedicine.medscape.com/article/417341-overview#showall.
- 109.Montalvo BM, LeBlang SD, Nuñez DB, Ginzburg E, Klose KJ, Becerra JL, Kochan JP. Color Doppler sonography in penetrating injuries of the neck. AJNR Am J Neuroradiol. 1996;17:943–951. [PMC free article] [PubMed] [Google Scholar]
- 110.Ginzberg E, Montalvo B, LeBlang S, Nunez D, Martin L. The use of duplex ultrasonography in penetrating neck trauma. Arch Surg. 1996;131:691–693. doi: 10.1001/archsurg.1996.01430190013002. [DOI] [PubMed] [Google Scholar]
- 111.Demetriades D, Theodorou D, Cornwell E, Berne TV, Asensio J, Belzberg H, Velmahos G, Weaver F, Yellin A. Evaluation of penetrating injuries of the neck: prospective study of 223 patients. World J Surg. 1997;21:41–47; discussion 47-48. doi: 10.1007/s002689900191. [DOI] [PubMed] [Google Scholar]
- 112.LeBlang SD, Nu-ez DB Jr, Rivas LA, Falcone S, Pogson SE. Helical computed tomographic angiography in penetrating neck trauma. Emerg Radiol. 1997;4:200–206. [Google Scholar]
- 113.LeBlang SD, Nunez DB. Noninvasive imaging of cervical vascular injuries. AJR Am J Roentgenol. 2000;174:1269–1278. doi: 10.2214/ajr.174.5.1741269. [DOI] [PubMed] [Google Scholar]
- 114.Scalf TC, Drose JA. Ultrasound diagnosis of a common carotid artery pseudoaneurysm. J Diagn Med Sonogr. 2000;16:202–204. [Google Scholar]
- 115.Ramesh A, Muthukumarassamy R, Karthikeyan VS, Rajaraman G, Mishra S. Pseudoaneurysm of internal carotid artery after carotid body tumor excision. Indian J Radiol Imaging. 2013;23:208–211. doi: 10.4103/0971-3026.120264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu CJ, Sun Y, Jeng JS, Huang KM, Hwang BS, Lin WH, Chen RC, Yip PK. Imaging in the diagnosis and follow-up evaluation of vertebral artery dissection. J Ultrasound Med. 2000;19:263–270. doi: 10.7863/jum.2000.19.4.263. [DOI] [PubMed] [Google Scholar]


