Abstract
The field of reproductive biology has undergone significant developments in the last decade. The notion that there is a fixed reserve pool of oocytes before birth was established by Zuckerman in 1951. However, in 2004, an article published in nature challenged this central dogma of mammalian reproductive biology. Tilly’s group reported the existence of ovarian germline stem cells (GSCs) in postnatal ovaries of mice and suggested that the bone marrow could be an extragonadal source of ovarian GSCs. These findings were strongly criticized; however, several independent groups have since successfully isolated and characterized ovarian GSCs in postnatal mice. The ovarian GSCs are located in the ovarian surface epithelium and express markers of undifferentiated GSCs. When transplanted into mouse ovaries, mouse ovarian GSCs could differentiate and produce embryos and offspring. Similarly, in a recent study, ovarian GSCs were found to be present in the ovaries of women of reproductive age. Conversely, there is increasing evidence that stem cells responsible for maintaining a healthy state in normal tissue may be a source of some cancers, including ovarian cancer. Cancer stem cells (CSCs) have been found in many tissues, including ovaries. Some researchers have suggested that ovarian cancer may be a result of the transformation and dysfunction of ovarian GSCs with self-renewal properties. Drug resistant and metastasis-generating CSCs are responsible for many important problems affecting ovarian cancer patients. Therefore, the identification of CSCs will provide opportunities for the development of new therapeutic strategies for treatments for infertility and ovarian cancer. In this article, we summarize the current understanding of ovarian GSCs in adult mammals, and we also discuss whether there is a relationship between GSCs and CSCs.
Keywords: Cancer, Cancer stem cell, Germline stem cell, Ovary, Reproductivity
Core tip: This review provides an overview on postnatal ovarian germinal stem cells (GSC) of mammals. The characteristics of these cells and the last developments in the field of oogenesis have been presented. We also discuss the relationship between ovarian GSCs and ovarian cancer stem cells. The identification and characterization of these two types of cells are essential for a better understanding of tumor initiation, progression and treatment.
INTRODUCTION
The field of the reproductive biology was revolutionized ten years ago when Johnson et al[1] published a study in Nature that challenged the long-held dogma established by Zuckerman[2] in 1951. Since that time, it has been generally believed that the ovaries of mammals do not possess renewable stem cells but instead contain a finite reserve of oocytes that diminishes through postnatal life. Although the existence of ovarian germline stem cells (GSCs) has been obviously demonstrated and fully accepted for adult females of non-mammalian species[3-5] and for adult males of a majority of species[6], the existence of ovarian GSCs in adult female mammals is still a subject of intense debate. In 2004, Johnson et al[1] demonstrated the existence of proliferative GSCs in the ovaries of adult mice, and recently, ovarian GSCs have been isolated and characterized in the ovaries of postnatal mice and reproductive-age women[7].
Amid the controversy created by Johnson et al[1], the presence of cancer stem cells (CSC) in ovarian cancer was established by Bapat et al[8], and accumulating data have provided substantial evidence for the involvement of CSCs in ovarian cancer[9-13]. Ovarian cancer (OC) is associated with enhanced tumor aggressiveness and metastasis, as well as drug resistance. The heterogeneous populations of cancer cells within an ovarian tumor tend to be more resistant to chemotherapeutic agents. In this context, the identification and characterization of CSCs in ovarian cancer is essential for a better understanding of the signaling pathways involved in tumor development and progression. In this review, we will focus on the latest developments in the field of oogenesis in the postnatal mammalian ovary. We will also discuss whether there is a link between ovarian GSCs and CSCs.
OVARIAN GSCS IN ADULT MAMMALS
Existence of ovarian GSCs
In 2004, Johnson et al[1] published a study that challenged the dogma established by Zuckerman[2] in 1951. The authors demonstrated that ovarian GSCs are present in the adult mouse ovary, contrary to the principle established more than 60 years ago (Table 1). In a first series of studies, Johnson et al[1] counted the numbers of healthy (non-atretic) and degenerating (atretic) follicles in ovaries of mice to study germ cell dynamics in female mammals. The numbers of non-atretic quiescent (primordial) and early growing (primary, preantal) follicles in ovary was higher than expected and their rate of clearance in the immature ovary (day 1-day 4) was less than expected. According to their experiments on the clearance of degenerative oocytes contained within immature follicles, from 1% to 33% of the immature follicle pool was atretic at any given time. The authors considered that the degeneration of this cell would deplete the primordial follicle reserve by young adulthood and that ovarian GSCs represent the source of oocytes produced de novo[1].
Table 1.
Articles in the field of ovarian germline stem cells and oogenesis
| Ref. | Main findings |
| Johnson et al[1] | Ovarian GSCs are observed within the ovarian surface epithelium and provide the adult mouse ovary with oocytes |
| Johnson et al[25] | GSCs are detected in mouse bone marrow and provide the postnatal mouse ovary with oocytes |
| Kerr et al[14] | The follicle numbers remain constant in ovaries of juvenile and early adult mice. Follicle renewal in postnatal and adult mouse ovaries is suggested |
| Zou et al[29] | Mouse ovarian GCS are isolated by immunoselection and characterized. Ovarian GCSs transplanted into ovaries of infertile mice undergo oogenesis and produce offspring |
| Pacchiarotti et al[30] | The adult mouse ovarian GSCs are isolated by FACS. Long-term expanded mouse ovarian GSCs maintain their characteristics, telomerase activity and express germ cell and stem cell markers |
| White et al[7] | The ovarian GSCs are isolated by FACS. Xenotransplantation of human GCSs into NOD-SCID mice leads to the formation of follicles containing oocytes |
| Zhang et al[32] | No mitotically active female GSCs exist in postnatal mouse ovaries |
| Lei et al[33] | The adult female mouse ovary does not contain active ovarian GSCs. The number of follicles produced during fetal development is sufficient to provide ovaries with oocytes in adult life |
| Park et al[39] | Existence of mitotically active germ cells in the postnatal mouse ovary demonstrated by a genetic approach coupled with a GSCs selection strategy |
GSCs: Germline stem cells; FACS: Fluorescence-activated cell sorting.
By using unbiased assumption-free stereological methods to count follicles in the mouse ovary, Kerr et al[14] demonstrated the presence of actively dividing surface epithelial cells in prepubertal mouse ovaries and supported the concept of follicle renewal in postnatal and adult ovaries in mice. This study, published in 2006, confirmed the data from Johnson et al[1] (Table 1).
Johnson et al[1] reported the presence of large ovoid cells in the surface epithelium of juvenile and young adult mouse ovaries by histological analysis. These cells seemed similar to the germline cells of fetal mouse ovaries. Immunohistochemical staining for Mouse Vasa Homologue (MVH, also called Deadbox 4 or Ddx4), a gene expressed exclusively in the germ cells in both vertebrate and invertebrate species[15], demonstrated that these large cells were of a germline lineage. Incorporation of 5-bromodeoxyuridine (BrdU) into the DNA of the MVH-positive cells demonstrated the proliferative capacity of these double-positive cells, which were localized to the ovarian surface epithelium. Moreover, postnatal ovarian expression of genes involved in the initiation of meiosis was determined by RT-PCR. Protein synaptonemal complex protein SCP3, the endonuclease Spo11, and the recombinase Dmc1 (required for the initiation of meiosis in mammals) were all detected at the mRNA level[1]. These results confirmed the presence of proliferative germ cells in postnatal mouse ovaries. Supplemental evidence of continuous folliculogenesis during post natal life was provided by grafting experiments. Ovarian fragments from wild-type mice were grafted onto hemi-ovaries of transgenic mice with ubiquitous expression of green fluorescent protein (GFP). After 3-4 wk, follicles containing GFP-positive oocytes were detected in the wild-type ovarian fragments surrounded by unlabeled granulosa cells. These results suggested that transgenic germ cells had migrated into the grafted ovarian fragment and formed new follicles in adult mice. Collectively, these findings demonstrated that proliferative GSCs exist in the postnatal mammalian ovary. The GSCs support oocyte and follicle production in the postnatal mammalian ovary. This new concept challenged the dogma of a fixed reserve of oocytes.
Following the publication of this landmark article, Johnson et al[1] were confronted by criticism and skepticism[16-20]. The main criticisms were related to the subjectivity of the scoring, the tissue fixation protocol, the mathematical model used to calculate the follicular dynamics, the toxicity of busulfan to follicles, the capacity of BrdU-MVH-double-positive cells for oocyte renewal, and the migration of GSCs to form new follicles in wild-type ovaries in the grafting studies.
Several groups criticized the methods used by Johnson et al[1]. Moreover, some of them proposed alternative explanations for Johnson’s findings. First, the numbers of preantral and antral immature follicles reported as atretic by Johnson et al[1] were very high and in contrast to previous studies[16]. Scoring atretic follicles represents a subjective and not reliable method for estimating the rates of follicular atresia[16,17]. Moreover, the morphological appearance of the follicles can be modified by the use of a harsh fixative[16,17]. Consequently, healthy and atretic immature follicles may have been misclassified in Johnson et al’s study, leading to an overestimation of the number of atretic immature follicles[17]. The mathematical model of the dynamics of follicle progression was also questioned by several authors. Johnson et al[1] were criticized for applying the rate of follicle disappearance from the CBA/Ca mouse strain, reported as a fast-depleting strain, in comparison to the C57Bl/6 strain[16]. To verify Johnson et al[1]’s results, Bristol-Gould et al[18] proposed examining whether the initial population of follicles was sufficient to support fertility in adulthood by using a mathematical model of the dynamics of follicle progression. These authors counted the follicles in each stage from postnatal day 6 through to 12 mo in mice. The dynamics of the follicle population were simulated according to two distinct models: a fixed pool model and a stem cell model in the mouse ovary. The fixed pool model accurately reflected the experimental decrease in follicle numbers and allowed the authors to refute the concept of GSC replenishment in the adult ovary[18]. Johnson’s assumption that busulfan was only toxic to GSCs and does not kill primordial follicles was questioned by two groups[16,17]. The loss of primordial follicles after busulfan treatment was considered to be a consequence of busulfan toxicity to primordial follicles and not due to the depletion of GSCs[17]. The evidence that BrdU and MVH double-positive cells in the epithelial layer of juvenile and young adult mouse ovaries represented functional GSCs was also challenged[16-20]. The critics of Johnson et al[1]’s findings suggested some alternative explanations for the detection of these cells in the ovarian surface epithelium. They asserted that these putative GSCs could be germ cells migrating out of the ovary, as had been reported for these cells in the past[16,21]. Alternatively, these double-labeled cells could be oocytes from primordial follicles, released by the ovary[16,20]. The grafting experiments were also subject to reinterpretation by opponents[16,20] of the findings from Johnson et al[1]. The plasticity of the mouse ovary has recently been demonstrated in experiments with chimeric ovaries[22]. In addition, the mouse ovarian tissue has been shown to reaggregate after injury[23]. The chimerism observed in the wild-type transplanted ovaries could result from the subsequent ovarian repair after the tissue trauma due to the transplantation rather than the generation of follicles de novo[16,20].
In response to the critics, in a new study, Tilly and his group counted the numbers of atretic and non-atretic follicles in wild-type (WT) female mice and caspase-6 knockout female mice[24]. They observed that the number of primordial follicles in caspase-6 deficient young adult females was nearly double the number of primordial follicles in WT females. As no decrease in the number of atretic follicles was detected, the authors interpreted the increase in the primordial follicle pool between birth and adulthood in caspase-6 mutant females as a consequence of neo-folliculogenesis.
Following this study, Skaznik-Wikiel et al[24] reinforced the validity of their findings and the concept of postnatal oocyte and follicle production in mammals.
Bone marrow as a source of GSCs
In 2005, Johnson et al[25] proposed a new concept that further challenged the dogma of a fixed reserve of oocytes in the ovaries of postnatal females. They suggested that the bone marrow (BM) could be a source of germ cells for the postnatal mouse ovary. The authors demonstrated that BM cells isolated from adult female mice expressed octamer-binding transcription factor-4 (Oct-4), MVH, deleted in azoospermia-like, a germ cell-specific RNA-binding protein (Dazl), Stella (a maternal factor) and Fragilis (Ifitm3, interferon-induced transmembrane protein 3), which are specific germline markers in mice. Transplantation of BM from adult wild-type female mice was performed with adult female mice treated with cyclophosphamide and busulfan to destroy the existing germ cell pools, and also into ataxia telangiectasia-mutated (Atm)-deficient female mice, which are unable to produce mature cells. After transplantation, oocyte-containing follicles at all stages of maturational development were detected in the ovaries of the chemotherapy-sterilized wild-type and Atm-mutant recipient mice. Moreover, oocytes and follicles were detected in the ovaries more than 11 mo after the initial BM transplantation. Johnson et al[25] concluded that the putative BM-derived germline cells were able to support long-term oocyte production.
Consequently, they hypothesized that the BM-derived germ cells could be transported by the peripheral blood (PB) to replenish the ovaries. Transplantation of PB mononuclear cells from transgenic female mice with GFP expression driven by an Oct-4 promoter was performed with chemotherapy-sterilized wild-type and atm-mutant female mice. GFP-positive oocytes were observed in the ovaries of the chemoablated adult wild-type and atm-mutant female mice. Therefore, PB was capable of generating oocytes. GSCs derived from the BM could circulate in the PB to colonize the ovaries. Johnson et al[25] concluded that the BM could be considered as a potential source of germ cells that could support oocyte production in adulthood.
The criticism of this new concept developed by Johnson et al[25] was again severe[26,27]. A group of 16 authors published a short correspondence in which they questioned Johnson et al[25]’s findings. Their main criticisms concerned the expression of germ cells markers on BM and PB cells, the lack of a reciprocal experiment showing that mononuclear PB cells from wild-type mice could produce oocytes in Atm-Oct4/GFP mutant mouse ovaries, the capacity of generated oocytes to sustain fertilization and subsequent embryo development, and the recurrent question of ovarian failure in women treated by chemotherapy and subsequent BM or PB transplantation.
Subsequently, Johnson et al[28] published documented responses to the critics to clarify the misinterpretations of their studies. Among the different points discussed by Telfer et al[26], they defended the germline specificity of the markers used in their studies. They also clearly explained that GFP could not have been absorbed by oocytes from the blood because blood cells from GFP-transgenic males did not induce the generation of GFP-expressing oocytes in wild-type female recipients. The authors reported some cases of fertility restoration in women treated with high-dose chemotherapy followed by BM or PB transplantation. They also suggested that the BM-derived stem cells involved in blood cell regeneration might be different from those that are able to promote de novo oogenesis, noting that the protocols are designed for the recovery of hematopoiesis and not oogenesis.
After the Johnson et al[25]’s publication, Eggan et al[27] performed parabiosis experiments with adult female mice to test the physiological relevance of BM-derived germ cells to contribute to oogenesis. To evaluate the capacity of circulating BM-derived GSCs to colonize ovaries, they examined ovulated oocytes from wild-type and ubiquitously-expressing GFP female mice that were surgically joined in parabiotic pairs at the age of 4-8 wk. The parabiotic pairs showed a high level peripheral blood chimerism after 6-8 mo (65% GFP-positive blood leukocytes in both mice).
After superovulation, no chimerism of the oocytes was observed in the parabiotic mice. Moreover, occasional GFP-positive cells associated with wild-type oocytes in the cumulus masses were positive for CD45, a pan-hematopoietic marker, demonstrating that these cells were circulating blood cells. The authors concluded that although these circulating cells could associate with ovulated oocytes in the ovary, they were not involved in the production of oocytes; the authors suggested that these hematopoietic cells may play an immune role in ovaries.
In subsequent studies, the investigators treated wild-type mice with cyclophosphamide and busulfan 1 d before joining them to GFP-expressing partners for a duration of 2 wk or 2 mo. In the treated and untreated control parabionts, PB and BM exhibited extensive leukocyte chimerism whereas no chimerism was observed in the oocytes. As indicated above, it was also shown that all GFP-positive cells associated with the ovulated cells were CD45 positive in both the treated and treated wild-type parabiotic mice. In addition, 2 mo after the chemoablative treatment, the ovaries still contained oocytes, revealing that the chemotherapy did not induce a complete depletion of oocytes in the treated parabionts.
The authors then suggested that a direct intravenous transplantation of bone marrow cells might introduce cells into the circulation that might be able to contribute to oogenesis. To investigate this possibility, wild-type mice were treated by chemotherapy (cyclophosphamide and busulfan) or sterilized by low-dose total body irradiation. The mice were injected or transplanted with GFP-transgenic BM cells and were superovulated after 2 mo. Chimerism was observed in hematopoietic stem cells in both chemotherapy-treated and irradiated recipient mice. However, no GFP-positive oocytes were detected. Furthermore, a small number of oocytes was ovulated in chemotherapy-treated mice. No oocytes were detected in irradiated mice.
In conclusion, these findings demonstrated that PB and BM cells did not directly contribute to the formation of ovulated oocytes or their recovery after chemotherapy. Additionally, the BM-derived cells combined with oocytes belonged to the hematopoietic lineage, and oocytes in the adult mammalian ovaries could not be regenerated by circulating germ cell progenitors.
Isolation and characterization of GSCs from mouse and human ovarian tissues
Despite the controversies, the existence of GSCs was finally reported in 2009 by Zou et al[29]. They developed a strategy to isolate GSCs from neonatal and young adult mouse ovaries by immunoselection with the antigen MVH (Figure 1). The isolated MVH-positive cells were morphologically similar to type A spermatogonial cells (SSCs). They possessed large cell bodies with little cytoplasm and large nuclei. These MVH-positive cells were present in the ovarian surface epithelium. The isolated MVH-positive cells were cultured on mitotically inactivated mouse embryonic fibroblast (MEF) cell feeders. The MVH-positive cells incorporated BrdU in culture, and the BrdU-MVH double-positive cells were thus considered to be ovarian GSCs. After several passages, the MVH-positive cells formed compact clusters with blurred cell boundaries that did not resemble embryonic stem (ES) cell colonies. The nGSCs from neonatal mice could be maintained in culture for more than 15 mo (68 passages) and maintained a morphology similar to that of freshly isolated nGSCs. GSC from adult (aGSCs) mice had been passaged in culture for more than 6 mo (more than 25 times). The cultured nGSCs and aGSCs expressed Oct-4, MVH, Dazl, Blimp-1 (a transcriptional repressor), Fragilis, Stella, and Rex-1 (zfp-42, zinc-finger protein 42), which are markers of undifferentiated GSCs (Figure 1). The cells did not express c-Kit (stem cell factor receptor), Figla (a meiosis-specific marker), Sox-2 (a key transcription factor that regulates stemness), Nanog (a pluripotency sustaining factor), synaptonemal complex protein 1,2,3 (Scp1-3), or zona pellucida protein 3 (ZP3), which are established markers of differentiated oocytes. This expression profile revealed that the cultured cells possessed undifferentiated GSC phenotypes. Moreover, the cultured cells showed high telomerase activity. After the transplantation of GFP-expressing GSCs into the ovaries of chemotherapy-ablated wild-type mice, the ovaries contained many GFP-positive oocytes at all stages of development. Moreover, the transplanted mice produced offspring after mating with a wild-type male. Eighty-two percent of nGSCs and 80% of aGSCs produced F1 offspring. The offspring were fertile and could also generate F2 GFP-transgenic progeny (Figure 1).
Figure 1.
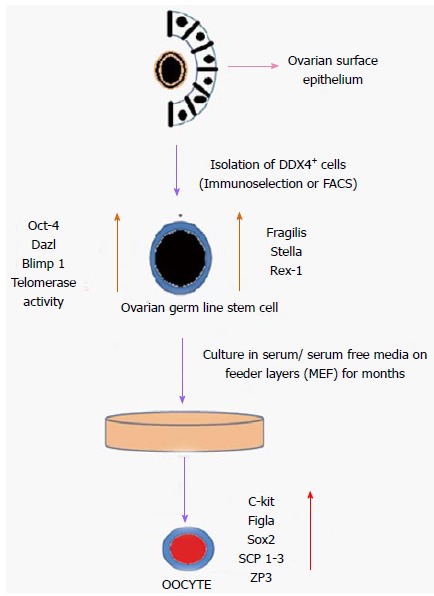
Schematic diagram of isolation and differentiation of ovarian germ stem cells. DDX4: DEAD box polypeptide 4; FACS: Fluorescence activated cell sorting; MEF: Mouse embryonic fibroblast; SCP 1-3: Synaptonemal complex protein 1,2,3; ZP3: Zona pellucida protein 3.
Furthermore, Pacchiarotti et al[30] used fluorescence-activated cell sorting (FACS) to isolate GSCs from the ovaries of a transgenic mouse model. In this model, GFP was expressed under the control of the Oct-4 promoter. The GFP-Oct-4-positive isolated cells were expanded on mouse MEFs for more than 1 year. GFP-Oct-4-positive cells formed round, flat colonies. These colonies maintained their telomerase activity and normal karyotype after 20 passages. The cells expressed germ cell and stem cell markers, such as germ cell nuclear antigen, c-kit, Oct-4, Nanog and GFR-α1 (the receptor for GDNF), and they possessed a normal karyotype. In the presence of growth factors, these GSC cells formed embryoid bodies (EB) and expressed specific markers for the three germ layers. In the absence of growth factors, EBs produced oocyte-like cells. The authors’ results thereby indicated the existence of GSCs in the postnatal mouse ovary.
In a recent study published in Nature Medicine, White et al[7] reported the isolation and characterization of ovarian germ stem cells from mouse and human ovarian tissue. GSCs were isolated and purified by FACS using the Ddx4 protein. Isolated mouse and human OSCs express high levels of Prdm1 (PR domain containing 1 with ZNF domain, referred to as Blimp1), Dppa3 (developmental pluripotency-associated 3, referred to as Stella), and Ifitm3, which identify primitive germ cell lines in mammals. Moreover, mouse and human OSCs express high levels of the catalytic subunit of telomerase (Tert), a feature of pluripotent stem cells and germ cells. The Ddx4-positive mouse and human ovarian GSCs were maintained on MEF-free cultures. After 10-12 and 4-8 wk of culture respectively, the mouse and human ovarian GSCs formed actively dividing germ cell colonies. Gene expression analysis of cultured cells confirmed the maintenance of early germ line markers. Moreover, mouse and human ovarian GSC cells cultured in vitro spontaneously underwent oogenesis 24-48 and 72 h after each passage, respectively. Ploidy analysis of the cultured mouse and human GSCs detected 4n, 2n, and 1n populations of cells. These 1n cells were purported to be haploid germ cells.
Transplantation of GFP-expressing mouse ovarian GSCs into ovaries of non-chemotherapy-conditioned wild-type mice resulted in the formation of developing follicles containing GFP-positive oocytes. In vitro fertilization of the GFP-expressing oocytes led to the formation of embryos expressing GFP. In co-cultures of human GFP-transduced ovarian GSCs with adult ovarian cortical tissue, GFP-positive oocytes were found to be enclosed by GFP-negative somatic granulosa cells; these were present in tightly compact structures that resembled follicles within 24 h after seeding. GFP-expressing human GSCs injected into fragments of human adult ovarian cortical tissues were also transplanted into NOD-SCID female mice. Human ovarian grafts contained primordial and primary follicles with GFP-negative oocytes and also immature follicles containing GFP-positive oocytes.
After the publication of this new study, Oatley and Hunt published a brief commentary to raise some questions regarding these latest findings[31]. They questioned whether the purported GSCs represented true stem cells. Oatley and Hunt also questioned the increase in haploid cells, indicating that oocytes completed both meiotic divisions in vitro and proposed that the oocytes underwent an abnormal maturation that does not occur in vivo. They also criticized the lack of studies on the normalcy of embryos derived from GFP-positive GSCs, arguing that the developmental potential and the genetic quality of the embryos needed to be tested.
Latest developments in the area of GSCs
After the publication of the above-mentioned studies, two different groups presented contradictory results[32,33]. These investigators refuted the existence of GSCs in the adult ovary and provided evidence supporting the dogma of a fixed pool of oocytes after birth.
In 2012, Zhang et al[32] claimed that no mitotically active female germline progenitors existed in postnatal mouse ovaries. In their study, they challenged the existence of ovarian GSCs using a genetic approach. They developed a multiple fluorescent Rosa26rbw/+;Ddx4-Cre germline reporter mouse model to trace the development of Ddx4-expressing ovarian cells in vitro and in vivo. This model expresses GFP ubiquitously. The Ddx4-expressing germline cells and the non-expressing Ddx4 somatic cells can be recognized by a difference in color. The authors tested the ability of adult mouse ovaries to support the formation of new follicles when provided with female primordial germ cells. Transplantation of enhanced GFP (EGFP)-expressing fetal ovarian cells into adult wild-type female mice led to the formation of fluorescent follicles at different stages of development in the cortex and the medulla of the recipient ovaries. Consequently, the adult mouse ovaries supported de novo follicular formation if progenitor cells that were able to differentiate into oocytes were provided. In addition, the adult mouse ovary cells did not transform into oocytes or granulosa cells, and no chimeric follicles were observed. Moreover, when the EGFP-expressing fetal ovarian cells were transplanted into the ovaries of chemotherapy-sterilized WT adult female mice, only newly formed EGFP-positive follicles were detected in the ovaries, which suggests that postnatal mouse ovaries are able to sustain de novo follicle formation when provided with exogenous germline progenitors.
The ovaries of Rosa26rbw/+;Ddx4 Cre postnatal mice were dissociated, and single cells were cultured to determine the proliferation capacity of Dxd4-expressing cells (Red FP positive cells, RFP).
None of the RFP-positive ovarian cells underwent any cell divisions, and they rapidly died off. In long-term cultures on feeders, RFP-positive ovarian cells did not form any colonies. Some Dxd-4-negative ovarian cells formed colonies of cells that were passaged stably, but the cells did not express germ cell markers. These cells did not participate in follicle formation after injection into adult wild-type ovaries. The authors concluded that these clonal cells presented a stem cell-like morphology, but they were not functional female germline progenitors.
Recently, another group published a study supporting the dogma of a fixed pool of oocytes after birth[33]. By using a sensitive lineage-labeling system created in transgenic mice (Rosa26-YFP mice), Lei and Spradling affirmed that the primordial follicles generated during fetal life in mice are sufficient to support adult oogenesis. Moreover, female mouse ovaries do not contain active GSCs, and they do not produce new oocytes in vivo[33].
In 2013, Woods et al[34], published a review on ovarian GSCs that took into consideration two commentaries[31,35] released after the publication of the studies by White et al[7] and by Zhang et al[32]. Woods et al[34] responded to the comments, analyzed the findings of the Zhang et al[32] study, and, more importantly, defended their point of view on the existence of ovarian GSCs and oogenesis in postnatal life in mammals.
Woods et al[34] considered their strategy for the purification of mammalian GSCs from the postnatal ovary to be a validated and reliable method. The protocol using an antibody against the extracellular epitope of the Ddx4 protein exposed on the surface of the plasma membrane of GSCs was also confirmed to isolate these cells from oocytes, which possess only cytoplasmic Ddx4. To respond to Oatley et al[31] about their doubts about the validity of the stem cell properties of the ovarian GSCs, Woods et al[34] explained that the GSCs expressed the same markers as primitive germ cells before and after culture, as described in their publication[7]. In cultures lacking somatic granulosa cells, GSCs-derived oocytes can exit meiotic arrest and complete both meiotic divisions; thereafter, they exhibit a haploid status, similar to spontaneous oocyte activation in vivo when the regulation by somatic granulosa cells is disrupted by ovulation[36]. With the support of data obtained from several groups[29,30] Woods et al[34] claimed that GSCs have the capacity to differentiate into fully mature eggs that can be fertilized to generate normal and viable embryos and offspring in response to the criticisms expressed by Oatley and Hunt.
In the second part of their article, Woods et al[34] also questioned the results reported by Zhang et al[32]. They affirmed that the transgenic mouse model used by Zhang et al[32] possessed a permanent genetic alteration in cells that have activated the Ddx4 gene promotor region. They stated that the fluorescent recombinant reporter gene would be expressed even if the endogenous Ddx4 gene is not active anymore. Zhang et al[32] did not isolate GSCs from the ovaries of their recombinant mice using the methods reported by other groups because they refuted the existence of a Ddx4 protein exposed on the extracellular surface of the plasma membrane. In experiments involving the transplantation of dissociated fetal ovarian cells into wild-type adult mice, Zhang et al[32] did not separate the fractions of purified germ line progenitors (primordial germ cells) and fetal follicular somatic cell progenitors to observe neofolliculogenesis in the adult ovary. A fetal somatic cell population or a fetal germ population transplanted separately could stimulate the adult ovary to recruit its own germ cell or somatic cell progenitors to sustain new follicle formation. Morphologic and genetic profiles of the RFP-positive postnatal ovarian observed in cultures were also questioned. The size of the Ddx4-expressing cells was significantly larger than that of the GSCs (5-8 mm in diameter). The method of selecting recombinant cells from mouse ovaries was discussed because Zhang et al[32] recovered the cells by the filtration of crude extracts of mice ovary with a size cutoff of 40 μm. Small immature oocytes present in postnatal mouse ovaries would not have been retained in the filter. The Ddx4-expressing cells observed in this study should have been considered as oocytes rather than putative GSCs, explaining their incapacity to proliferate in cultures. Identification of the Ddx4-expressing cells needed to be addressed by polymerase chain reaction (PCR)-based analysis of the gene expression patterns of GSCs and oocytes, which would have certainly given positive results for oocyte-specific genes.
Finally, in the discussion of their article, Woods et al[34] compared their findings on ovarian stem cells with the observations established for the germinal stem cells in females of non-mammalian species, such as Drosophila and in males of mammalian species, such as mice. Although the reproductive period in women has a limited duration of 3 decades, Woods et al[34] argued that the presence of germinal stem cells in the ovaries should be accepted as it is in Drosophila, in which the GSCs persist after the failure of reproductive life[37], or in male mice, in which the presence of germinal spermatogonial stem cells has been detected after spermatogenic failure[38]. The authors defended the idea that mammalian females should not be excluded from that standard.
Following the puzzling questions about the Zhang et al[32] study, Park et al[39] decided to carry on experiments using transgenic Ddx4-Cre mice crossed with mice carrying a Rosa26tdTm/tdTm fluorescent gene reporter to identify GSCs in adult mouse ovaries[39]. In experiments performed according to the protocol used in the study by Zhang et al[32], Park et al[39] confirmed that cells collected from crudely dispersed ovaries from recombined mice at postnatal day 8 and selected by filtration were immature oocytes based on PCR analysis of oocyte-specific markers. Moreover, the tdTM/Ddx4 double-positive cell population purified by FACS from dissociated Ddx4-Cre;Rosa26tdTm/+ ovaries expressed the primitive germ cell markers Prdm1, Dppa3, Ifitm3, Tert, and Dazl but did not express the oocyte-specific markers newborn ovary homeobox (Nobox ), ZP3, and growth differentiation factor 9 (Gdf9). In addition, Ddx4 mRNA was detected in freshly purified tdTM/Ddx4 double-positive cells, although it was present at a low level. Cultures of the double-positive cells resulted fluorescent germ cell colonies that were mitotically active. In this study, Park et al[39] demonstrated that a genetic approach associated with a FACS cell selection based on extracellular Ddx4 was a reliable and pertinent strategy for the isolation of GSCs from the adult mouse ovary.
From the studies described above, we can infer that the presence of GSCs in the adult ovary remains a subject of intense debate and controversy, and the field of ovarian biology is undergoing profound changes. Progress in ovarian biology has also been made since the discovery of ovarian CSC in 2005[8]. The characterization of specific ovarian CSCs based on markers of stemness has also generated intense research and discussion. In the following section, we will focus on the characteristics of ovarian CSCs and their relationship with GSCs.
OVARIAN CANCER STEM CELLS: IS THERE ANY RELATIONSHIP BETWEEN GSCS AND CANCER STEM CELLS
To date, the question concerning the cellular origin of cancer is still debated. Two major hypotheses have been proposed regarding the formation of cancer: (1) Clonal evolution, or a stochastic model; and (2) a CSC model[40]. In the first model, every cell within a tumor mass has the equivalent self-renewal capacity and multilineage potency to drive tumor development. In the CSC model, cancer originates from a small rare population of undifferentiated stem cells with self-renewal capacity. Given this theory, it is not surprising that the CSC hypothesis has gained considerable attention in recent years.
As it is known, CSCs share similar biological properties with normal somatic stem cells, including an extensive self-renewal capacity, expression of specific stem cell surface markers and genes and common signaling pathways. However, CSCs differ significantly from normal stem cells in their drug resistance and metastatic activities. Therefore, recent developments in the field of stem cell biology have led to the reconsideration of the definition of cancer. Many researchers suggest that cancer is a stem cell disease and focus on the CSCs theory to understand which cancer cells are responsible for the development of tumors, chemoresistance and high rates of recurrence.
Epithelial ovarian cancer (EOC) is the most lethal of all gynecological malignancies worldwide and the seventh leading cause of cancer-related death[41]. It is generally often diagnosed at late stages of the disease. Greater than 90% of EOCs arise from the ovarian surface epithelium (OSC). The molecular and cellular mechanisms underlying EOC remain poorly understood because of the complexity and heterogeneity of the tumor development process[42].
Accumulating experimental data have demonstrated that EOC is a stem cell-driven disease[43,44]. Over the last few years, several studies have focused on the isolation, identification, and characterization of stem cells from ovarian cancer cell lines, primary tumor tissues and ascites from cancer patients. Although no universal single marker has been found to isolate ovarian CSCs, several putative markers have been identified by several groups (Table 2). However, the isolation and characterization of CSCs have been the subject of extensive studies and the experimental strategies are still a matter of debate.
Table 2.
Markers of ovarian cancer stem cells
| Marker | Ref. |
| Side population | Szotek et al[9] |
| CD44/CD117 | Zhang et al[10] |
| Natriureticpeptide receptor A | Kong et al[11] |
| CD133 | Ferradina et al[45] |
| CD24 | Gao et al[13] |
| DEAD box polypeptide 4 | Hashimoto et al[12] |
DEAD: Asp-Glu-Ala-Asp.
The first evidence for the existence of a CSC population in EOC patients was reported by Bapat et al[8] The authors isolated a single tumorigenic clone from the ascites fluid of an ovarian cancer patient and defined the characteristics of this cell clone, which was identified as a stem/progenitor cell. They suggested that ovarian cancer may be the result of transformation and dysfunction of stem cells in the ovary.
Subsequently, Szotek et al[9] identified stem cell-like cells, or side population (SP) cells, in the murine transgenic epithelial ovarian cancer cell line MOVCAR-7, the human ovarian cancer cell lines OVCAR 3, SKOV-3 and IGROV-1 and ascites from a small group of ovarian cancer patients using the dye efflux marker SP. Then, they went on to characterize the cell surface markers of these cells by flow cytometry. The authors found that c-kit/117 and CD44 were expressed in the mouse SP cells. However, human SP cells were not further functionally characterized in this study.
In 2008, Zhang et al[10] isolated and identified a self-renewing subpopulation of cancer-initiating cells from human ovarian primary tumor tissues for the first time. They showed that these cells expressed various stem cell markers, including stem cell factor, Nanog, ATP Binding Cassette G-member protein (ABCG2), and Oct-4. These cells also demonstrated chemoresistance to cancer therapeutics and were able to initiate tumors in mice, as determined by evaluating the CD44+/CD117+ phenotype. Subsequent studies have demonstrated that CD133(+) cells have higher clonogenic and proliferative potential than CD133(-) cells in primary ovarian tumors[45,46]. This result is not consistent with a recently published study indicating the CD133 expression in tumorigenic ovarian cancer cells is heterogeneous[47]. Similarly, contradictory results have been obtained using CD24 for the characterization of ovarian CSCs[13,48].
Although the origin of CSCs has been debated for a long time, a common consensus on this issue has not been reached. While some authors support the hypothesis that CSCs originate from normal stem cells undergoing malignant transformation[49,50], in vitro cancer models have shown that CSCs do not arise from normal stem cells[51]. Although several putative ovarian CSC markers have been identified, these markers can also be found on normal stem cells[52]. To understand the relationship between CSCs and normal stem cells, the gene expression profiles in human ovarian stem cells and ovarian cancer was analyzed, and the results showed that the expression of some genes is significantly reduced in malignant ovarian tumors but relatively unchanged in benign tumors compared to normal ovary[53]. Surprisingly, in a more recent study, a link between normal stem cells and CSCs in ovarian cancer was established[54]. The authors analyzed the expression patterns of the ovarian GSC marker DDX4 and the ovarian CSC marker CD133 in ovarian cancers using tissue microarrays. They found that the levels of both DDX4 and CD133 were significantly increased in ovarian cancer, and the expression pattern of DDX4 was similar to that of CD133. Even more interesting, they showed that almost all CD133-positive cancer cells also expressed DDX4 whereas the CD133-negative cells did not.
The genetic program regulating the self-renewal and differentiation of CSCs plays a key role in cancer initiation, invasion and migration. A great body of evidence suggests that epigenetic changes and chromosomal alterations are responsible for tumor development including colorectal[55], breast[56], prostate[57], and hepatocellular carcinoma[58]. Epigenetic changes include altered DNA methylation, chromatin remodeling and non-coding small RNA (miRNA) expression. Recent developments in the understanding of the role of epigenetic mechanisms have shed new light on carcinogenesis. Taken together, similar to other cancers, determination of the epigenetic mechanisms involved in ovarian cancer opens promising new therapeutic avenues for treatment.
It has been emphasized that DNA methylation might play a seminal role in tumor formation, development and metastasis. DNA methylation of the cytosine residues of CG (also designated as CpG) dinucleotides is the most well-known epigenetic mechanism. Long-term silencing of genes in ES cells is under the control of the polycomb complex of proteins (PcG), and recent findings have shown that stem cell PcG targets are up to 20-fold more likely to have cancer-specific promoter methylation than nontargets, supporting a stem cell origin of cancer, including ovarian cancer[59,60]. Moreover, Baba et al[52] demonstrated that CD133 expression, which is a somatic stem cell marker, in ovarian cancer is directly controlled by both histone modifications and promoter methylation. Additionally, specific methylated DNA markers can be detected in the serum, plasma and peritoneal fluid of ovarian cancer patients[61].
MicroRNAs (miRNAs) are small noncoding RNAs that control gene expression post-transcriptionally and play pivotal roles in stem cell biology. Interestingly, emerging evidence suggests that some miRNAs are differentially expressed in CSCs and somatic cells and even in cancer cells, which indicates that these miRNAs may be involved in the regulation of cancer-related processes[62-65] Recently, the involvement of miRNAs in the tumorigenesis, metastasis and drug resistance of EOC has been increasingly reported; therefore, it has been proposed that miRNAs could present a novel therapeutic strategy for the management of ovarian cancer.
Guo et al[65] showed that miR-9 was downregulated in human ovarian cancer compared to normal ovary, and overexpression of miR-9 repressed cell growth. Li et al[66] examined the role of miR-27a in the development of drug resistance in ovarian cancer cells, and they found that inhibition of miR-27a decreased the expression levels of the multi drug resistance 1 (MDR1) gene and increased paclitaxel-induced apoptosis. Another recent study reported aberrant miRNA expression in ovarian cancers compared to normal ovary. Iorio et al[67] reported the overexpression of miR-200a, miR-141, miR-200c, and miR-200b, as well as the downregulation of miR-199a, miR-140, miR-145, and miR-125b1, in ovarian cancer cell lines.
In light of these findings, it is clear that the CSCs play an important role in the development and maintenance of EOC, and epigenetic changes are responsible for the behavior of cancer progenitor cells and their progeny.
CONCLUSION AND FUTURE PERSPECTIVE
The field of reproductive biology is still divided over the possibility of neo-oogenesis in the mammalian adult ovary between the supporters of this new theory[1,7,29,30] and the opponents who subscribe to the long-held dogma of a fixed number of follicles at birth[32,33,68]. In addition, a recurrent question concerns the physiological role of GSCs in the adult mammalian ovary. Nonetheless, the isolation and characterization of GSCs from human ovaries open new perspectives to design protocols for producing mature, competent oocytes in vitro for clinical application in assisted reproduction techniques (ART).
During the past years, attempts to generate competent oocytes from ES cells and induced pluripotent stem cells have failed to meet expectations, and fertilization of ESC-derived oocytes remains to be demonstrated[69,70]. Recently, stem cells derived from newborn mouse skin have been shown to produce oocytes when cultured in an appropriate environment, but these oocytes were not able to undergo complete maturation[71]. Therefore, as a pure stem cell population that can be easily isolated, female GSCs provide a model to explore postnatal oogenesis in mammals. In addition, these cells may plausibly contribute to the preservation of sterility in women.
Many unanswered questions in the development of oocytes from GSCs remain to be addressed. Cultures of GSCs facilitate the characterization of factors that contribute to the development and regulation of oocytes[72]. Importantly, the epigenetic program and modifications involved in de novo oocyte formation in the adult ovary need to be further established[73].A better understanding of these mechanisms of regulation may provide a means to stimulate the generation of oocytes in adult human ovaries in vivo.
The question of menopause also remains to be addressed. If ovaries can generate new oocytes during adulthood, it can therefore be suggested that the process of menopause may be the consequence of GCS and ovarian somatic cell aging and not the result of depletion of the oocyte pool. Aging GSCs may arrest to differentiate into oocytes, and/or the environment of the GCS niche may fail to sustain oocyte generation. Therefore, the issue of menopause occurrence is not incompatible with the concept of postnatal neo-oogenesis.
Translational applications of germinal stem cell-based strategies in the field of infertility treatment in women may be achievable in the near future. Before the use of treatments that negatively impact fertility in women, samples taken from the ovaries could facilitate the isolation of GSCs for cryopreservation and/or for ex vivo expansion and subsequent differentiation into oocytes. Cultured GSCs could be transferred back into the ovaries after treatment to support neo-folliculogenesis, or GSC-derived cultured oocytes could be fertilized in a program of in vitro fertilization[74].
Although these potential clinical applications give hope for the future, we are a considerable way from using female GSCs in ART to treat infertility in women.
OC is the most lethal of all gynecological malignancies worldwide. To better understand cancers, researchers are now focusing on identifying the relationship between CSCs and normal stem cells. While both stem cell types have the ability to self-renew, common signaling pathways and similar gene expression profiles, these important properties make it difficult to isolate and distinguish these cells from each other. Additionally, the field faces major challenges, including the absence of specific cell surface markers, inadequate isolation and differentiation protocols, the paucity of genome-wide studies, and also ethical restrictions.
Taken together, it remains uncertain which ovarian cancer phenotype follows the stem cell model due to the complexity and heterogeneity of EOC. On the other hand, the initiation and progression of EOC is characterized by the activation of oncogenes and the switching of tumor suppressor gene expression by epigenetic mechanisms[75-77]. More importantly, there is evidence on the epigenetic regulation of pluripotency transcription factors, signaling mechanisms and self-renewal pathways that play a critical role in the transformation of GSCs into CSCs in EOC. This knowledge provides us with not only a better understanding of how cancer cells may arise from germinal stem cells, but it also contributes to the development of new technologies or strategies in the management of EOC.
Over the last several years, a great body of evidence supports the idea that ovarian CSCs are responsible for metastasis and drug resistance after chemotherapy in this life-threatening disease. The lack of markers for early detection is the main obstacle to effective treatment strategies. A better understanding of the molecular pathogenesis of EOC is needed to develop new drug therapies and/or diagnostic biomarkers.
Footnotes
Conflict-of-interest: The authors declare that they have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 29, 2014
First decision: November 27, 2014
Article in press: March 18, 2015
P- Reviewer: Hutz RJ, Teresa Valenti M S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
References
- 1.Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- 2.Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;6:63–108. [Google Scholar]
- 3.Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Kobayashi K, Nishimura T, Higashijima S, Tanaka M. Identification of germline stem cells in the ovary of the teleost medaka. Science. 2010;328:1561–1563. doi: 10.1126/science.1185473. [DOI] [PubMed] [Google Scholar]
- 5.White YA, Woods DC, Wood AW. A transgenic zebrafish model of targeted oocyte ablation and de novo oogenesis. Dev Dyn. 2011;240:1929–1937. doi: 10.1002/dvdy.22695. [DOI] [PubMed] [Google Scholar]
- 6.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 9.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong X, Wang X, Xu W, Behera S, Hellermann G, Kumar A, Lockey RF, Mohapatra S, Mohapatra SS. Natriuretic peptide receptor a as a novel anticancer target. Cancer Res. 2008;68:249–256. doi: 10.1158/0008-5472.CAN-07-3086. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto H, Sudo T, Mikami Y, Otani M, Takano M, Tsuda H, Itamochi H, Katabuchi H, Ito M, Nishimura R. Germ cell specific protein VASA is over-expressed in epithelial ovarian cancer and disrupts DNA damage-induced G2 checkpoint. Gynecol Oncol. 2008;111:312–319. doi: 10.1016/j.ygyno.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672–2680. doi: 10.1038/onc.2010.35. [DOI] [PubMed] [Google Scholar]
- 14.Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132:95–109. doi: 10.1530/rep.1.01128. [DOI] [PubMed] [Google Scholar]
- 15.Noce T, Okamoto-Ito S, Tsunekawa N. Vasa homolog genes in mammalian germ cell development. Cell Struct Funct. 2001;26:131–136. doi: 10.1247/csf.26.131. [DOI] [PubMed] [Google Scholar]
- 16.Gosden RG. Germline stem cells in the postnatal ovary: is the ovary more like a testis. Hum Reprod Update. 2004;10:193–195. doi: 10.1093/humupd/dmh023. [DOI] [PubMed] [Google Scholar]
- 17.Greenfeld C, Flaws JA. Renewed debate over postnatal oogenesis in the mammalian ovary. Bioessays. 2004;26:829–832. doi: 10.1002/bies.20094. [DOI] [PubMed] [Google Scholar]
- 18.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, Woodruff TK. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298:149–154. doi: 10.1016/j.ydbio.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Byskov AG, Faddy MJ, Lemmen JG, Andersen CY. Eggs forever. Differentiation. 2005;73:438–446. doi: 10.1111/j.1432-0436.2005.00045.x. [DOI] [PubMed] [Google Scholar]
- 20.Albertini DF. Micromanagement of the ovarian follicle reserve--do stem cells play into the ledger. Reproduction. 2004;127:513–514. doi: 10.1530/rep.1.00247. [DOI] [PubMed] [Google Scholar]
- 21.Motta PM, Makabe S. Germ cells in the ovarian surface during fetal development in humans. A three-dimensional microanatomical study by scanning and transmission electron microscopy. J Submicrosc Cytol. 1986;18:271–290. [PubMed] [Google Scholar]
- 22.Eppig JJ, Wigglesworth K. Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cell components. Biol Reprod. 2000;63:1014–1023. doi: 10.1095/biolreprod63.4.1014. [DOI] [PubMed] [Google Scholar]
- 23.Gosden RG. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum Reprod. 1990;5:499–504. doi: 10.1093/oxfordjournals.humrep.a137132. [DOI] [PubMed] [Google Scholar]
- 24.Skaznik-Wikiel M, Tilly JC, Lee HJ, Niikura Y, Kaneko-Tarui T, Johnson J, Tilly JL. Serious doubts over “Eggs forever”. Differentiation. 2007;75:93–99. doi: 10.1111/j.1432-0436.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, Forkert R, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–315. doi: 10.1016/j.cell.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Andersen CY, Anderson R, Braw-Tal R, Clarke H, Gougeon A, et al. On regenerating the ovary and generating controversy. Cell. 2005;122:821–822. doi: 10.1016/j.cell.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- 28.Johnson J, Skaznik-Wikiel M, Lee HJ, Niikura Y, Tilly JC, Tilly JL. Setting the record straight on data supporting postnatal oogenesis in female mammals. Cell Cycle. 2005;4:1471–1477. doi: 10.4161/cc.4.11.2186. [DOI] [PubMed] [Google Scholar]
- 29.Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
- 30.Pacchiarotti J, Maki C, Ramos T, Marh J, Howerton K, Wong J, Pham J, Anorve S, Chow YC, Izadyar F. Differentiation potential of germ line stem cells derived from the postnatal mouse ovary. Differentiation. 2010;79:159–170. doi: 10.1016/j.diff.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Oatley J, Hunt PA. Of mice and (wo)men: purified oogonial stem cells from mouse and human ovaries. Biol Reprod. 2012;86:196. doi: 10.1095/biolreprod.112.100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci USA. 2012;109:12580–12585. doi: 10.1073/pnas.1206600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci USA. 2013;110:8585–8590. doi: 10.1073/pnas.1306189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woods DC, White YA, Niikura Y, Kiatpongsan S, Lee HJ, Tilly JL. Embryonic stem cell-derived granulosa cells participate in ovarian follicle formation in vitro and in vivo. Reprod Sci. 2013;20:524–535. doi: 10.1177/1933719113483017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Telfer EE, Albertini DF. The quest for human ovarian stem cells. Nat Med. 2012;18:353–354. doi: 10.1038/nm.2699. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod. 1997;57:743–750. doi: 10.1095/biolreprod57.4.743. [DOI] [PubMed] [Google Scholar]
- 37.Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7:344–354. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Ebata KT, Robaire B, Nagano MC. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74:119–124. doi: 10.1095/biolreprod.105.045591. [DOI] [PubMed] [Google Scholar]
- 39.Park ES, Tilly JL. Use of DEAD-box polypeptide-4 (Ddx4) gene promoter-driven fluorescent reporter mice to identify mitotically active germ cells in post-natal mouse ovaries. Mol Hum Reprod. 2015;21:58–65. doi: 10.1093/molehr/gau071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- 41.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 42.Godwin AK, Testa JR, Hamilton TC. The biology of ovarian cancer development. Cancer. 1993;71:530–536. doi: 10.1002/cncr.2820710207. [DOI] [PubMed] [Google Scholar]
- 43.Yan HC, Fang LS, Xu J, Qiu YY, Lin XM, Huang HX, Han QY. The identification of the biological characteristics of human ovarian cancer stem cells. Eur Rev Med Pharmacol Sci. 2014;18:3497–3503. [PubMed] [Google Scholar]
- 44.Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, et al. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506–514. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 46.Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci USA. 2011;108:6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi MF, Jiao J, Lu WG, Ye F, Ma D, Dong QG, Xie X. Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cell Mol Life Sci. 2010;67:3915–3925. doi: 10.1007/s00018-010-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 50.Blair A, Hogge DE, Sutherland HJ. Most acute myeloid leukemia progenitor cells with long-term proliferative ability in vitro and in vivo have the phenotype CD34(+)/CD71(-)/HLA-DR- Blood. 1998;92:4325–4335. [PubMed] [Google Scholar]
- 51.Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 52.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 53.Ismail RS, Baldwin RL, Fang J, Browning D, Karlan BY, Gasson JC, Chang DD. Differential gene expression between normal and tumor-derived ovarian epithelial cells. Cancer Res. 2000;60:6744–6749. [PubMed] [Google Scholar]
- 54.Kim KH, Kang YJ, Jo JO, Ock MS, Moon SH, Suh DS, Yoon MS, Park ES, Jeong N, Eo WK, et al. DDX4 (DEAD box polypeptide 4) colocalizes with cancer stem cell marker CD133 in ovarian cancers. Biochem Biophys Res Commun. 2014;447:315–322. doi: 10.1016/j.bbrc.2014.03.144. [DOI] [PubMed] [Google Scholar]
- 55.Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 56.Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 57.Rennie PS, Nelson CC. Epigenetic mechanisms for progression of prostate cancer. Cancer Metastasis Rev 1998- 1999;17:401–409. doi: 10.1023/a:1006121219097. [DOI] [PubMed] [Google Scholar]
- 58.Bakker J, Lin X, Nelson WG. Methyl-CpG binding domain protein 2 represses transcription from hypermethylated pi-class glutathione S-transferase gene promoters in hepatocellular carcinoma cells. J Biol Chem. 2002;277:22573–22580. doi: 10.1074/jbc.M203009200. [DOI] [PubMed] [Google Scholar]
- 59.Widschwendter M, Fiegl H, Egle D, Mueller-Holzner E, Spizzo G, Marth C, Weisenberger DJ, Campan M, Young J, Jacobs I, et al. Epigenetic stem cell signature in cancer. Nat Genet. 2007;39:157–158. doi: 10.1038/ng1941. [DOI] [PubMed] [Google Scholar]
- 60.Fiegl H, Windbichler G, Mueller-Holzner E, Goebel G, Lechner M, Jacobs IJ, Widschwendter M. HOXA11 DNA methylation--a novel prognostic biomarker in ovarian cancer. Int J Cancer. 2008;123:725–729. doi: 10.1002/ijc.23563. [DOI] [PubMed] [Google Scholar]
- 61.Ibanez de Caceres I, Battagli C, Esteller M, Herman JG, Dulaimi E, Edelson MI, Bergman C, Ehya H, Eisenberg BL, Cairns P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64:6476–6481. doi: 10.1158/0008-5472.CAN-04-1529. [DOI] [PubMed] [Google Scholar]
- 62.Aydoğdu E, Katchy A, Tsouko E, Lin CY, Haldosén LA, Helguero L, Williams C. MicroRNA-regulated gene networks during mammary cell differentiation are associated with breast cancer. Carcinogenesis. 2012;33:1502–1511. doi: 10.1093/carcin/bgs161. [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie Z, Cao L, Zhang J. miR-21 modulates paclitaxel sensitivity and hypoxia-inducible factor-1α expression in human ovarian cancer cells. Oncol Lett. 2013;6:795–800. doi: 10.3892/ol.2013.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M, Li X, Tang H. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-kappaB1. FEBS J. 2009;276:5537–5546. doi: 10.1111/j.1742-4658.2009.07237.x. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L, Wang Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol. 2010;119:125–130. doi: 10.1016/j.ygyno.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 67.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 68.Notarianni E. Reinterpretation of evidence advanced for neo-oogenesis in mammals, in terms of a finite oocyte reserve. J Ovarian Res. 2011;4:1. doi: 10.1186/1757-2215-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tedesco M, Farini D, De Felici M. Impaired meiotic competence in putative primordial germ cells produced from mouse embryonic stem cells. Int J Dev Biol. 2011;55:215–222. doi: 10.1387/ijdb.103108mt. [DOI] [PubMed] [Google Scholar]
- 70.Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Höög C. Mouse embryonic stem cells form follicle-like ovarian structures but do not progress through meiosis. Stem Cells. 2006;24:1931–1936. doi: 10.1634/stemcells.2005-0520. [DOI] [PubMed] [Google Scholar]
- 71.Dyce PW, Liu J, Tayade C, Kidder GM, Betts DH, Li J. In vitro and in vivo germ line potential of stem cells derived from newborn mouse skin. PLoS One. 2011;6:e20339. doi: 10.1371/journal.pone.0020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park ES, Woods DC, Tilly JL. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertil Steril. 2013;100:1468–1475. doi: 10.1016/j.fertnstert.2013.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–349. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woods DC, Tilly JL. The next (re)generation of ovarian biology and fertility in women: is current science tomorrow’s practice. Fertil Steril. 2012;98:3–10. doi: 10.1016/j.fertnstert.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Zhao FJ, Chen LL, Wang LQ, Nephew KP, Wu YL, Zhang S. MiR-373 targeting of the Rab22a oncogene suppresses tumor invasion and metastasis in ovarian cancer. Oncotarget. 2014;5:12291–12303. doi: 10.18632/oncotarget.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao M, Sun J, Zhao Z. Distinct and competitive regulatory patterns of tumor suppressor genes and oncogenes in ovarian cancer. PLoS One. 2012;7:e44175. doi: 10.1371/journal.pone.0044175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feng Q, Deftereos G, Hawes SE, Stern JE, Willner JB, Swisher EM, Xi L, Drescher C, Urban N, Kiviat N. DNA hypermethylation, Her-2/neu overexpression and p53 mutations in ovarian carcinoma. Gynecol Oncol. 2008;111:320–329. doi: 10.1016/j.ygyno.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]


