Abstract
The reconstitution of a fully organized and functional hair follicle from dissociated cells propagated under defined tissue culture conditions is a challenge still pending in tissue engineering. The loss of hair follicles caused by injuries or pathologies such as alopecia not only affects the patients’ psychological well-being, but also endangers certain inherent functions of the skin. It is then of great interest to find different strategies aiming to regenerate or neogenerate the hair follicle under conditions proper of an adult individual. Based upon current knowledge on the epithelial and dermal cells and their interactions during the embryonic hair generation and adult hair cycling, many researchers have tried to obtain mature hair follicles using different strategies and approaches depending on the causes of hair loss. This review summarizes current advances in the different experimental strategies to regenerate or neogenerate hair follicles, with emphasis on those involving neogenesis of hair follicles in adult individuals using isolated cells and tissue engineering. Most of these experiments were performed using rodent cells, particularly from embryonic or newborn origin. However, no successful strategy to generate human hair follicles from adult cells has yet been reported. This review identifies several issues that should be considered to achieve this objective. Perhaps the most important challenge is to provide three-dimensional culture conditions mimicking the structure of living tissue. Improving culture conditions that allow the expansion of specific cells while protecting their inductive properties, as well as methods for selecting populations of epithelial stem cells, should give us the necessary tools to overcome the difficulties that constrain human hair follicle neogenesis. An analysis of patent trends shows that the number of patent applications aimed at hair follicle regeneration and neogenesis has been increasing during the last decade. This field is attractive not only to academic researchers but also to the companies that own almost half of the patents in this field.
Keywords: Adult stem cells, Skin grafts, Epidermis, Multipotential differentiation, Tissue regeneration, Dermal papilla, Epithelial-mesenchymal interactions
Core tip: Loss of hair follicles caused by injuries or pathologies affects the patients’ psychological well-being and endangers inherent functions of the skin. Different experimental strategies and approaches to obtain mature hair follicles have been designed based upon current knowledge of the epithelial and dermal cells involved in embryonic hair generation and adult hair cycling, and in the epithelial-mesenchymal interactions among them. This review summarizes the current advances in hair follicle neogenesis and regeneration, with emphasis on those involving neogenesis of hair follicles in adults from isolated cells and tissue engineering as well as an analysis on patent trends in this field.
INTRODUCTION
Regenerative medicine aims to create living, functional tissues that repair or replace lost or damaged organ function resulting from disease, injury, congenital defects or aging.
The main challenge of tissue engineering is the reconstitution of fully organized and functional organ systems from dissociated cells that have been propagated under defined tissue culture conditions.
Skin is the largest organ in the human body, acting as a barrier with protective, immunologic and sensorial functions. Deep skin injuries produce a complete destruction of skin regenerative elements. These wounds heal by contraction, with epithelization from the edges only and extensive scarring, resulting in reduced joint movements and cosmetic defects[1]. Moreover, if these lesions are too extensive, the healing process is unsuccessful and they become life-threatening for the patient. Tissue engineering has emerged as a new interdisciplinary field combining scaffolds, cells and biomolecular signals towards the treatment of skin lesions. This useful strategy may contribute not only to the treatment of deep skin injuries but also to the understanding of skin regeneration.
The main goal of tissue-engineered skin grafts is to restore lost barrier function. Many dermal-epidermal composites or skin equivalents have been described for use in the clinic[2] but the inability of these skin constructs to regenerate skin appendages as hair follicles has limited their use. Although the ability to reconstitute adult skin with functional skin appendages has long been a major clinical objective, the regeneration of epidermal appendages, such as hair follicles, and sebaceous and sweat glands, is a challenge that is still pending.
Recent studies clearly demonstrate that there are multipotent stem cells with the capability to regenerate hair follicles and sebaceous glands in adult mammalian skin and this multipotency can be maintained in cell culture[3,4]. The hair-differentiation potentiality of epidermal stem cells can be activated by inductive dermal cells. As will be extensively reviewed in this article, co-grafting of those cellular components from mice allows complete hair reconstitution[5]. The successful regeneration of hair follicles in immunodeficient mice suggests that creating human hair follicles in tissue-engineered skin grafts is feasible. However, regeneration of human hair in a similar manner has not been reported.
Skin appendages cannot be restored in healed wounds by current tissue-engineered skin grafts[6] mainly because of their limited self-regeneration capability in adults[7], the lack of appendage structures in the human skin grafts and probably an improper molecular microenvironment. Many strategies still need to be explored, particularly enriching isolated cells with trichogenic capability, maintaining this capability during processing, and providing the cells with proper environmental signals.
The lack of skin appendages by skin wounds and by pathologies such as different kinds of alopecia not only affects the patients’ psychological well-being[8], but also endangers the inherent functions of the skin.
Skin appendages cannot regenerate on their own after damage[7]. For the fully functional regeneration of ectodermal organs, it has been proposed that a bioengineered organ could be developed by reproducing the embryonic processes of organogenesis.
This review summarizes current advances in the different experimental strategies to regenerate or neogenerate hair follicles, in vitro and in vivo, with emphasis on approaches that include the neogenesis of hair follicles in adults from isolated cells and by tissue engineering as well as an analysis on patent trends in this field.
ANATOMY AND BIOLOGY OF HAIR FOLLICLES
The skin consists of three layers: epidermis, dermis, and hypodermis. The epidermis is in fact a multi-layered epithelium extending from the basement membrane that separates it from the dermis to the air. The dermis is located below the epidermis and is a connective tissue comprised of extracellular matrix, fibroblasts, vascular endothelial cells, and skin appendages.
Skin appendages, including hair follicles, sebaceous glands and sweat glands, are linked to the epidermis but project deep into the dermal layer. A human has around five million hair follicles with two types of hair, terminal hair (long, thick pigmented) and vellus hair (thin, unpigmented)[9].
Hair follicles are composed of an outer root sheath (ORS) that is contiguous with the epidermis, an inner root sheath (IRS) and the hair shaft (HS). The matrix surrounding the dermal papilla (DP), in the hair root, contains actively dividing and relatively undifferentiated cells that are essential for follicle formation.
In embryogenesis, the skin begins as a single layer of epidermal stem cells. Then, mesenchymal cells populate the skin to form the underlying collagenous dermis[10]. Human hair follicles start to develop through complex morphogenetic processes resulting from highly coordinated series of bidirectional epithelial-mesenchymal interactions[11]. Hair follicle development is initiated by the appearance of a thickening in the embryonic ectoderm called placode resulting from the condensation of the underlying mesoderm that will form the DP[12]. The DP becomes a permanent part of the follicle base[13] enveloped by the hair bulb. It is considered the commander of the hair follicle determining the hair thickness, length, and life cycle[8]. Signals from the condensed mesoderm induce the proliferation of the placode which forms mature hair follicles by a systematic series of differentiation and proliferation processes of epithelial cells. The hair follicle becomes fully mature when its bulb nears the bottom of the dermis. At this point, the proliferative cells (matrix) at the follicle base continue to divide, producing progeny cells that terminally differentiate to form the growing hair that emerges from the skin surface. The inner layers begin to differentiate into concentric cylinders to form the central HS and the surrounding channel, the IRS.
These tissues undergo continual rejuvenation and, in response to injury, they must be prepared for wound repair. The capability of the skin for maintaining tissue homeostasis, regenerating hair, and repairing the epidermis after injury resides in its stem cells.
HAIR FOLLICLE STEM CELLS AND THE HAIR CYCLE
Stem cells are undifferentiated cells that are ultimately responsible for the constant renewal of the skin due to their distinguishing properties of self-renewal for the entire life span of an organism and their ability to differentiate into a variety of specialized cells.
A pool of progenitor cells is located on the basement membrane of the skin. These cells contribute to epidermal homeostasis undergoing continuous self-renewal and differentiation to keratinocytes that migrate towards the surface of the skin where they undergo terminal differentiation and maturation providing the skin’s barrier properties.
Another reservoir of slow-cycling multipotent stem cells that gives rise to a range of differentiated cell types in skin is located in a specialized region of the ORS in the hair follicle, known as the bulge[3,4,14-16]. Early work showed that these slow cycling multipotent stem cells not only contribute to the growth of hair follicles themselves and of the sebaceous glands[3] but also can be activated and migrate out of hair follicles in order to repair the damaged epithelium[17]; however, they contribute little to the turnover of the intact epidermis. In the absence of these hair follicle stem cells (HFSC), hair follicle and sebaceous gland morphogenesis is blocked, and epidermal wound repair is compromised[18]. The epithelial-mesenchymal interactions with the underlying DP play a pivotal role in embryonic hair genesis[12], in the regulation of post-natal hair follicle cyclical activity, and in the repair of wounded skin[19-22].
Unlike most organs, hair follicles do not reach homeostasis once they mature. Each mature hair follicle is a regenerating system, which physiologically undergoes cycles of growth (anagen), regression (catagen), and rest (telogen) numerous times in adult life[23]. In catagen, hair follicle stem cells are maintained in the bulge. Then, the resting follicle re-enters anagen (regeneration) when proper molecular signals are provided. During late telogen to early anagen transition, signals from the DP stimulate the hair germ and quiescent bulge stem cells to become activated[24]. Many paracrine factors are involved in this crosstalk at different hair cycle stages and some signaling pathways have been implicated[3,19,25]. In anagen, stem cells in the bulge give rise to hair germs, then the transient amplifying cells in the matrix of the new follicle proliferate rapidly to form a new hair filament[26].
Even if it is generally believed that hair follicles do not form after birth in humans[27], the formation of vellus hair follicles from the reconstituted epidermis of an abraded area of facial skin was described in 1956[28]. However, the great challenge of skin tissue engineering and skin wound regeneration remains to be its inability to reliably reconstitute skin appendages, most notably hair follicles and sweat glands. The information about the mechanisms that generate and maintain skin appendages provided by recent studies with skin-derived progenitor cells, may be the basis of new therapies that could help to overcome these limitations.
CAUSES OF HAIR LOSS AND REGENERATION OF THE HAIR FOLLICLE
Hair loss responds to different causes ranging from mild traumas, such as hair fiber plucking, to severe traumas such as partial- or full-thickness skin lesions. Pathological processes, such as cicatricial or non cicatricial alopecia, can also be responsible for hair loss. After plucking, if HFSC and DP remain, a new hair filament would be spontaneously regenerated. Some label retaining studies have shown that hair follicle stem cells in the bulge remain intact after hair plucking[29,30] whereas others reported bulge stem cells suffering apoptosis after plucking which are replaced by label retaining cells coming from hair germ[31]. It was also observed that the slow-cycling bulge cells (CD34 positive cells in mouse) are involved in normal hair homeostasis and wound healing, whereas regeneration after hair plucking involved actively cycling cells from the lower ORS[26]. However, the cell dynamics in this process is less clear than in the physiological renewal and further studies are required to understand this process.
However, when the cellular niches are completely lost, it is necessary to generate a completely new hair follicle in a process called hair follicle neogenesis.
Based on the knowledge on the epithelial and dermal cells, and their interactions, during the embryonic hair generation and adult hair cycling, different experimental approaches have been designed to regenerate hair follicles or generate new ones by the neogenesis process.
These hair regeneration and neogenesis attempts are recapitulated in this article, and can be classified into 4 categories: (1) reversion of pathological intra- and/or extra-follicular environment, for instance androgenetic alopecia (AGA); (2) regeneration of complete hair follicles from the recombination of hair follicle parts; (3) neogenesis of hair follicles from isolated cells; and (4) neogenesis of hair follicles by tissue engineering.
Reversion of pathological intra- and/or extra-follicular environment
Hair follicle cycle and growth can be affected and deregulated by paracrine factors from the follicle itself and/or from the surrounding dermal tissue, or by endocrine factors[32,33] leading to hair loss. Many factors have been described as molecular mediators of hair follicle growth [11]. Some of them, such as PDGF[33] and Wnt proteins[34], are positive regulators while others, such as BMPs[35], suppress hair growth. Many paracrine factors involved in the epithelial-mesenchymal crosstalk at different stages of the hair cycle, and some implicated signaling pathways, have also been studied[3,19,25].
In addition, endocrine factors, such as sexual hormones, have both physiological and pathological effects on hair growth as in human male beard, and androgenetic alopecia respectively. Androgenetic alopecia[36] is a common, progressive disorder in which large, terminal scalp hairs are gradually replaced by smaller hairs following a defined pattern. The DP is the site through which androgens act on follicle cells by altering the regulatory paracrine factors involved in the differentiation and proliferation of HF stem cells. DPs from balding scalp have higher levels of androgen receptor and 5-alpha reductase. Dihydrotestosterone can induce DP to secrete factors including, TGF-beta1 and DKK-1 which down regulate keratinocytes growth[37,38].
A functional crosstalk between the androgen receptor and Wnt signaling pathways in DPCs has been described in target tissues[39]. Moreover, our group has demonstrated that androgens regulate secreted factors involved in normal HF stem cell differentiation via the inhibition of the canonical Wnt signaling system in androgen sensitive DPCs. We provided evidence that androgen activation of GSK-3β would be responsible for the inhibition of Wnt/β-catenin signaling[40]. The current pharmacological treatment for AGA includes androgen metabolism modulators, such as the 5-alpha reductase inhibitor finasteride and the antiandrogen agent flutamide. Nevertheless, the results of these treatments are not only variable and patient-dependent, but can also cause undesirable side effects. Therefore, therapies acting on specific molecular targets are necessary to treat this pathology downstream from the actual antiandrogenetic pharmaceutical modulators. The crosstalk between the androgen receptor (AR) and Wnt/β-catenin signaling pathways could be one alternative therapeutic target, among many others recently reviewed[41].
In these experimental approaches, modulation of the intra-follicular microenvironment and the extra-follicular macroenvironment could contribute to promote growth of the existing hair follicles but not to increase their number by the formation of new ones.
Regeneration of hair follicles by recombination of hair-follicle parts
Taking into account the inductive ability of DP and the current knowledge on epithelial-mesenchymal interactions, different recombination of dermal and epidermal tissue have been employed for hair follicle regeneration. In the early 1960s, researchers showed that whole follicle end bulbs and isolated whole whisker papillae (containing dermal and epidermal components) remain viable and may produce whisker hair follicles in a new site[42]. The most surprising finding was that isolated DPs (without epidermal components) were capable of inducing the generation of new hair follicles recruiting epithelial cells from the implantation sites. The same results were obtained when epidermis from different origins were transplanted together with whisker DP[43]. Regeneration of hair follicles was also observed in both mice[43-45] and humans[46] when dermal sheath tissue was used, which was sufficient to regenerate also the DP structure. After implantation, the whisker DP was capable of inducing hair follicle regeneration retaining the information to determine hair fiber type and follicle size[47].
Neogenesis of hair follicles in adult individuals from isolated cells
Grafting of dermal-inductive tissue is limited by the fact that it is not possible to generate more hair follicles than the obtained from the donor tissues. To overcome this limitation different approaches and experimental models using freshly or cultured isolated cells from both dermal and dermal/epidermal origin were tested. Most of them involved neonatal and embryonic murine cells. One of the first models used was the chamber assay (Figure 1) in which dermal and epithelial cells are seeded inside a chamber consisting of a cylinder inserted through a full-thickness skin lesion in mouse dorsal skin, covered by a dome. In former studies using this device in nude mice[48], it was showed that hair buds obtained from neonatal mice combined with fresh neonatal dermal cells or with immortalized clones from vibrissae rat DPCs produced mature and cycling hair follicles. Using the same chamber assay, hair bud preparations without any additional inductive dermal component were unable to form hair follicles resulting in a scarring reparative tissue, a thin epidermis and a dermis without appendages[49]. On the other hand, the combination of hair buds with fresh dermal cell preparations or early passages of cloned vibrissae cell lines resulted in skin with normal epidermal and dermal layers.
Figure 1.
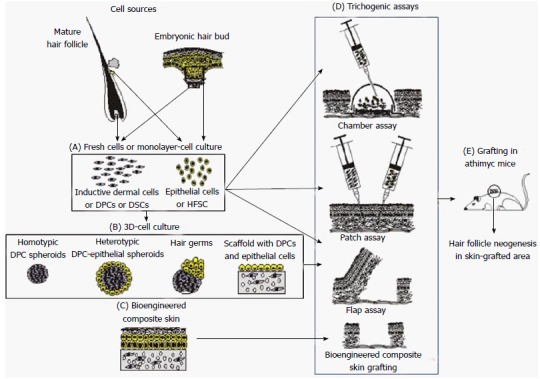
Hair follicle neogenesis using isolated epithelial and dermal cells. Inductive-dermal and Epithelial Cells can be obtained from adult tissue or embryonic sources. Isolated cells by enzymatic digestion of living tissues, or monolayer cell cultures (A) can be used in the trichogenic assays (D): Chamber assay; Patch assay; and flap assay. Moreover, isolated cells can be cultured in 3D-conditions, allowing them to create their own extracellular matrix as in the case of homotypic and heterotypic spheroids and hair germs (B). Also, cells can be seeded on a precast scaffold used for cellular support or used to produce a bioengineered composite skin (C). Any of these constructs can be evaluated by the trichogenic assays (D): Flap assay; and Bioengineered composite skin grafting. All these trichogenic assays are mainly performed in the back-skin of athymic mice in order to avoid immune rejections and to take advantage of its bare-skin (E). DPCs: Dermal papilla cells; DSCs: Dermal sheath cells; HFSC: Hair follicle stem cells.
Although these studies confirmed the trichogenic potential of dissociated dermal cells, particularly DPCs, the conditions of the chamber assay correspond to a wound healing environment and do not represent the physiological status. Other approaches allow studying the trichogenic potential of isolated cells in a more physiological environment, as is the case of the patch assay (Figure 1). This assay consists in injecting dissociated murine neonatal epidermal and dermal cells hypodermically into adult mouse skin. This system, originally described by Zheng et al[50], showed the formation of cell clusters that resulted in “infundibular cysts” which generated hair germs, followed by pegs that grew to differentiate into cycling mature pilosebaceous units. Surprisingly, whereas patch hair follicles were in anagen phase, the host skin hair follicles were in telogen and vice versa, suggesting that follicle cycling depends on the trichogenic cells and not on the host skin hair cycling. Even when the shaft grows inside an intradermal cyst that tends to extinction by inflammatory processes, none of the other already described systems at that moment showed to be so efficient to evaluate the trichogenic potential of isolated cells. A variation of this method is called tracheal grafting method. It consists in inoculating rat tracheas with fresh or cultured keratinocytes from newborn mice together with newborn fibroblasts or cultured DPCs and implanting them subcutaneously in athymic mice for four weeks. In this assay hair follicles and sebaceous glands were generated using cultured keratinocytes from newborn mice and fresh newborn fibroblasts[51]. Although adult mice keratinocytes successfully produced a two layered epithelium, they failed to generate hair follicles or sebaceous glands[51].
Other strategy to evaluate the hair inductive capability of dermal cells is the “flap assay” (Figure 1). This approach originally described by Qiao et al[52] consists in: (1) generating a skin flap in the dorsal skin of nude mice; (2) locating an embryonic epidermis supported by a silicone sheet in direct contact with the muscle with the basal side of the embryonic epidermis facing upwards; (3) seeding embryonic murine dermal cells or cultured DPCs onto the basal face of the graft-epidermis; and (4) pulling over the graft and suturing the flap edges. In this configuration the graft-dermal cells were layered between the graft-epidermis and the host connective tissue. When fresh embryonic dermal cells were used, abundant hair was developed four weeks after grafting. The induced hairs were oriented vertically down towards the silicone sheet. After surgical flap inversion, the formed hairs were exposed in the host skin surface and survived for more than 12 mo.
Neogenesis of hair follicles by tissue engineering
Although the assays described above provided much information respect to the hair neogenesis process and the cells involved in it, the high number of cells required, the need of in vivo animal models and the poor results obtained with human cellular sources make these procedures not applicable for clinical uses in the near future. The development of new strategies has become necessary and begun to emerge. They are mainly focused on tissue engineering-based follicle neogenesis, including three-dimensional (3D) cell culture conditions generated by the cells themselves or by the use of biocompatible scaffolds.
Lee et al[53] developed a simplified procedure to reconstitute hair-producing skin. They obtained both epidermal and dermal cells from newborn mice, and mixed them in different ratios. A high density cellular suspension was prepared in drops of minimal volume on tissue culture inserts or wells. They allowed the cells to settle until gel consistency was obtained. Alternatively, they seeded the cells on the collagen-side of the IntegraTM matrix (porous matrix of cross-linked bovine tendon collagen and glycosaminoglycan and a silicone layer). Both constructs were grafted in full thickness skin wounds generated on the back of athymic mice. After grafting, the epidermal cells formed a basal layer and some epidermal microcysts could be observed. Dermal cells started to form dermal condensations adjacent to the epidermal layer or cysts. Eight days after grafting hair germ started to appear and progressed to hair peg, observing complete hair follicles four days later that kept the ability to continuously cycle for at least one year. This method would allow preparing constructs with specific sizes and shapes useful to treat alopecia by tissue implants.
Two separate reports[54,55] showed hair follicle neogenesis using hair germs obtained by the previously described “organ germ method”[56]. One of them[54] generated bioengineered hair germs mixing epithelial and mesenchymal cells derived from mouse embryos within a collagen gel drop. In these culture conditions, the two cellular types generated a structure with two cellular layers separated by a translucent region they called hair germ. When these hair germs were transplanted ectopically into subrenal capsules, the presence of mature hair follicles was observed. These bioengineered hair follicles had a normal histological structure with the concentric epithelial layers of ORS and IRS, DP, hair matrix, and sebaceous glands. These follicular units transplanted intracutaneously in nude mice (Figure 1), were connected to the arrector pili muscle and nerve fibers and were able to produce hair shafts and cycling.
In addition, a fully functional orthotopic hair regeneration was demonstrated via intracutaneous transplantation of bioengineered hair follicle germs generated by epithelial and mesenchymal cells derived from mouse embryos[55] (Figure 1). These bioengineered hairs responded to hair cycles and had the correct structure of natural hair follicles and shafts, and the proper connections with surrounding host tissues. Qiao et al[57], using embryonic dermal cells and hair-peg-derived keratinocytes, generated cellular aggregates by hanging drop methods. These aggregates cultured in methylcellulose coated wells elongated, and after seven days, acquired hair follicle features. These structures termed “proto hairs”, showed a morphological structure more developed respect the previously described “hair germs”[54,55]. They presented hair-like characteristics as an inner mass of cells similar to the DP structure surrounded by matrix-like keratinocytes and a partially keratinized substance that could represent intent to produce a hair shaft. After implantation into shallow incisions in nude mice (Figure 1), black-pigmented hair fibers were observed emerging from epidermis. These hairs persisted throughout the experimental period (6 mo) and were shown to regrow after plucking.
Although embryonic tissues are used as cellular sources, these studies showed the potentiality of dissociated cells to form hair follicle germs or proto-hairs able to generate mature hair follicles both by ectopic and orthotopic implantation. Collectively, these studies provide a proof of concept for the use of Follicular Cell Implantation to restore hair in patients suffering from hair loss.
As it was already mentioned, dermal cellular components from the skin, such as DPCs or dermal sheath cells (DSCs), have the potential to induce hair formation in in vivo assays and also induce in vitro differentiation of hair follicle stem cells to hair lineage[25,40]. Even if it is possible to isolate and culture DPCs, the inductive ability tends to fade with time in 2D cultures[6,58]. Considering that the hair follicle neogenesis requires a large number of DPCs, it is necessary to look for new strategies to maintain their inductive potential in culture in order to use them in skin bioengineering and hair follicle neogenesis.
The niche generated by the extracellular matrix (ECM) of DP probably plays a key role in keeping DPC inductive activity. Accordingly, the loss of ECM throughout culture time could be responsible for the fading of DPC inductive properties.
DPCs are naturally aggregated at the hair-bulb base. In proper culture conditions, these cells tend to aggregate[13,59] and their inductive activity is preserved after they are grafted in this state. It has been demonstrated that it is possible to generate dermal papilla spheroidal microtissues using different methods including rotation, two-step rotation and flotation, and hanging drop[60-62]. Osada et al[63] artificially prepared DPC spheres by aggregation of mouse vibrissae follicle DPCs in a round-bottom 96-well low-binding plate. These spheres expressed higher amounts of versican, an indicator of inductive capability, than DPCs in monolayer cultures. In a “patch assay” (Figure 1), together with embryonic-epidermal cells, they induced hair follicle neogenesis, keeping their inductive capability for at least twenty-six passages.
However, these methods are labor intensive and the microtissues obtained have a very variable size and cell number content, which reduces reproducibility.
DPCs can self-assemble into dense spheroids when seeded on controlled biomaterial surfaces such as poly (ethylene-co-vinyl alcohol) (EVAL)[64] or polyvinyl alcohol (PVA) membranes[65]. The DPC microtissues generated on EVAL surface[64] expressed DPC-markers such as neural cell adhesion molecule (NCAM)[66] and α-smooth muscle actin[67]. Alkaline phosphatase activity, which correlates with hair follicle inductive ability of DPC[68], was higher in spheroid microtissues than in monolayer cultured cells. Finally, the spheroids mixed with newborn mouse epidermal cells and injected into the hypodermis of nude mice in a “patch assay” (Figure 1) were able to generate new hair follicles.
The use of PVA as substratum material enhanced DPC aggregation[65], preventing cellular attachment and spreading. Using this material and reducing culture surface it is possible to control the number, size and compaction of spheroidal microtissues. DPCs quickly aggregated into a single spheroid whose diameter decreased progressively due to tissue compaction. The expression of DPC-inductive capability markers, versican[69] and alkaline phosphatase[68], were present in spheroids of various sizes. All these experiments were made both with adult DPCs from rat vibrissae, and human scalp hairs, giving similar results. The hypodermal injection in nude mice of eighty spheroids altogether with keratinocytes from newborn mice induced HF neogenesis. These results are very promising given that transplantation of dispersed cultured human DPCs had shown very low efficiency in inducing new hair follicles[70]. Using the same approach, heterotypic folliculoid microtissues or hair follicle-like organ germs were produced using dissociated adult epithelial and mesenchymal cells by self-assembly on EVAL coated wells[71]. Keratinocytes and DPCs seeded together grew into floating or loosely attached multicellular spheroids. Histological analysis showed that the spheroids are composed by a core of DPCs surrounded by keratinocytes. These keratinocytes expressed keratin 6, a cytokeratin preferentially expressed by ORS in vivo[72], and DPCs expressed versican, a marker associated to HF-inductive ability. Using “patch assays” (Figure 1) disperse DPCs and keratinocytes did not form new hair follicles, whereas heterotypic folliculoid microtissues did. These last experiments would contribute to the development of new strategies for scalable production of organoid microtissues of epithelial organs for hair follicle neogenesis by bioengineering approaches.
Altogether, these results strongly indicate that the hair-inductive capability of DPCs can be restored by three-dimensional spheroid cultures.
Hair follicle neogenesis using human cells
During embryogenesis, dermal mesenchymal cells drive HF development and a condensate of these cells remains during adult life forming hair follicle DP. These cells retain the capability to induce hair follicle regeneration and neogenesis[21,73] as reviewed by Ohyama et al[5] and Yang et al[6]. However, most of these experiments were performed using murine and embryonic cells, whereas DPC from human origin showed many difficulties to maintain inductive capability in culture[74].
As discussed above, rat cultured DPCs self-aggregate within the dermis forming a papilla-like structure that synthesizes its own extracellular matrix[13]. However, such behavior was not observed with cultured human DPCs, highlighting specie-specific differences. Human keratinocytes from ORS and DPCs cultured in MatrigelTM (3D network of extracellular matrix proteins and collagen) have shown to form tubule-like structures in this skin-equivalent in vitro[75] and organize themselves into epidermoid cyst-like spheroids[72,76], but did not form complete hair follicles. Similarly, Sriwiriyanont et al[77], observed neofollicle formation in nude mice grafted with engineered skin substitutes containing murine DPC and human keratinocytes in a collagen-glycosaminoglycan matrix, but not in those containing human DPC and human keratinocytes.
Ehama et al[78] reported the formation of hair follicle-like structures using human primary cultures of foreskin- or adult-derived epidermal cells co-grafted with murine DPCs by a chamber assay (Figure 1). The innermost regions were similar to the hair cortex and medulla of mature human follicles. Hair shaft-like fibers occasionally emerged at the skin surface, and structures, reminiscent of the DP, at the follicle bottom were also observed. However, these structures showed no bulge region, nor all the follicular epithelial layers, and the DP-like tissue failed to express the anagen DP marker versican. These hair follicle-like structures correspond to the initial follicle formation suggesting that the differentiation process was altered.
Using the “flap assay”[52] (Figure 1), Qiao et al[79] showed that DPCs from human scalp combined with epidermis from mouse embryo produced mature hair follicles that persisted and grew. The authors reported that the inductive potential of DPCs could be maintained using keratinocyte-conditioned medium. These DPCs were able to form DP and dermal sheath in vivo.
Spheroids microtissues were also obtaining by culturing human DPCs in a 96-well low-binding plate, and implanted intradermically into nude mice using the “patch assay” (Figure 1)[80]. This combination of human DPC spheres with murine neonatal epidermal cells generated new hair follicles. In contrast, hair follicles were never observed when monolayer DPC cultures were used.
Cultured human DPCs do not induce hair neogenesis unless changes in the culture conditions are made. In this sense, the formation of DPC-microsphere tissues has shown to improve hair-inductive properties of rodent cells as well as the expression of cell-markers associated with these properties[63-65]. Therefore, all these observations encouraged investigators to look for new strategies intended to achieve the first step toward human hair follicle neogenesis that is, providing culture conditions that confer hair-inductive ability to human DPCs. Higgins et al[74] evaluated the human DPC-transcriptome observing that monolayer DPC cultures showed the most important changes immediately after early outgrowths from DP explants. These changes involved 3729 transcripts, including several involved in hair follicle development. This large number of differentially expressed transcripts shows the physiological changes that 2D culture conditions do on the molecular signature of DPCs. The generation of DPC spheroids by the hanging drop method partially restores the intact DP signature. These human-DPC spheres induced hair follicle neogenesis when placed in between foreskin epidermis and dermis and grafted onto nude mice (Figure 1). These hair follicles showed a DP and dermal sheath highly positive for alkaline phosphatase, hair specific keratin markers disposed in concentric layers and blood vessel formation around follicles. Nevertheless, neither sebaceous glands nor hair fibers emerging from the skin surface were seen. These results suggested that human DPC spheroids would be able to initiate hair follicle morphogenesis but the production of a complete hair follicle requires additional signals.
Recently, Miao et al[81] produced DPC-spheroids from cultured DPCs on a MatrigelTM scaffold. As reported by Higgins et al[74], the DPC spheres were shown to restore DP-signature gene expression of NCAM, versican and α-smooth muscle actin (α-SMA), lost during monolayer culture. The authors also claimed that these DPC-spheres, combined with hair germinal matrix cells, onto Matrigel-coated plates produced colorless fiber-like structures in vitro.
Other alternative to culture dermal mesenchymal cells in 3D conditions is the organotypic method consisting in culturing cells inside a scaffold. Using this method, Wu et al[82] prepared a collagen gel with human DPCs or dermal sheath cells (DSCs). This gel was seeded with keratinocytes from interfollicular skin, superior ORS or inferior ORS obtaining in vitro a bilayered skin. Nonetheless, only the constructs containing superior ORS keratinocytes showed hair follicle-like structures. That is, an outer root sheath, nearly ten layers of concentric epithelial cells, a middle layer IRS-like and some keratinoid substances in the center, similar to a hair fiber. When these constructs were transplanted into nude mice (Figure 1), only the organotypic cultured tissue containing superior ORS keratinocytes and DPCs exhibited hair follicle structures.
In a recent study[83], composite skin substitutes were generated by seeding human neonatal foreskin keratinocytes onto a dermal equivalent structure cultured in air-liquid interface condition. This dermal equivalent was composed of DPCs from human scalp contained in a collagen-I gel. Eight weeks after grafted onto nude mice (Figure 1), these constructs presented hair follicles showing bulb, dermal sheath, hair matrix and cortex. Histological analysis showed concentric layers of IRS and ORS, sebaceous glands and hair shaft. Inmuno-histochemistry assays revealed that both epithelial and dermal cells from neo-follicles were from human origin, and that DPCs and DSCs expressed human nestin and versican.
The analysis of all these reports leads to the conclusion that hair follicle neogenesis using human epithelial and dermal cells is a very difficult task that requires special culture conditions, somehow recreating the normal or embryonic skin environment, and the use of embryonic or neonatal cells.
Very recently, we prepared in our laboratory a dermal-epidermal skin substitute by seeding an acellular dermal matrix with cultured hair follicle epithelial stem cells and DPCs, both obtained from adult human scalp[84]. These constructs were grafted into a full-thickness wound generated on nude mice skin (Figure 1). In only fourteen days, histological structures reminiscent of many different stages of embryonic hair follicle development were observed in the grafted area. These structures showed concentric cellular layers of human origin, and expressed k6hf, a keratin present in epithelial cells of the companion layer. Although the presence of fully mature hair follicles was not observed, these results represent, up to our knowledge, the first report showing that both epithelial and dermal cultured cells from adult human scalp in a dermal scaffold were able to produce in vivo structures that recapitulate embryonic hair development.
ANALYSIS OF PATENTS TRENDS IN HAIR FOLLICLE REGENERATION AND NEOGENESIS
So far, we have made a concise review of the scientific literature describing the attempts to regenerate the hair follicle. We have also mentioned a number of reviews on academic articles dealing with hair regeneration or wound induced follicle neogenesis that have been published in recent years[85]. However, reviews on tissue engineering and hair regeneration patents are less common. Many of these strategies have been combined in different methods intended for use in active applications. In this section, we review and analyze published patents on hair regeneration[86-150], as a measure of the interest in the industry for this area of research, identifying the currently available technical developments, favorite research strategies and main points of interest. Parameters analyzed include not only chronological patent publishing trends, but also the most cited patents, top patent owners (assignees), most cited documents, and the classification of patents among technology areas according to the International Patent Classification (IPC). Moreover, hair regeneration applications that have been patented throughout the years are screened (Figure 2).
Figure 2.
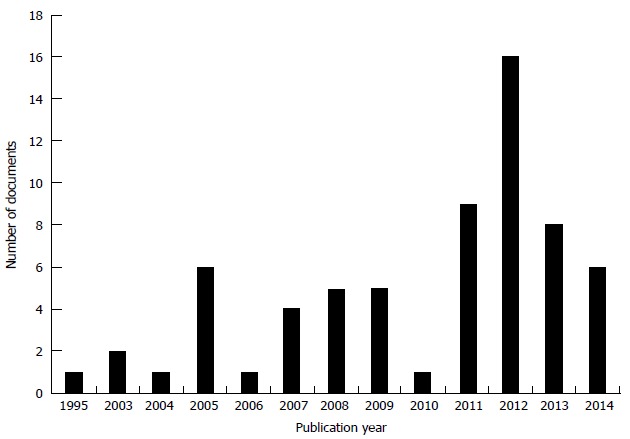
Evolution of the number of patent applications related to hair regeneration (time frame 1990-2014). Own investigation using patent data base Thomson Innovation.
Patent search was performed using the Thomson Innovation Database, a collaboaration platform for searching and analyzing global patents, integrated with analytics and workflow tools which allow access to more than 40 databases from different countries. For each particular group of hair regeneration, specific keywords (descriptors) were selected. Descriptors were chosen based on the synergy of two approaches: the search of specific keywords and concepts used in academic publications, and the selection of additional descriptors from specific patent vocabulary. Therefore, the keywords are: Stem cell; progenitor cell; precursor cell; repair; growth; wound; regeneration; neogenesis; skin; tissue engineering; dermis; epidermis; dermal; hair follicle; sebaceous gland and sebaceous unit. The presence of the chosen descriptors was checked in the title, abstract and claims of existing patents, considering documents published between years 1990 and 2014. More than forty technology patent and patent application databases were used for patent search, including the Spanish OEPM, the American USPTO, the worldwide WIPO, the European EPO, the patent offices of France, Germany, Great Britain and the Far-Eastern patent offices of Japan, China and South Korea. Besides the keywords, the search process was completed with the use of the IPC codes under which the patents of interest could be framed. The combination of the search approaches previously mentioned offered a universe of about 475 patent families, which were manually reviewed and filtered, setting aside those directed to gene therapies, mesenchymal, embryonic stem cell, osteoblast, neuron and hematopoietic cell and others. The result of this process was the identification of 65 patent families[86-150]. The selection of the document to be considered in patent trends was the first published. It is important to note that patent applications can be published in different countries under different national numbers. This is called the family of the patent. In the search performed for the present study, care was taken to include just one patent per family.
Tables showing patents that are most frequently cited take into account all the patents in the family, since it may occur that the earliest document is not necessarily the most cited one.
Competitive dynamics
The competitive dynamics indicator shows the distribution of patents among the different organizations, providing a general picture of the tissue repair regeneration industry and their relative positioning. On the basis of this information we can identify the most prolific applicants for hair regeneration technology patents in the world, by establishing the number of applications filed by each company.
Table 1 lists the applicants that have filed more than one application, which will be our “Reference Group”. Most of the members of this group are manufacturing companies, indicating that this technology belongs to the industrial sector. The highest percentile is shared by a small number of applicants with five patents each, while a second group includes a wide range of companies with no clear technological leadership.
Table 1.
Owners of hair regeneration patents and patent applications matching all the descriptors found in academic literature
| Assignee | No of patent applications | Percentage |
| Shiseido Co Ltd | 5 | 7.69% |
| Aderans Research Institute Inc | 5 | 7.69% |
| Hospital for Sick Children | 2 | 3.08% |
| Biointegrence Inc, phoenixbio Co Ltd | 2 | 3.08% |
| Follica Inc | 2 | 3.08% |
| Organ technologies Inc | 2 | 3.08% |
| Universitiy of Jilin | 2 | 3.08% |
| University of Southern California | 2 | 3.08% |
| National University of Taiwan | 2 | 3.08% |
| Aderans Research Institute Inc, Bioamide Inc | 1 | 1.54% |
| Agency for science, technology and research | 1 | 1.54% |
| Alvi Armani Genomics Inc | 1 | 1.54% |
| Anticancer Inc, Li L, Yang M | 1 | 1.54% |
| Beijing Yonghe Hair Transplant Technology | 1 | 1.54% |
| Biomaster KK | 1 | 1.54% |
| Chen B, Gao Q, Zeng Q | 1 | 1.54% |
| Chinese Academy of Science Institute of Zoology | 1 | 1.54% |
| Total No of patent applications | 65 | 100.00% |
| No of Entities | 50 |
Own investigation using patent data base Thomson Innovation.
The group of companies of the first percentile shown in Table 1, are the leading hair regeneration manufacturers Shiseido Co ltd and Aderans Research Institute, which own more than five published patents each, and which own almost 20% of all filed applications in this field.
The second percentile of institutions having one or two applications each could offer a favorable scenario for technology transfer.
It should be highlighted that there are over 20 institutions or about 40% patent applications from non-profit institutions including universities and foundations. The most prolific institutions are: Jilin University, University of Pennsylvania, University of Southern California and National Taiwan University, which gives an idea that this field is very prolific in academic institutions.
Technology trends
The patent technology trends indicator introduces the serial number of patent applications showing how technology has evolved over time as well as its appropriation by companies and non-profit institutions in the field of hair regeneration. This technological area has experienced constant growth. In the 90’s an average of 2.5 patent applications were filed per year, and in the first decade of the 21th century the average increased to 6 patent applications per year (Figure 2).
The years 2011 and 2012 stand out as the ones with the most patent applications, 10 and 16 respectively.
Aderans Corp Company filed most patent applications from 2005 to 2008. Shiseido and Follica, as well as the University of Pennsylvania have filed patent applications in recent years.
From this general perspective, it can be inferred that the technological field is, at present time, in a developmental stage, with an annual growth rate of 10%, based on the number of patent documents filed since 2010.
From another perspective, Figure 3 shows the cumulative patent families published in the last 20 years. As it can be seen, since 2005 there has been a remarkable increase of attraction in the area: while 50% of the patents required 15 years to be generated, the remaining 50% has been filed over the past five years.
Figure 3.
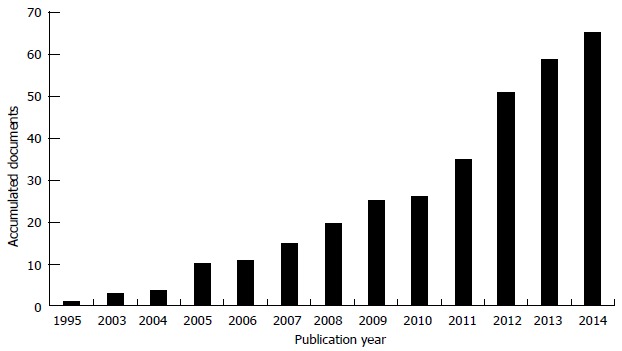
Evolution of accumulated patent families. Tendency line. Own generation based on patent databases.
The analysis of patent application-trends shows that, at the present time, the development of hair regeneration technology is evolving positively, making this technological field attractive to companies that start producing and selling products as a result of the development of hair regeneration technology.
Technological leadership indicator
The technology leadership indicator identifies specific companies with the most consolidated and developed technologies in this specific field. The Patents which have received the highest number of citations are considered to have the highest degree of technological progress or impact. The analysis of patent citations allows the identification of patents which have been most useful for later researchers and for the development of newer technologies and may illustrate the diffusion of technical knowledge and its different practical applications throughout the years.
Just as those patents that have received most citations are not always the most relevant ones, and those most relevant are not always the most cited ones; a number of empirical studies demonstrate a strong correlation between those variables. In Table 2 the 10 most cited institutions as references are listed.
Table 2.
Most cited patents from 1990 to present
| Publication number | Title | Assignee | Inventor | Publication year | Count of citing patents | Ref. |
| WO1995001423A1 | Methods of culturing and modulating the growth of hair follicular stem cells | New York State Univ, Pennsylvania Univ | Lavker RM, Sun T, Yang J | 1995 | 29 | [86] |
| WO2005053763A1 | Hair growth method | Biointegrence Inc, Phoenixbio Co Ltd | Matsunaga M, Matsunaga MC, Shimada T, Shimada TC, Toyoshima K, Toyoshima KC, Yoshizato K | 2005 | 17 | [94] |
| WO2003024406A2 | Nestin-expressing hair follicle stem cells | Anticancer Inc | Li L, Lingna L, Meng Y, Ri R, Yang M | 2003 | 17 | [87] |
| WO2005071063A1 | Methods of making and using skin-derived stem cells | Hospital For Sick Children | Biernaskie J, Fernandes K, Fernandez K, Mckenzie I, Miller F, Miller FD | 2005 | 16 | [91] |
| WO2002060396A2 | Hair follicle neogenesis by injection of follicle progenitor cells | Aderans Res Inst Inc, Bioamide Inc | Barrows TH | 2002 | 16 | [88] |
| US20050214344A1 | Tissue engineered biomimetic hair follicle graft | Aderans Res Inst Inc | Barrows T, Barrows TH, Cochran S, Cochran SA, Marshall B | 2005 | 15 | [90] |
| US20070092496A1 | Method of delivering cells to the skin | Aderans Res Inst Inc | Du X, Stenn K, Stenn KS, Washenik K, Washenik KJ, Zhang Y, Zheng Y | 2007 | 10 | [99] |
| WO2003104443A2 | Hair follicle mesenchymal stem cells and use thereof | Torico Sci Innovations Inc, Trichoscience Innovations Inc | Hoffmann R, Mcelwee KJ | 2003 | 9 | [89] |
| US20070122387A1 | Hair grafts derived from plucked hair | Aderans Res Inst Inc | Barrows TH, Cochran SA, Marshall B, Schlicher R, Su Y | 2007 | 9 | [105] |
| WO2007062386A2 | Hair follicle graft from tissue engineered skin | Aderans Res Inst Inc | Barrows TH, Macintyre P, Washenik KJ | 2007 | 8 | [98] |
Own investigation using patent data base Thomson Innovation.
Up to the present, New York University and Pennsylvania State University have received the highest number of citations with 29 citations which reflects the importance of the works of Lavker et al[86] previously described. Following these institutions, companies likse Biointegrence Co and Phoenixbio Co Inc, and Anticancer Inc have 17 citations. The Hospital for Sick Children, Aderans Research Institute Inc, Sci Torico Innovations Inc and Trichoscience Innovations Inc are among the most innovative companies.
Skin substitutes were among the earliest products to be developed using principles of tissue engineering, and their success is evident in the clinical use of several commercially available products. However, skin substitutes capable of performing all the functions of normal skin are not currently available, which limits their use in patients. Hair follicle neogenesis is not observed using current skin substitutes. Thus, the efforts in hair and skin bioengineering continue to be in a leading place in regenerative medicine. Furthermore, hair restoration is one of the fastest growing areas of cosmetic therapies for both men and women.
Hair follicles are important not only for appearance, skin hydration, barrier formation, and protection against pathogens but also in wound healing. As hair follicles store epidermal stem cells, skin with hair follicles heals faster. In addition, bulge stem cells are less susceptible to loss through minor trauma and damage through ultraviolet light. Thus, treatments that involve neogenesis of normal hair follicles would find much wider application for restoring normal skin function and appearance.
Methods and compositions capable of generating morphologically-correct, fully-developed human hair follicles, useful for treating conditions such as full- or partial-thickness skin loss, wounds, burns, scars, and hair loss have been developed in order to fulfill this necessity. In this analysis, only patents aiming to hair follicle regeneration or neogenesis have been selected. As reviewed, it has been determined that both epithelial cells and mesenchymal cells are essential for hair follicle regeneration. The regeneration of chimeric hair follicles comprised of mouse DPC and human epithelial cells has been shown to be possible, however it is still not possible to regenerate completely human hair follicles. One of the reasons for this is the difficulty to obtain an adequate number of human DPCs having the ability to induce hair follicle formation to be used for transplantation. Consequently, most of the patents filed in this field provide cellular compositions capable of hair neogenesis and regeneration or methods for isolating progenitor skin cells as well as the culture conditions that will allow them to keep their inductive properties to promote hair neogenesis or regeneration.
The first most cited patent listed in Table 2 (WO1995001423), published in 1995, described a method of culturing and modulating subpopulations of follicular keratinocytes from upper portions of hair follicle to be used for identifying agents which stimulate hair growth or prevent hair loss and useful for follicular reconstruction and transplantation as well as wound coverage in burns and skin ulcer[86] .
In 2005 a patent application (WO2005053763A1) developed a method for transplanting the dermal papilla or dermal papilla cells and outer skin epidermis tissue or epidermal cells to hairless scalp to regenerate hair[94].
Different tissue engineered-hair follicle grafts were also patented; a scaffold constructed to mimic the architecture of the native hair follicle and for percutaneous implantation facilitating the follicle neogenesis process[90] (US20050214344), a hair graft comprising plucked hairs having adhered epidermal stem cells and follicular dermal cells for implantation into wounded skin[105] (US20070122387A1) and a hair graft comprising a scaffold, and tissue engineered epidermal and dermal layer with hair follicle progenitor cells (WO2007062386A2)[98].
Several methods for isolating skin-derived precursor stem cells from hair follicles or dermal papillae as well as the culture conditions or the delivery into the skin of these cells in order to induce hair growth are also among the most cited patents (WO2002060396A, WO2005071063A1 and US20070092496A1)[88,91,99].
Finally, among the most cited patents are those describing methods for isolating hair follicle mesenchymal stem cells useful for treating alopecia and gene therapy (WO2003104443A2)[89] or useful for hair loss, burns or skin replacement as well as for treating neurological or degenerative disorders (WO2003024406A2)[87].
The rest of the patents analyzed whose content is not mentioned, can be found in the References section[86-150] at the end of this review.
CONCLUSION
Although a successful strategy to generate human hair follicles from adult cells has not yet been reported, the results presented in this review suggest the issues that need to be addressed before success can be achieved. Perhaps the most important of those issues is to provide cells with a three-dimensional structure that simulates the natural scaffold. Herein the cells should develop and interact among themselves or with other cell types in the epithelial-mesenchymal interactions. Improving culture conditions that allow the expansion of these cells without losing their natural properties, as well as selecting the appropriate epithelial stem cells, should give us the tools needed to face the challenge of regenerating human hair follicles.
These efforts in hair and skin bioengineering are also visible in the growing number of patent applications filed during the last decade, indicating that this technological field is not only attractive to academic research but also to the companies that own almost half of these patents.
Footnotes
Supported by the Agencia Nacional de Producción Científica y Tecnológica (ANPCyT), No. ANR BIO 0032/10.
Conflict-of-interest: The authors state no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 28, 2014
First decision: September 4, 2014
Article in press: February 9, 2015
P- Reviewer: Kiselev SL, Shawcross SG, Wakao H S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Papini R. Management of burn injuries of various depths. BMJ. 2004;329:158–160. doi: 10.1136/bmj.329.7458.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface. 2010;7:229–258. doi: 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 5.Ohyama M, Zheng Y, Paus R, Stenn KS. The mesenchymal component of hair follicle neogenesis: background, methods and molecular characterization. Exp Dermatol. 2010;19:89–99. doi: 10.1111/j.1600-0625.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- 6.Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 8.Paus R, Foitzik K. In search of the “hair cycle clock”: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 9.Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol. 2004;122:14–19. doi: 10.1046/j.0022-202X.2003.22110.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 11.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 12.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 13.Jahoda CA, Oliver RF. Vibrissa dermal papilla cell aggregative behaviour in vivo and in vitro. J Embryol Exp Morphol. 1984;79:211–224. [PubMed] [Google Scholar]
- 14.Christiano AM. Epithelial stem cells: stepping out of their niche. Cell. 2004;118:530–532. doi: 10.1016/j.cell.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 18.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8:46–55. doi: 10.1046/j.1523-1747.2003.12171.x. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs E, Merrill BJ, Jamora C, DasGupta R. At the roots of a never-ending cycle. Dev Cell. 2001;1:13–25. doi: 10.1016/s1534-5807(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 21.Gharzi A, Reynolds AJ, Jahoda CA. Plasticity of hair follicle dermal cells in wound healing and induction. Exp Dermatol. 2003;12:126–136. doi: 10.1034/j.1600-0625.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 22.Jahoda CA, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet. 2001;358:1445–1448. doi: 10.1016/S0140-6736(01)06532-1. [DOI] [PubMed] [Google Scholar]
- 23.Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006;119:391–393. doi: 10.1242/jcs.02793. [DOI] [PubMed] [Google Scholar]
- 24.Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roh C, Tao Q, Lyle S. Dermal papilla-induced hair differentiation of adult epithelial stem cells from human skin. Physiol Genomics. 2004;19:207–217. doi: 10.1152/physiolgenomics.00134.2004. [DOI] [PubMed] [Google Scholar]
- 26.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider MR, Schmidt-Ullrich R, Paus R. The hair follicle as a dynamic miniorgan. Curr Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Kligman AM, Strauss JS. The formation of vellus hair follicles from human adult epidermis. J Invest Dermatol. 1956;27:19–23. doi: 10.1038/jid.1956.71. [DOI] [PubMed] [Google Scholar]
- 29.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 30.Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, Kizawa K, Toyoda M, Morohashi M. Label-retaining cells in the bulge region are directed to cell death after plucking, followed by healing from the surviving hair germ. J Invest Dermatol. 2002;119:1310–1316. doi: 10.1046/j.1523-1747.2002.19644.x. [DOI] [PubMed] [Google Scholar]
- 32.Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 35.Chen CC, Chuong CM. Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci. 2012;66:3–11. doi: 10.1016/j.jdermsci.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Randall VA, Hibberts NA, Thornton MJ, Merrick AE, Hamada K, Kato S, Jenner TJ, de Oliveira I, Messenger AG. Do androgens influence hair growth by altering the paracrine factors secreted by dermal papilla cells. Eur J Dermatol. 2001;11:315–320. [PubMed] [Google Scholar]
- 37.Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–1969. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 38.Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, Kim JC. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–269. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- 39.Grisouard J, Mayer D. Specific involvement of glycogen synthase kinase-3 in the function and activity of sex steroid hormone receptors reveals the complexity of their regulation. J Steroid Biochem Mol Biol. 2009;117:87–92. doi: 10.1016/j.jsbmb.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166:1035–1042. doi: 10.1111/j.1365-2133.2012.10856.x. [DOI] [PubMed] [Google Scholar]
- 41.Valente Duarte de Sousa IC, Tosti A. New investigational drugs for androgenetic alopecia. Expert Opin Investig Drugs. 2013;22:573–589. doi: 10.1517/13543784.2013.784743. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. The transplantation of individual rat and guineapig whisker papillae. J Embryol Exp Morphol. 1961;9:117–127. [PubMed] [Google Scholar]
- 43.Oliver RF. The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. J Embryol Exp Morphol. 1970;23:219–236. [PubMed] [Google Scholar]
- 44.Oliver RF. Ectopic regeneration of whiskers in the hooded rat from implanted lengths of vibrissa follicle wall. J Embryol Exp Morphol. 1967;17:27–34. [PubMed] [Google Scholar]
- 45.Horne KA, Jahoda CA. Restoration of hair growth by surgical implantation of follicular dermal sheath. Development. 1992;116:563–571. doi: 10.1242/dev.116.3.563. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, Christiano AM, Jahoda CA. Trans-gender induction of hair follicles. Nature. 1999;402:33–34. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 47.Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development. 1992;115:1103–1109. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- 48.Lichti U, Weinberg WC, Goodman L, Ledbetter S, Dooley T, Morgan D, Yuspa SH. In vivo regulation of murine hair growth: insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101:124S–129S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 49.Prouty SM, Lawrence L, Stenn KS. Fibroblast-dependent induction of a murine skin lesion with similarity to human common blue nevus. Am J Pathol. 1996;148:1871–1885. [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–876. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 51.Gilmour SK, Teti KA, Wu KQ, Morris RJ. A simple in vivo system for studying epithelialization, hair follicle formation, and invasion using primary epidermal cells from wild-type and transgenic ornithine decarboxylase-overexpressing mouse skin. J Invest Dermatol. 2001;117:1674–1676. doi: 10.1046/j.0022-202x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 52.Qiao J, Philips E, Teumer J. A graft model for hair development. Exp Dermatol. 2008;17:512–518. doi: 10.1111/j.1600-0625.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee LF, Jiang TX, Garner W, Chuong CM. A simplified procedure to reconstitute hair-producing skin. Tissue Eng Part C Methods. 2011;17:391–400. doi: 10.1089/ten.tec.2010.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asakawa K, Toyoshima KE, Ishibashi N, Tobe H, Iwadate A, Kanayama T, Hasegawa T, Nakao K, Toki H, Noguchi S, et al. Hair organ regeneration via the bioengineered hair follicular unit transplantation. Sci Rep. 2012;2:424. doi: 10.1038/srep00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, Hasegawa T, Irié T, Tachikawa T, Sato A, Takeda A, et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun. 2012;3:784. doi: 10.1038/ncomms1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 57.Qiao J, Turetsky A, Kemp P, Teumer J. Hair morphogenesis in vitro: formation of hair structures suitable for implantation. Regen Med. 2008;3:683–692. doi: 10.2217/17460751.3.5.683. [DOI] [PubMed] [Google Scholar]
- 58.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 59.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–562. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 60.Ihara S, Watanabe M, Nagao E, Shioya N. Formation of hair follicles from a single-cell suspension of embryonic rat skin by a two-step procedure in vitro. Cell Tissue Res. 1991;266:65–73. doi: 10.1007/BF00678712. [DOI] [PubMed] [Google Scholar]
- 61.Takeda A, Matsuhashi S, Shioya N, Ihara S. Histodifferentiation of hair follicles in grafting of cell aggregates obtained by rotation culture of embryonic rat skin. Scand J Plast Reconstr Surg Hand Surg. 1998;32:359–364. doi: 10.1080/02844319850158435. [DOI] [PubMed] [Google Scholar]
- 62.Kelm JM, Fussenegger M. Microscale tissue engineering using gravity-enforced cell assembly. Trends Biotechnol. 2004;22:195–202. doi: 10.1016/j.tibtech.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Osada A, Iwabuchi T, Kishimoto J, Hamazaki TS, Okochi H. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975–982. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- 64.Young TH, Lee CY, Chiu HC, Hsu CJ, Lin SJ. Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly(ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials. 2008;29:3521–3530. doi: 10.1016/j.biomaterials.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 65.Huang YC, Chan CC, Lin WT, Chiu HY, Tsai RY, Tsai TH, Chan JY, Lin SJ. Scalable production of controllable dermal papilla spheroids on PVA surfaces and the effects of spheroid size on hair follicle regeneration. Biomaterials. 2013;34:442–451. doi: 10.1016/j.biomaterials.2012.09.083. [DOI] [PubMed] [Google Scholar]
- 66.Müller-Röver S, Peters EJ, Botchkarev VA, Panteleyev A, Paus R. Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J Histochem Cytochem. 1998;46:1401–1410. doi: 10.1177/002215549804601209. [DOI] [PubMed] [Google Scholar]
- 67.Jahoda CA, Reynolds AJ, Chaponnier C, Forester JC, Gabbiani G. Smooth muscle alpha-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci. 1991;99(Pt 3):627–636. doi: 10.1242/jcs.99.3.627. [DOI] [PubMed] [Google Scholar]
- 68.McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 69.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci U S A. 1999;96:7336–7341. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soma T, Fujiwara S, Shirakata Y, Hashimoto K, Kishimoto J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signalling activation. Exp Dermatol. 2012;21:307–309. doi: 10.1111/j.1600-0625.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 71.Yen CM, Chan CC, Lin SJ. High-throughput reconstitution of epithelial-mesenchymal interaction in folliculoid microtissues by biomaterial-facilitated self-assembly of dissociated heterotypic adult cells. Biomaterials. 2010;31:4341–4352. doi: 10.1016/j.biomaterials.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 72.Havlickova B, Bíró T, Mescalchin A, Tschirschmann M, Mollenkopf H, Bettermann A, Pertile P, Lauster R, Bodó E, Paus R. A human folliculoid microsphere assay for exploring epithelial- mesenchymal interactions in the human hair follicle. J Invest Dermatol. 2009;129:972–983. doi: 10.1038/jid.2008.315. [DOI] [PubMed] [Google Scholar]
- 73.Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- 74.Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM. Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc Natl Acad Sci USA. 2013;110:19679–19688. doi: 10.1073/pnas.1309970110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chermnykh ES, Vorotelyak EA, Gnedeva KY, Moldaver MV, Yegorov YE, Vasiliev AV, Terskikh VV. Dermal papilla cells induce keratinocyte tubulogenesis in culture. Histochem Cell Biol. 2010;133:567–576. doi: 10.1007/s00418-010-0691-0. [DOI] [PubMed] [Google Scholar]
- 76.Limat A, Breitkreutz D, Hunziker T, Klein CE, Noser F, Fusenig NE, Braathen LR. Outer root sheath (ORS) cells organize into epidermoid cyst-like spheroids when cultured inside Matrigel: a light-microscopic and immunohistological comparison between human ORS cells and interfollicular keratinocytes. Cell Tissue Res. 1994;275:169–176. doi: 10.1007/BF00305384. [DOI] [PubMed] [Google Scholar]
- 77.Sriwiriyanont P, Lynch KA, Maier EA, Hahn JM, Supp DM, Boyce ST. Morphogenesis of chimeric hair follicles in engineered skin substitutes with human keratinocytes and murine dermal papilla cells. Exp Dermatol. 2012;21:783–785. doi: 10.1111/exd.12003. [DOI] [PubMed] [Google Scholar]
- 78.Ehama R, Ishimatsu-Tsuji Y, Iriyama S, Ideta R, Soma T, Yano K, Kawasaki C, Suzuki S, Shirakata Y, Hashimoto K, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127:2106–2115. doi: 10.1038/sj.jid.5700823. [DOI] [PubMed] [Google Scholar]
- 79.Qiao J, Zawadzka A, Philips E, Turetsky A, Batchelor S, Peacock J, Durrant S, Garlick D, Kemp P, Teumer J. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen Med. 2009;4:667–676. doi: 10.2217/rme.09.50. [DOI] [PubMed] [Google Scholar]
- 80.Kang BM, Kwack MH, Kim MK, Kim JC, Sung YK. Sphere formation increases the ability of cultured human dermal papilla cells to induce hair follicles from mouse epidermal cells in a reconstitution assay. J Invest Dermatol. 2012;132:237–239. doi: 10.1038/jid.2011.250. [DOI] [PubMed] [Google Scholar]
- 81.Miao Y, Sun YB, Liu BC, Jiang JD, Hu ZQ. Controllable production of transplantable adult human high-passage dermal papilla spheroids using 3D matrigel culture. Tissue Eng Part A. 2014;20:2329–2338. doi: 10.1089/ten.tea.2013.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu JJ, Zhu TY, Lu YG, Liu RQ, Mai Y, Cheng B, Lu ZF, Zhong BY, Tang SQ. Hair follicle reformation induced by dermal papilla cells from human scalp skin. Arch Dermatol Res. 2006;298:183–190. doi: 10.1007/s00403-006-0686-9. [DOI] [PubMed] [Google Scholar]
- 83.Thangapazham RL, Klover P, Wang JA, Zheng Y, Devine A, Li S, Sperling L, Cotsarelis G, Darling TN. Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal-epidermal composites. J Invest Dermatol. 2014;134:538–540. doi: 10.1038/jid.2013.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leirós GJ, Kusinsky AG, Drago H, Bossi S, Sturla F, Castellanos ML, Stella IY, Balañá ME. Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl Med. 2014;3:1209–1219. doi: 10.5966/sctm.2013-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chueh SC, Lin SJ, Chen CC, Lei M, Wang LM, Widelitz R, Hughes MW, Jiang TX, Chuong CM. Therapeutic strategy for hair regeneration: hair cycle activation, niche environment modulation, wound-induced follicle neogenesis, and stem cell engineering. Expert Opin Biol Ther. 2013;13:377–391. doi: 10.1517/14712598.2013.739601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lavker RM, Sun TT, Yang JS, inventors; New York State Univ, Pennsylvania Univ, assignees . Methods of culturing and modulating the growth of hair follicular stem cells. WO1995001423A1. 1995. p. Jan 12. [Google Scholar]
- 87.Li LN, Yang M, inventors; AntiCancer Inc, assignee . Nestin- expressing hair follicle stem cells. WO2003024406A2. 2003. p. Mar 27. [Google Scholar]
- 88.Barrows Thomas H, inventor; Aderans Res Inst Inc, Bioamide Inc, assignee . Hair follicle neogenesis by injection of follicle progenitor cells. WO2002060396A2. 2002. p. Aug 08. [Google Scholar]
- 89.Hoffmann R, Mcelwee Kevin J, inventors; Torico Sci Innovations Inc, Trichoscience Innovations Inc, assignee . Hair follicle mesenchymal stem cells and use thereof. WO2003104443A2. 2003. p. Dec 18. [Google Scholar]
- 90.Barrows Thomas H, Cochran Stephen A, Marshall B, inventors; Aderans Res Inst Inc, assignee . Tissue engineered biomimetic hair follicle graft. United States patent US20050214344A1. 2005. p. Sep 29. [Google Scholar]
- 91.Miller Freda D, Fernandez K, Biernaskie J, Mckenzie I, inventors; Hospital For Sick Children, assignee. Methods of making and using skin-derived stem cells. WO2005071063A1. 2005. p. Aug 08. [Google Scholar]
- 92.Kitano M, Shirakawa T, Tamaoki Tomoko, Sasahara Yusuke, inventors; Technology Seed Incubation KK, assignee. Cultured dermal cell, transplanting material and material for gene analysis using the same and method for preparation of cultured dermal cell. Japanese patent JP2005218445A. 2005. p. Aug 18. [Google Scholar]
- 93.Jahoda CAB, Reynolds AJ, Whitehouse CJ, Hole N, inventors; Durham Univ, assignee . Differentiated cells. WO2005059119A3. 2005. p. Aug 11. [Google Scholar]
- 94.Yoshizato K, Shimada T, Toyoshima K, Matsunaga M, inventors; Biointegrence Inc, Phoenixbio Co Ltd, assignees . Hair growth method. WO2005053763A1. 2005. p. Jun 16. [Google Scholar]
- 95.Kishimoto J, Ehama R, Ideta R, Arai T, Yano K, Soma T, inventors; Shiseido Company Ltd, assignee . Method of preparing hair papilla cell preparation, composition and method for regenerating hair follicle and animal having regenerated hair follicle.WO2005033302A1. 2005. p. Apr 14. [Google Scholar]
- 96.Zheng Y, Du XB, Wang W, Boucher M, Parimoo S, Stenn KS, inventors; Aderans Res Inst Inc, assignee . Organogenesis from dissociated cells. WO2006020958A2. 2006. p. Feb 23. [Google Scholar]
- 97.Li XL, Zhang Y, Hua YQ, Guo Y, Zhang WH, Bai RX, inventors; Tianjin Hospital, assignee . Follicular stem cell originated from human hair and its amplifying preparation process. Hair follicle stem cell from human hair and method of proliferating and preparing it. Chinese patent CN101045916A. 2007. p. Oct 3. [Google Scholar]
- 98.Barrows Thomas H, Macintyre P, Washenik KJ, inventors; Aderans Res Inst Inc, assignee . Hair follicle graft from tissue engineered skin. WO2007062386A2. 2007. p. May 31. [Google Scholar]
- 99.Zheng Y, Du XB, Washenik K, Stenn KS, inventors; Aderans Res Inst Inc, assignee . Method of delivering hair follicle progenitor cells to the skin. United States patent US20070092496A1. 2007. p. Apr 26. [Google Scholar]
- 100.Toyoshima K, Matsunaga M, Yoshizato K, inventors; Phoenixbio Co Ltd, Biointegrence Inc, assignees . Method for cultivation of hair follicular dermal sheath cell. WO2007037486A1. 2007. p. Apr 5. [Google Scholar]
- 101.Miller Freda D, Biernaskie J, inventors; Hospital For Sick Children, assignee. Skin-derived precursor cells and uses thereof. WO2008148218A1. 2008. p. Dec 11. [Google Scholar]
- 102.Steinberg D, Pojasek K, Prouty S, Cotsarelis G, Ito M, inventors; Follica Inc, Pennsylvania Univ, assignees . Methods, kits, and compositions for generating new hair follicles and growing hair. WO2008042216A2. 2008. p. Apr 10. [Google Scholar]
- 103.Kang KS, Ra JC, inventors; Univ Seoul Nat Ind Found, Modecell Ltd, Rnl Bio Co Ltd, assignees . Method for isolation of a hair follicle stem cell and a composition for hair reproduction reproduction. WO2008004819A1. 2008. p. Jan 10. [Google Scholar]
- 104.Jung SH, inventor; Everbridge Co Ltd, assignee . Method for culturing human hair follicle component cells, method for manufacturing bioartificial skin, and method for in vitro induction of hair structure using the cultured hair follicle component cells sheath cells, hair papilla cells, lower dermal sheath cells, melanocytes, and pilomatrix cells, a method for producing bioartificial skin from the cultured hair follicle component cells and a method for in vitro induction of hair follicle structure from the cultured hair follicle component cells. Korean patent KR2008071656A. 2008. p. Aug 5. [Google Scholar]
- 105.Cochran Stephen A, Marshall B, Barrows Thomas H, Su YD, Schlicher R, inventors; Aderans Res Inst Inc, assignee . Hair grafts derived from plucked hair. United States patent US20070122387A1. 2007. p. May 31. [Google Scholar]
- 106.Lindner G, Lauster R, inventors; Berlin Tech Univ, assignee . Methods for producing hair microfollicles and de novo papillae and their use for in vitro tests and in vivo implantations.WO2009118283A1. 2009. p. Oct 1. [Google Scholar]
- 107.Philips EJ, Qiao J, Teumer J, inventors; Intercytex Ltd, assignee . Cell co-culture for hair follicle production. United States patent US20090198336A1. 2009. p. Aug 6. [Google Scholar]
- 108.Fujiwara S, Kishimoto J, Soma T, inventors; Shiseido Company Ltd, assignee . A separating and culturing method of hair follicle stem cells. Korean patent KR2009021340A. 2009. p. Mar 3. [Google Scholar]
- 109.Park JK, Yoon HH, Yoo BY, Kim YJ, Shin YH, inventors; Univ Dongguk Ind Academic Coop Found, assignee. Method for the preparation of dermal papilla tissue employing mesenchymal stem cells. WO2009014272A1. 2009. p. Jan 29. [Google Scholar]
- 110.Sherley James L, King J, inventors; Massachusetts Inst Technology, assignee. Methods for ex vivo propagation of somatic hair follicle stem cells. WO2005121323A3. 2009. p. Apr 4. [Google Scholar]
- 111.Yoshimura K, Inoue K, Sato T, inventors; Biomaster Inc, assignee . The cell transplant method for hair regeneration. Japanese patent JP2010082339A. 2010. p. Apr 15. [Google Scholar]
- 112.Lee L, Chuong CM, Garner W, Jiang TX, inventors; Southern California University, assignee . Compositions and methods to generate pilosebaceous units. United States patent US20110321180A1. 2011. p. Dec 29. [Google Scholar]
- 113.Darling Thomas N, Li S, Thangapazham R, inventors; Jackson H M Found Military Med, assignee . Hair follicle neogenesis. WO2011160055A2. 2011. p. Dec 22. [Google Scholar]
- 114.Armani A, Armani S, Seneviratne C, Nazari R, inventors; Alvi Armani Genomics Inc, assignee . Cell Compositions and Methods for Hair Follicle Generation. United States patent US20110305671A1. 2011. p. Dec 15. [Google Scholar]
- 115.Lederman Seth M, Kellogg Scott C, Ju W, inventors; Follica Inc, assignee . Combination therapy. WO2011123218A1. 2011. p. Oct 6. [Google Scholar]
- 116.Jin Y, Zhang YJ, Yang L, inventors; Chinese PLA Fourth Military Medical University, Xi’an Engineering Technology Research Centre of Tissue Engineering, assignees. Tissue engineering skin with sebaceous gland-like structure and preparation method thereof. Chinese patent CN102172337A. 2011. p. Sep 7. [Google Scholar]
- 117.Huang YY, Hsieh CH, Wang JL, inventors; Taiwan Nat Univ, assignee . Method and apparatus for culturing tissue. United States patent US20110293573A1. 2011. p. Dec 1. [Google Scholar]
- 118.Mize N, inventor; Genogen Inc, assignee . Methods and compositions for skin regeneration. WO2011044367A1. 2011. p. Apr 14. [Google Scholar]
- 119.Naughton GK, Ziegler F, Baumgartner M, Nickey K, inventors; Histogen Inc, assignee . Conditioned medium and extracellular matrix compositions from cells cultured under hypoxic conditions. WO2011006107A1. 2011. p. Jan 13. [Google Scholar]
- 120.Jin Y, Fan XJ, Zhang YJ, Wang AJ, inventors; Shaanxi Ruisheng Biotechnology Co Ltd, Chinese Pla Fourth Military Medical Univ, assignees. Method for preparing tissue engineering skin containing hair follicle. WO2011094964A1. 2011. p. Aug 11. [Google Scholar]
- 121.Cotsarelis G, Ito M, inventors; Pennsylvania Univ, assignee . Methods for generating new hair follicles, treating baldness and hair removal. Australian patent AU2012204059A1. 2012. p. Jul 26. [Google Scholar]
- 122.Mahjour SB, Wang H, inventors; Mahjour, Seyed Babak, Wang , Hongjun , assignees . Creation of hair follicles in tissue-engineered skin grafts. United States patent US20120276154A1. 2012. p. Nov 1. [Google Scholar]
- 123.Sun G, Zhang X, Harmon John W, Gerecht S, inventors; Johns Hopkins Univ, assignee . Skin and hair regeneration using polysaccharide-based hydrogels. WO2012158312A2. 2012. p. Nov 22. [Google Scholar]
- 124.Chen L, Liu DQ, Lv Y, Pei XT, Shi W, Xie XY, Yao HL, Yue W, Xi JF, Zhang XY, inventors; Field Operation Blood Transfusion Institute of PLA Sciences Academy of Military, assignee. Cell culture medium and application thereof. Chinese patent CN102676448A. 2012. p. Sep 19. [Google Scholar]
- 125.Toyoshima KE, Tsuji T, inventors; Organ Technologies Inc, assignee. Method of preparing regenerated hair follicle germ for transplantation in which hair color is controlled, composition including regenerated hair follicle germ for transplantation, and method of transplanting regenerated hair follicle germ. WO2012115079A1. 2012. p. Aug 30. [Google Scholar]
- 126.Yan QG, inventor; Beijing Yonghe Hair Transplant Technolog, assignee . Culture method of hair follicle stem cell for treating baldness, as well as syringe. Chinese patent CN102586177A. 2012. p. Jul 18. [Google Scholar]
- 127.Toyoshima KE, Tsuji T, inventors; Organ Technologies Inc, assignee. Method for producing regenerative organ primordium provided with guide for transplantation, composition containing regenerative organ primordium provided with guide for transplantation produced thereby, and method for transplanting regenerative organ. WO2012108069A1. 2012. p. Aug 16. [Google Scholar]
- 128.Liu J, Li Y, inventors; Jilin Univ, assignee . Culture method for amplifying large numbers of hair follicle stem cells in vitro. WO2012009958A1. 2012. p. Jan 26. [Google Scholar]
- 129.Ishizaka S, Oji Y, inventors; Nara Medical Univ, assignee . The cultivation method of a hair follicle stem cell. Japanese patent JP2012249556A. 2012. p. Dec 20. [Google Scholar]
- 130.Maya SB, inventors; Newcastle-Upon-Tyne Univ, assignee . Expansion and directed differentiation of epidermal neural crest stem cells. WO2011144901A4. 2012. p. Mar 1. [Google Scholar]
- 131.Duan EK, Zhang SB, inventors; Chinese Acad Sci Zoology Inst, assignee. A separating and culturing method of hair follicle stem cells. Chinese patent CN102344906A. 2012. p. Feb 8. [Google Scholar]
- 132.Lederman Seth M, Kellogg Scott C, Barman Shikha P, inventors; Follica Inc, assignee . Hair growth treatment WO2012067630A1. 2012. p. May 24. [Google Scholar]
- 133.Yoshida Y, Soma T, Fujiwara S, inventors; Shiseido Company Ltd, assignee . Composition for regenerating follicle which contains cd36-expressing connective tissue sheath cells.WO2012042618A1. 2012. p. Apr 5. [Google Scholar]
- 134.Yamanishi H, Soma T, Yoshida Y, Ishimatsu Y, inventors; Shiseido Company Ltd, assignee . Method for promoting hair growth or hair regeneration by maintaining or increasing expression of cell-adhesion factor. United States patent US8334136B2. 2012. p. Dec 18. [Google Scholar]
- 135.Kurita K, inventor; Lion Corp, assignee . Artificial skin. Japanese patent JP05097387B2. 2012. p. Dec 12. [Google Scholar]
- 136.Ehama R, Kishimoto J, Ideta R, Soma T, inventors; Shiseido Company Ltd, assignee . Method of regenerating follicles by inhibiting gene capable of inhibiting follicle formation or activating gene capable of inducing follicle formation. Korean patent KR1186716B1. 2012. p. Sep 27. [Google Scholar]
- 137.Wan ACA, Lim TC, Leong MF, Ying JY, Toh JKC, inventors; Agency Science Tech & Res, assignee. Fibrous structure. WO2013137829A1. 2013. p. Sep 19. [Google Scholar]
- 138.Liu HQ, Guo XC, Wu MJ, inventors; PLA Second Military Medical University, assignee . Method of modifying mesenchymal stem cell by JAM1 gene and application thereof. CN103031278A. 2013. p. Apr 10. [Google Scholar]
- 139.Kobielak K, Kandyba E, inventors; Southern California Univ, assignee . Hair follicle stem cells and methods of use same. WO2013040248A1. 2013. p. Mar 21. [Google Scholar]
- 140.Alenfall J, Dunér P, Hultgårdh NA, inventors; Follicum AB, assignee . Novel compositions and uses thereof. WO2013021212A2. 2013. p. Feb 14. [Google Scholar]
- 141.Li Y, Liu J, inventors; Jilin Univ, assignee . Culturing method for amplifying large amount of hair follicle stem cells in vitro. Taiwanese patent TW201321513A. 2013. p. Jun 1. [Google Scholar]
- 142.Gao Q, Chen BL, Zeng Q, inventors; Gao Qing, Chen Bing-Lai, Zeng Qiao, assignee . Method for culturing amplified human hair follicle stem cells and reprogramming amplified human hair follicle stem cells to induced pluripotent stem cells. Chinese patent CN102994447A. 2013. p. Mar 27. [Google Scholar]
- 143.Fu XB, Huang S, inventors; Chinese Pla No 1 Hospital Affiliated Gen, assignee. Construction method for tissue engineering skin containing complete skin appendage. Chinese patent CN102949752A. 2013. p. Mar 6. [Google Scholar]
- 144.Lin SJ, Chan CC, Yen CM, inventors; Taiwan Nat Univ, assignee . Method for the manufacture of microtissues for inducing the growth of a hair follicle. United States patent US8492112B2. 2013. p. Jul 23. [Google Scholar]
- 145.Huang Z, Lin X, Zhang YD, Chen SZ, inventors; Fujian Normal University, assignee . Method for differentiating human keratinized stem cell into human follicular cell through induction in vitro. Chinese patent CN103667180A. 2014. p. Mar 26. [Google Scholar]
- 146.Gho CG, inventor; Hair Science Institute, assignee. Method for in vivo hair multiplication. WO2014092571A1. 2014. p. Jun 19. [Google Scholar]
- 147.Keyes W, Doles J, inventors; Fundacion Privada Ct De Regulacio Genomica Crg, assignee . Jak inhibitors for activation of epidermal stem cell populations. WO2014013014A1. 2014. p. Jan 23. [Google Scholar]
- 148.Xu SC, Quan RF, Huang ZM, Zheng X, inventors; Hangzhou City Xiaoshan Distr Traditional Chinese Medical Hospital, assignee. Separation culture method of rat hair follicle stem cells. Chinese patent CN103710297A. 2014. p. Apr 9. [Google Scholar]
- 149.Aljitawi O, Hopkins R, Detamore M, Garimella R, inventors; Childrens Mercy Hospital, Kansas Univ, assignees . Generating ck19-positive cells with hair-like structures from whartons jelly. United States patent US20140148915A1. 2014. p. May 29. [Google Scholar]
- 150.Christiano AM, Jahoda CAB, inventors; Columbia New York Univ, assignee . The method for compact aggregation of a dermis cell. Japanese patent JP05386180B2. 2014. p. Jan 15. [Google Scholar]


