Abstract
The aims of this study were to enhance the solubility and dissolution rate of nimodipine (ND) by preparing the inclusion complexes of ND with sulfobutylether-b-cyclodextrin (SBE-β-CD) and 2-hydroxypropyl-b-cyclodextrin (HP-β-CD) and to study the effect of the preparation method on the in vitro dissolution profile in different media (0.1 N HCl pH 1.2, phosphate buffer pH 7.4, and distilled water). Thus, the inclusion complexes were prepared by kneading, coprecipitation, and freeze-drying methods. Phase solubility studies were conducted to characterize the complexes in the liquid state. The inclusion complexes in the solid state were investigated with differential scanning calorimetry (DSC), X-ray diffractometry (X-RD), and Fourier transform infrared spectroscopy (FT-IR). Stable complexes of ND/SBE-β-CD and ND/HP-β-CD were formed in distilled water in a 1:1 stoichiometric inclusion complex as indicated by an AL-type diagram. The apparent stability constants (Ks) were 1334.4 and 464.1 M−1 for ND/SBE-β-CD and ND/HP-β-CD, respectively. The water-solubility of ND was significantly increased in an average of 22- and 8-fold for SBE-β-CD and HP-β-CD, respectively. DSC results showed the formation of true inclusion complexes between the drug and both SBE-β-CD and HP-β-CD prepared by the kneading method. In contrast, crystalline drug was detectable in all other products. The dissolution studies showed that all the products exhibited higher dissolution rate than those of the physical mixtures and ND alone, in all mediums. However, the kneading complexes displayed the maximum dissolution rate in comparison with drug and other complexes, confirming the influence of the preparation method on the physicochemical properties of the products.
Keywords: 2-hydroxypropyl-b-cyclodextrin, dissolution rate, inclusion complexes, nimodipine, sulfobutylether-b-cyclodextrin
INTRODUCTION
Nimodipine (3-(2-methoxyethyl) 5-propan-2-yl 2, 6-dimethyl-4-(3-nitrophenyl)-1, 4-dihydropyridine-3, 5-dicarboxylate; Fig. 1a) is an effective second generation calcium channel blocker with a molecular weight of 418.4 Da and a molecular formula of C21H26N2O7 (http://www.drugbank.ca/drugs/DB00393). It is used in the treatment of cerebrovascular spasms following subarachnoid hemorrhage [1, 2] and has been the most widely studied calcium channel block in the treatment of acute cerebral ischemia [3]. It is highly crystalline and practically insoluble in water (24.3 mg/L at 25°C). Since it has a high permeability, but has a low solubility, ND has been categorized as a Biopharmaceutical Classification System (BCS) class II compound. The lower bioavailability of nimodipine, which averages 13% after oral administration, might be due to high hepatic first pass effects and poor aqueous solubility and this slower its dissolution rate.
Fig. 1.

Molecular structures. Nimodipine (a) and CD derivative (b), where R is 2-hydroxypropyl (CH2CHOHCH3) or sulfobutylether ((CH2)4 SO3 Na) for HP-Β-CD and SBE-Β-CD, respectively
In order to enhance the dissolution characteristics of poorly soluble drugs, such as nimodipine, and improve its solubility, complexation with oligosaccharide derivatives-cyclodextrins (CD) appears to be one of the most effective methods [4–6].
Cyclodextrin molecules are crystalline, are cyclic (a-1, 4)-linked oligosaccharides composed of seven a-d-glucopyranose units, which together form a rigid cone-shaped structure (Fig. 1b). This structure possesses a hydrophobic inner cavity and hydrophilic outer surface which allows the formation of inclusion complexes in which lipophilic compounds are noncovalently bound within the cavity. Cyclodextrins are popular for their ability to form inclusion complex and has been extensively used to enhance the aqueous solubility, stability, and dissolution rate of poorly water-soluble drugs [7–9]. Beta cyclodextrins (β-CDs) are most commonly used because of lower toxicities than other CDs, and their cavity size is suitable for a wide range of guest molecules. However, with no substituent on the glucopyranose unit, β-CD has poor water solubility and is parenterally unsafe due to its nephrotoxicity [10]. Recently, several synthetically modified β-CD s, such as hydroxypropyl-b-cyclodextrin (HP-β-CD) and sulfobutyl ether-b-cyclodextrin (SBE-β-CD) have been made to improve the inclusion capacity and the physicochemical properties of parent CDs. They have been reported on account of their high solubility in water (excess 70 g/100 ml for SBE-β-CD and 65 g/100 ml for HP-β-CD at 25°C) and minimal toxicity [11–13]. Especially, SBE-β-CD, which is currently used in five commercially approved injectable products in the USA, has been recently been used to stabilize drugs and is currently undergoing extensive chronic safety assessment [14].
Researchers have established the potential of various approaches to improve the solubility, dissolution rate, and/or oral bioavailability of ND, such as solid dispersions of ND with hydroxypropyl methylcellulose, polyvinylpyrrolidone/vinyl acetate copolymer, and ethyl acrylate, methyl methacrylate polymer [15], preparation of solid lipid nanoparticles and nanostructured lipid carriers of nimodipine in the presence of electrolytes [16] and particularly the formation of inclusion complexes with β-cyclodextrin and hydroxypropyl-β-cyclodextrin [17–19].
However, until today, complexation of ND with SBE-β-CD has been less studied and to our knowledge no detailed characterization studies of this complex are present in literature.
Thus, the aims of this work were to study the interaction of nimodipine with SBE-β-CD, in both solution and solid state, in order to improve the aqueous solubility of the drug and to enhance its dissolution rate and its inclusion capacity was compared with that of HP-β-CD. The complexes with both β-CD derivatives were prepared by different methods: kneading, coevaporation, and freeze drying at various stoichiometric ratios. Both the preparation method of solid drug–CD complexes and the nature of the particular CD play important roles in the performance of a drug–CD formulation application [20].
The complexation of nimodipine with SBE-β-CD and HP-β-CD was characterized in the liquid state through the use of phase solubility diagrams. Solid-state complexation was examined through differential scanning calorimetry (DSC), X-ray diffractometry (X-RD), Fourier-transform infrared spectroscopy (FTIR), and the improvement in solubility and dissolution rate of the solid drug complex. The dissolution properties of the solid complexes were evaluated and compared with those of nimodipine alone and of the corresponding physical mixtures.
MATERIALS AND METHODS
Materials
Nimodipine was purchased from Chang Yi Pharmaceutical Co., Ltd. (Guangdong, China). Sulfobutyl ether7 β-cyclodextrin (CAPTISOL, degree of substitution, 0.9, average Mw = 2163 Da) and 2-Hydroxypropyl-b-cyclodextrin (average degree of substitution, 0.6, average Mw = 1541.5 Da) were purchased from Giant Ring Technology Co., Ltd. (Nanjing, China) and Rick Chemicals Co., Ltd. (Kunshan, China), respectively. All other chemicals and solvents were of analytical reagent grade, double distilled water was used throughout the study.
Phase Solubility Studies
The effects of CDs on the solubility of nimodipine were investigated according to the phase solubility technique established by Higuchi and Connors [21]. Excess amounts of nimodipine (50 mg) were added to 15 ml of distilled water containing increasing concentrations of SBE-β-CD and HP-β-CD (2–16 mM) in 25 ml stoppered glass bottles. The obtained suspensions were shaken at 37 ± 0.5°C for 3 days in a thermostatically controlled shaking water bath (Model SHY-2A, Jintan, China). After equilibrium attainment, aliquots were withdrawn, filtered through a 0.45 μm membrane filter, and assayed spectrophotometrically (UV-2000, Hangzhou, China) for nimodipine content at 239 nm against blank solutions containing the same concentrations of cyclodextrins. Each experiment was carried out in triplicate. Solubility diagrams were constructed by plotting the molar concentration of nimodipine dissolved (solubility) versus the molar concentration of the CD. From these diagrams, the stability rate constants for the complexation of nimodipine with CDs were calculated.
The UV spectrophotometric method for nimodipine was validated. The calibration curve was linear in the working range of 1–10 μg/mL with a slope of 0.0657 (r2 = 0.9996) in distilled water.
In addition, absorption spectra were used to confirm the formation of inclusion complex. The maximum absorbance of the phase solubility samples was measured against a reagent blank which was prepared with identical reagent concentration but without nimodipine.
A UV–Visible spectrophotometer (Agilent 8453, USA) was used to record the maximum absorption spectra of nimodipine-free and its inclusion complex with SBE-β-CD and HP-β-CD between 200 and 400 nm.
Formulation of Solid Nimodipine–Cyclodextrin Complexes
Solid inclusion complexes of ND with SBE-β-CD and HP-β-CD were prepared by kneading, coprecipitation, and freeze-drying procedures in a 1:1 M ratio. Physical mixtures (PM) were prepared in the same molar ratios for comparative purpose, where the ND and the different CDs were put together and thoroughly mixed in a mortar for 20 min without kneading or freezing them. The three different methods employed for the preparation of solid complexes are described:
Kneading Method (Kn)
The calculated amounts of nimodipine and cyclodextrin were accurately weighed, transferred to a mortar, and kneaded for 45 min. During this process, a small volume of methanol:water (50:50 v/v) solution was added to the mixture to maintain a suitable consistency. The final product was dried at 40°C for 48 h and then was ground to powder, passed through a 100-mesh sieve, and stored in a sealed glass vial.
Coprecipitation Method (CoP)
Quantities of drug and cyclodextrin, in the required molar ratio (1:1), were dissolved in methanol:water, respectively. The ND solution was added dropwise into CD solution. The mixture was vigorously stirred for 6 h at 50°C. The solvent was removed using a rotary evaporator. The obtained product was dried at around 45–50°C and stored for further measurements.
Freeze-Drying Method (FD)
A 1:1 M ratio of ND and CD were dissolved in methanol and distilled water, respectively. The resulting solutions were mixed for 24 h at 25°C using a magnetic stirrer. The final solution was frozen at −70°C, and subsequently freeze-dried for 48 h at −50°C using a freeze-dryer (FD-1C-50; Beijing, China). The lyophilized powder was passed through a 100-mesh sieve and stored in a sealed glass vial.
Characterization of Nimodipine-Cyclodextrin Inclusion Complexes
Differential Scanning Calorimetry
Nimodipine and its complexes with SBE-β-CD and HP-β-CD were analyzed by differential scanning calorimeter (NETZSCH DSC 204(Selb/Bavaria). ND (2.5 mg) and PM, Kn, CoP, FD samples (equivalent to 2.5 mg of nimodipine) were sealed using aluminum pans and scanned at 10°C/min between 30 and 300°C at a frequency modulation of 60 Hz. DSC analysis was carried out under nitrogen gas flow of 40 mL/min.
Powder X-ray Diffractometry
The powder X-ray diffractometry (X-RD) patterns of nimodipine (200 mg), CDs, and PM, Kn, CoP, and FD formulations (containing an equivalent amount of nimodipine) were recorded at room temperature using a Bruker D8 Advance X-Ray diffractometer (Bruker, Germany). The samples were irradiated with Cu Kα radiation, at 40 kV voltage and 40 mA current. The scanning rate employed was 4°/min over a diffraction angle of 2θ and range of 3°–40°.
Fourier-Transform Infrared Spectroscopy
The FTIR spectra were recorded using a Bruker FTIR spectrophotometer (Model Tensor 27, Bruker, Germany). Spectra were measured with a KBr disk and performed in the scanning range of 4000–500 cm at ambient temperature.
Molecular Modeling Studies
Molecular modeling was performed to elucidate the intermolecular interaction between nimodipine and HP-β-CD/SBE-β-CD into the inclusion complex.
The 3D structures of nimodipine and β-cyclodextrin were retrieved from the Drug Bank and the Protein Data Bank, respectively. The structure of HP-β-CD was built by adding four 2-hydroxypropyl groups on the hydroxyl groups of β-CD [22], whereas SBE-β-CD was constructed by adding seven sulfobutyl groups to parent β-CD [23] using the Chembio3D ultra (Version 8.0, CambridgeSoft com., USA). Geometrical energies for cyclodextrins and drug structures were minimized using the MM2 method and force field.
The inclusion model of nimodipine with HP-β-CD/SBE-β-CD was set up using the MOE (Molecular Operating Environment) software. The search for the lowest energy structure for the inclusion system of nimodipine into HP-β-CD/SBE-β-CD cavity has been adopted, and the optimal complex structure was then established in Chembio3D ultra 8.0.
In Vitro Dissolution Studies
Dissolution experiments were performed on a ZRS-4 Intelligent Dissolution Tester (Tianjin, China) according to the dispersed amount method [24]. Ten milligrams of nimodipine or the CD formulation (kneaded mixture, coevaporated, or freeze dried formulation) equivalent to 10 mg of nimodipine were added to 900 mL of three different mediums: simulated gastric fluid (pH 1.2), phosphate buffer (pH 7.4), and distilled water. The stirring speed was 100 rpm, and the temperature was maintained at 37 ± 0.5°C. At fixed time intervals (5, 10, 15, 20, 30, 40, 60, 90, and 180 min), 5 mL samples were drawn with a filter syringe, and the nimodipine content was determined spectrophotometrically at 239 nm. A correction was applied for the cumulative dilution caused by replacement of the sample with an equal volume of fresh medium.
The dissolution profiles were evaluated on the basis of the dissolution efficiency parameter at 30 min (DE30, %) and at 180 min (DE180, %). The dissolution efficiency parameters were calculated from the area under the dissolution curves and expressed as a percent of the area of the rectangle described by 100% dissolution in the same time [25]. All dissolution tests were executed six times.
RESULTS AND DISCUSSION
Inclusion Complexation in the Liquid State
Phase Solubility Studies
The phase solubility diagram of nimodipine as a function of the concentration of various CDs is shown in Fig. 2. The phase solubility diagram was of AL according to Higuchi and Connors. The aqueous solubility of nimodipine was increased linearly as a function of the concentration of HP-β-CD/SBE-β-CD with a slope of <1 showing that the increase in the solubility was due to the formation of 1:1 M complex. The apparent solubility constant (K1:1) obtained from the slope and the intercept (S0) of the linear portion of the phase solubility diagram was found to be 1410.6 and 495.1 M−1 for SBE-β-CD and HP-β-CD, respectively. These values of stability constant indicate that complexes formed are quite stable and that they seem suitable for improving the dissolution rate-limited gastrointestinal absorption and oral bioavailability [26]. If the complex were too weak, there is a little improvement in the solubility of the drug. On the other hand, if the complex was too strong, as indicated by a stability constant greater than 10,000 M−1, the complex may not dissociate appreciably, and the complex itself is not absorbed [26].
Fig. 2.
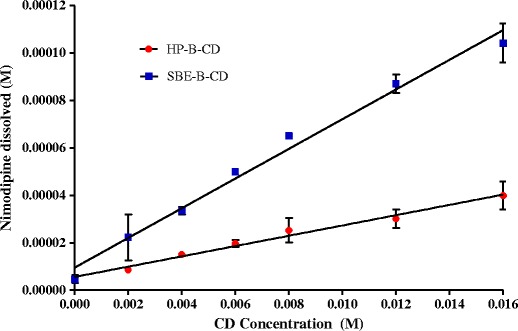
Phase solubility diagrams of nimodipine with SBE-β-CD and HP-β-CD in distilled water (∼37°C). (n = 3)
In this study, the solubility of nimodipine in the presence of SBE-β-CD increased more than in the presence of HP-β-CD in distilled water. The highest K1:1 value exhibited by SBE-β-CD could be ascribed to the presence of the four-carbon butyl chain coupled with repulsion of the end group negative charges which allows for an “extension” of the hydrophobic region of the CD cavity and thus increase its affinity towards nimodipine [27]. Which is in complete agreement with the findings of De Chasteigner (1996), suggesting that the longer the hydrophobic chain linked to the β-CD, the higher the association of drug within the complex [28].
The complexing ability and, therefore, solubility improvement by SBE-β-CD is generally higher than HP-β-CD for many drug molecules, for example, DY-9760e [29], Bentazon [30], Quercetin [31], and Piroxicam [32].
The absorption spectra of inclusion complex at different concentrations range of SBE-β-CD and HP-β-CD are shown in Figs. 3 a and b. The maximum concentration of nimodipine in aqueous solution determined in the absence of CDs at 37°C was of 4.75 × 10−6 M. It is observed that the absorbance value increased with increasing SBE-β-CD/HP-β-CD concentrations. It indicates the solubility of guest molecule (nimodipine) increases upon forming the inclusion complex [33].
Fig. 3.

a UV–Vis spectra of nimodipine free (a) and in the presence of increasing amount of SBE-β-CD b 2 × 10−3 M, c 4 × 10−3 M, d 6 × 10−3 M, e 8 × 10−3 M, f 12 × 10−3 M, and g 16 × 10−3 M. b UV–Vis spectra of nimodipine free (a) and in the presence of increasing amount of HP-β-CD b 2 × 10−3 M, c 4 × 10−3 M, d 6 × 10−3 M, e 8 × 10−3 M, f 12 × 10−3 M, and g 16 × 10−3 M
As shown in Fig. 2, solubility increase was highest when SBE-β-CD was used, being 22-fold higher than that of nimodipine with 16 mM SBE-β-CD. In the other hand, HP-β-CD at the same concentration (16 Mm) displayed an eightfold solubility increase compared to the drug alone.
Inclusion Complexation in the Solid State
The inclusion complexes of nimodipine with CDs were prepared and characterized in the solid state. The kneaded formulations and the physical mixtures were analyzed to determine if inclusion complexes were formed. The existence of a nimodipine–CD complex in the solid state was confirmed by DSC, X-RD, and FTIR.
Differential Scanning Calorimetry
The thermal behavior of ND-SBE-β-CD and ND-HP-β-CD inclusion complexes was studied using DSC to confirm the formation of the solid complex. DSC thermograms of ND, SBE-β-CD, HP-β-CD, physical mixtures, and inclusion complexes from kneaded method are shown in Fig. 4.
Fig. 4.

a Differential scanning calorimetry thermograms of nimodipine (ND), SBE-β-CD, physical mixture (PM), coprecipitated (CoP), freeze-dried (FD), and kneaded complexes (Kn). b Differential scanning calorimetry thermograms of nimodipine (ND), HP-β-CD, physical mixture (PM), coprecipitated (CoP), freeze-dried (FD), and kneaded complexes (Kn)
The DSC thermogram of pure nimodipine revealed a unique representative endothermic melting peak at the onset temperature of 124.6°C. The DSC trace of SBE-β-CD and HP-β-CD showed a broad endothermic effect ranging between 40 and 100°C, which may be attributed to a dehydration process. The broad endotherm displayed by SBE-β-CD thermogram around 275°C was due to the beginning of decomposition events. The physical mixtures of nimodipine with SBE-β-CD and HP-β-CD also show the endothermic peak that is characteristic of nimodipine, (124.2°C), even though some size reduction and broadening of endothermic peak of drug was observed (Fig. 4a, b), indicating that there was partial ND-SBE-β-CD/HP-β-CD interaction in physical mixture. Similar results were reported for other drugs with SBE-β-CD and HP-β-CD [34, 35]. The upward diagram of physical mixture was analogous to the curves of SBE-β-CD and HP-β-CD, respectively.
For the ND-SBE-β-CD formulations, the characteristic melting point peak of nimodipine almost completely disappeared in the kneading complex, while the freeze-dried formulation showed a slight endothermic peak, which is indicative of some drug–CD interaction resulting in a loss of ND crystallinity and suggests that the freeze drying method did not produce a complete inclusion complex. On the contrary, the DSC thermogram of the coprecipitated formulation showed the persistence of the endothermic peak of nimodipine, indicating that a true inclusion complex had not formed at 1:1 molar ratio in the solid state.
Similar results were observed for ND–HP-β-CD complexes, where the endothermic peak was also completely absent for the kneaded complex. This suggested a solid-state interaction of HP-β-CD and ND by the kneading method.
These results indicate that the inclusion complexes prepared by kneading exist in the new solid state, which confirms that kneading method was the best method for the preparation of inclusion complexes [30, 36].
X-ray Diffractometry
The nimodipine cyclodextrins complexes from kneading were evaluated using X-ray diffraction (Fig. 5a, b). The diffractograms of nimodipine exhibited a series of strong and well-defined peaks at 20.3°, 17.3°, and 12.8° (2 ) with peak intensities of 1514, 1574, and 1807, respectively, which are indicative of the crystalline nature of the drug (Fig. 5). Both pure SBE-β-CD and HP-β-CD showed a halo-pattern demonstrating their amorphous states as evidenced from the absence of diffraction peaks in Fig. 5 a and b, respectively. Similar observations have been reported by other authors [37–39]. As shown in Fig. 5, the diffraction patterns of physical mixtures of ND with SBE-β-CD and HP-β-CD correspond to the superposition of those of the pure components, with lower intensities of the diffraction peaks compared with the X-RD pattern of pure ND. The diffractogram of the physical mixture ND-SBE-β-CD shows a higher reduction in peak intensities: 332, 399, and 286, respectively, whereas the ND-HPBCD PM diffractograms presents the typical ND peaks at ∼20.3°, 17.3°, and 12.8° with decreased intensities of 586, 310, and 745, respectively. This could be explained by the particle size reduction during physical mixing [37] and some interaction between ND and SBE-β-CD/HP-β-CD resulting in reduction in crystalline nature of ND [40].
Fig. 5.

a X-ray diffraction analysis of nimodipine (ND), SBE-β-CD, physical mixtures (PM), and kneaded complexes (Kn). b X-ray diffraction analysis of nimodipine (ND), HP-β-CD, physical mixtures (PM), and kneaded complexes (Kn)
As presented in Fig. 5, the diffraction pattern of an inclusion complex clearly differs from that of uncomplexed cyclodextrins when complex formation is indicated [41].
In the kneaded complex formulations, the crystallinity of ND was found to be reduced to a greater extent, in the inclusion complexes of ND with SBE-β-CD and HP-β-CD evidenced by complete disappearance of intense of peaks of ND (Fig. 5a, b). It also differs much from the diffractograms of the corresponding physical mixtures indicating that nimodipine and SBE-β-CD/HP-β-CD form true inclusion complexes in solid state [41, 42]. The peak of high intensity at ∼3.55 (2 ) appearing in some of the diffractograms in both Fig. 5a and b is probably due to diffraction from the planes of the aluminum sample holder. This is a common error incurred in the recording of X-ray diffraction patterns and is not an indication of potential crystalline characteristics of a compound [43].
Fourier-Transform Infrared Spectroscopy
FTIR spectroscopy has been carried out to assess the interaction between drug and cyclodextrin molecules in the solid state. Figure 6 illustrates the FTIR spectra of nimodipine, HP-β-CD, SBE-β-CD, physical mixtures, and complexes from kneading method.
Fig. 6.

a The Fourier transform-infrared (FTIR) spectra of nimodipine, SBE-β-CD; physical mixture, and kneaded complex. b The Fourier transform-infrared (FTIR) spectra of nimodipine, HP-β-CD; physical mixture and kneaded complex
Nimodipine spectra showed the presence of the following main peaks at ∼3297 cm−1 (N–H stretching), ∼3097 cm−1 (C–H aromatic stretching), ∼2933 cm−1 (C–H aliphatic stretching), ∼1695 cm−1 (C = O stretching in ester), ∼1648 cm−1 (C = C stretching), ∼1621 cm−1 (C = C aromatic), ∼1523 cm−1 (−NO2), ∼1381 cm−1 (−C–CH3), and ∼1134 cm−1 (−C–O-ester).
The FTIR spectrum of SBE-β-CD is mainly characterized by intense bands at 3446.4 cm−1 due to the O–H stretching vibration. The band at 2939.4 cm−1 correspond to the C–H stretching, whereas the peaks at 1204.7 and 1040.7 cm−1 are respectively ascribed to C–H and C–O stretching vibrations. C = C–O asymmetric stretching and O–H bending, while that of HP-β-CD (Fig. 6b) shows prominent peaks at 3387 cm−1 (O–H), 2930 cm−1 (C–H), and 1655 cm−1 (H–O–H bending).
The FTIR spectra of both physical mixtures contain all the contributions coming from the nimodipine and SBE-β-CD, HP-β-CD without showing any significant differences. This demonstrates that the drug remains intact and that there is no interaction between the drug and the cyclodextrin. On the contrary, when compared to that of the physical mixture, the spectrum of the inclusion complex exhibits relevant changes in center-frequencies, intensities, and widths of the characteristic absorption peaks, revealing the formation of a new chemical bond between the drug and the cyclodextrin [44].
As shown in Fig. 6, the characteristic absorption band of carbonyl group (C = O) of pure nimodipine, which appears at 1695.4 cm−1, is shifted to a higher frequency and a narrower shape for both inclusion complexes. The curve of the ND-SBE-β-CD complex was moved from 1695.4 to 1696.4 cm−1, while it was shifted from 1695.4 to 1695.7 cm−1 for the ND-HP-β-CD complex. This fact, which has been reported for similar systems [45, 46], has been attributed to the disruption of the strong hydrogen bonds in nimodipine, and their replacement by a less intense association [47].
Moreover, the absorption band at 3331 cm−1 ascribed to stretching vibration of the N–H bond in the dihydropyridine ring was broadened, slightly shifted, or nearly disappeared in inclusion complex spectra (3300.8 and 3302.1 cm−1 for the ND-SBE-β-CD and ND-HP-β-CD, respectively). Similar effects were observed and explained previously [48].
Thus, one can conclude that the functional groups mainly involved in the inclusion process are the N–H and C = O molecular groups. In both cases, the peak maxima are shifted to higher frequencies for the inclusion complex system as compared to the pure nimodipine, which indicate that the nimodipine molecule is included in the SBE-β-CD and HP-β-CD cavity through the dihydropyridine ring. During the complexation process, some water molecules are expelled from the inner part of the CD truncated conus, being demonstrated by the intensity decrease in the high frequency wing 1040.7 cm−1 of the ν(O-H) profile of SBE-β-CD and HP-β-CD spectra.
Molecular Modeling Studies
Molecular structure of the inclusion complex was established using computational modeling technology to visually illustrate the formation of ND-HP-β-CD/ND-SBE-β-CD complexes. The focal benefit of performing Molecular Modeling calculations is to provide some insights on the inclusion process that can support the experimental findings. Based on the lowest energy structure for the inclusion system, the most stable geometry found for the inclusion complexes can be observed in Fig. 7 a and b. It was evident that the drug has been included in the cyclodextrin cavity in a 1:1 M ratio, which is in agreement with the conclusions of the DSC, X-ray, and FTIR studies.
Fig. 7.

a Three-dimensional structures of the least energy structure of ND-HP-β-CD inclusion complex. b Three-dimensional structures of the least energy structure of ND-SBE-β-CD inclusion complex
In Vitro Dissolution Studies
The dissolution curves of ND, physical mixtures and ND–SBE-β-CD, ND-HP-β-CD various binary systems in distilled water, 0.1 N HCl (pH 1.2) and phosphate buffer (pH 7.4), at 37 ± 0.5°C are shown in Fig. 8 a–f.
Fig. 8.
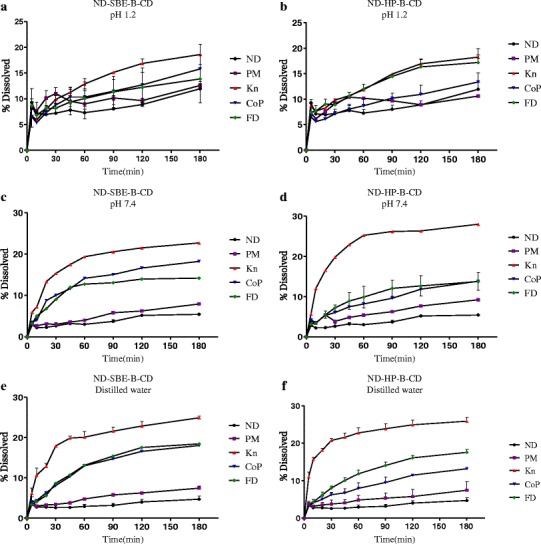
a In vitro dissolution profile of nimodipine-SBE-β-CD inclusion complex (kneading, coprecipitation, and freeze-dried method) and its physical mixture in simulated gastric fluid (pH 1.2). b In vitro dissolution profile of nimodipine-HP-β-CD inclusion complex (kneading, coprecipitation, and freeze-dried method) and its physical mixture in simulated gastric fluid (pH 1.2). c In vitro dissolution profile of nimodipine-SBE-β-CD inclusion complex (kneading, precipitation, and freeze-dried method) and its physical mixture in phosphate buffer (pH 7.4). d In vitro dissolution profile of nimodipine-HP-β-CD inclusion complex (kneading, coprecipitation, and freeze-dried method) and its physical mixture in phosphate buffer (pH 7.4). e In vitro dissolution profile of nimodipine-SBE-β-CD inclusion complex (kneading, precipitation, and freeze-dried method) and its physical mixture in distilled water. f In vitro dissolution profile of nimodipine-HP-β-CD inclusion complex (kneading, coprecipitation, and freeze-dried method) and its physical mixture in distilled water
In all mediums, it was evident that no complete dissolution was achieved for pure nimodipine, only 5% (Fig. 8c–f) and 10% (Fig. 8a, b) even after 180 min, under the specified dissolution conditions. The hydrophobic property of the drug prevented its contact with the dissolution medium causing it to float on the surface, and consequently hindering its dissolution.
Dissolution rates of physical mixtures of both cyclodextrins were slightly higher than that of ND alone (p > 0.05), this improvement can be mainly attributed to the hydrophilic effect of CD which increase the drug solubility in the solid–liquid interface and can reduce the interfacial tension between ND and the dissolution medium, thus leading to a higher dissolution rate [49].
It can be observed (Fig. 8a–f) that dissolution rate was significantly improved by ND complexation with SBE-β-CD and HP-β-CD, showing that all the prepared binary systems exhibit a drug dissolution rate higher than that of the pure ND, in all the mediums. The extent of the enhancement of the dissolution rate was found to be dependent on the preparation method, since physical mixture, coevaporated, and freeze-dried systems showed lowest dissolution rates than the kneaded complex (kneaded > freeze–dried > coevaporated > physical mixture > ND).
To evaluate the effect of cyclodextrin complexation on the dissolution rate of nimodipine, two parameters, dissolution efficiency calculated at 30 and at 180 min (DE30 and DE180), were measured for all products studied (Table I).
Table I.
The Dissolution Parameters (DE30 and DE180) of Pure Nimodipine, Physical Mixtures, Coevaporated, Freeze-Dried, and Kneaded Products in Distilled Water, 0.1 M HCl (pH 1.2) and Phosphate Buffer (pH 7.4) at 37 ± 0.5°C (n = 6)
| Product | Medium | Dissolution efficiency (0–30 min) | Dissolution efficiency (0–180 min) |
|---|---|---|---|
| Nimodipine | DW | 2.93 ± 0.402 | 5.70 ± 1.380 |
| pH 1.2 | 6.42 ± 0.935 | 10.10 ± 0.859 | |
| pH 7.4 | 2.94 ± 0.460 | 5.29 ± 0.020 | |
| Effect of HP-β-CD | |||
| Physical mixture | DW | 4.51 ± 0.080 | 8.71 ± 0.480 |
| pH 1.2 | 8.32 ± 1.710* | 9.75 ± 0.422* | |
| pH 7.4 | 4.67 ± 0.770 | 8.45 ± 1.060 | |
| Co-precipitation | DW | 6.38 ± 1.160 * | 12.37 ± 0.890* |
| pH 1.2 | 6.75 ± 0.475** | 13.92 ± 0.560** | |
| pH 7.4 | 5.70 ± 0.530* | 12.84 ± 1.080* | |
| Freeze-drying | DW | 8.37 ± 0.413** | 17.81 ± 1.126** |
| pH 1.2 | 10.97 ± 1.356*** | 17.69 ± 1.938 *** | |
| pH 7.4 | 7.18 ± 0.760** | 15.58 ± 2.190** | |
| Kneading | DW | 20.12 ± 0.198*** | 27.63 ± 1.058*** |
| pH 1.2 | 9.63 ± 2.530** | 18.27 ± 2.320** | |
| pH 7.4 | 18.18 ± 0.785*** | 27.16 ± 1.495*** | |
| Effect of SBE-β-CD | |||
| Physical mixture | DW | 3.75 ± 1.085 | 7.58 ± 1.955 |
| pH 1.2 | 9.24 ± 1.095** | 11.04 ± 1.275** | |
| pH 7.4 | 3.08 ± 0.250 | 7.10 ± 0.510 | |
| Co-precipitation | DW | 7.20 ± 0.560** | 17.31 ± 0.964** |
| pH 1.2 | 9.66 ± 0.874*** | 15.88 ± 0.840*** | |
| pH 7.4 | 9.43 ± 0.570** | 17.45 ± 0.440** | |
| Freeze-drying | DW | 7.12 ± 0.654** | 17.99 ± 1.120** |
| pH 1.2 | 8.41 ± 0.334*** | 17.21 ± 0.760*** | |
| pH 7.4 | 7.95 ± 0.540** | 14.09 ± 0.990** | |
| Kneading | DW | 15.81 ± 0.675*** | 24.65 ± 0.395*** |
| pH 1.2 | 8.74 ± 0.770** | 19.15 ± 0.680** | |
| pH 7.4 | 14.35 ± 1.120*** | 22.11 ± 0.975*** | |
*p < 0.05; **p < 0.01; ***p < 0.001 versus nimodipine
The kneaded complexes of both SBE-β-CD and HP-β-CD showed higher dissolution rate of the drug compared with the other methods (followed in order by freeze-drying, coprecipitation, and physical mixing Fig. 8b, d and e, f) indicating a better interaction of the drug with CD by the kneading method, as expected from the physicochemical characterization. The HP-β-CD kneading complexes showed rapid dissolution in distilled water (Fig. 8f); about 25% of the drug dissolved in 30 min. The DE30 and DE180 were about seven- and fivefolds, respectively, higher than the ND alone (P < 0.001), whereas the DE30 and DE180 of the SBE-β-CD kneaded complexes were about five- and fourfolds higher than those of ND (p < 0.001) Fig. 8 e and f.
The lower dissolution rates exhibited by the coevaporated and freeze-dried systems in all the mediums could be explained by the partial formation of a true inclusion complex in these methods, as seen in the DSC results.
The enhancement in dissolution of the kneaded complex could be attributed mainly to the formation of soluble inclusion complexes in the solid state with reduction in the crystallinity of ND [50], as confirmed by DSC, FTIR, and X-RD studies. The interactions between the hydrophobic part of the guest and the apolar cavity causes dehydration of the hydrophobic guest molecule and its transfer into the cavity, thereby increasing the affinity toward water and hence increasing the dissolution [51].
The highest dissolution rate exhibited by the kneaded complexes comparing to the other methods could be due to greater hydrophilicity, higher wetting effect, decrease of drug crystallinity obtained with the sample mechanical treatment, which increased the drug–carrier contact surface and ability to form stable inclusion complex of the SBE-β-CD and HP-β-CD [26]. This suggests that the enhancement of the dissolution rate is dependent on the preparation method since coevaporated and freeze-dried complexes showed lowest dissolution rates.
Although a higher solubility of ND is reached using SBE-β-CD, the increment in drug dissolution from ND–HP-β-CD systems was slightly higher than the corresponding ones with SBE-β-CD. Similar observations have been reported by other authors with no explanation provided [52, 53]. However, this phenomenon could be attributed to the difference in water volume used in the phase solubility and dissolution studies. Since the inclusion complex of ND-β-CD is actually a mixture of both, inclusion complex and free ND, CDs [30], the hydrophilic effect of both SBE-β-CD and HP-β-CD on ND solubility is more pronounced in small volume due to the encircling of ND by uncomplexed CDs forming drug-CD aggregates which reduce the interfacial tension between ND and water, thus leading to a higher solubility. In the other hand, when large water volume is used such as in dissolution studies, this phenomenon is unlikely to occur since nimodipine floats on the surface, hindering its contact with CDs, and therefore its dissolution.
CONCLUSION
In this work, inclusion complexes of nimodipine with SBE-β-CD and HP-β-CD have been successfully prepared. The solubility of ND in water was significantly increased in an average of 22- and 8-fold for SBE-β-CD and HP-β-CD, respectively. Results showed that the kneading method yielded the higher degree of amorphous entities suggesting the formation of true inclusion complexes between ND and SBE-β-CD/HP-β-CD. Phase solubility studies indicated stable inclusion complexes with association rate constants (K1:1) of 1410.6 and 495.1 M−1 for SBE-β-CD and HP-β-CD, respectively. In all mediums (0.1 N HCl pH 1.2, phosphate buffer pH 7.4, and distilled water), dissolution profiles showed that all the products exhibit higher dissolution rate than those of the physical mixtures and ND alone. The dissolution of ND was substantially higher for SBE-β-CD and HP-β-CD inclusion complexes prepared by the kneading method. Thus, the method of preparing complexes played a key role in enhancing the dissolution rate. No significant difference was found between the two cyclodextrins at p < 0.05. Therefore, the kneaded system of ND with SBE-β-CD prepared at a molar ratio of 1:1 could be chosen for the formulation of nimodipine tablets for better therapeutic efficacy due to the better solubilization capacity of SBE-β-CD, compared to HP-β-CD.
Acknowledgments
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Langley M, Sorkin EM. Nimodipine: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in cerebrovascular disease. Drugs. 1989;37:669–99. doi: 10.2165/00003495-198937050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Mück W, Bode H. Bioanalytics of nimodipine—an overview of methods. Pharmazie. 1994;49:130–9. [PubMed] [Google Scholar]
- 3.Reynolds Martindale JEF. The extra pharmacopeia (31sted) London: Royal Pharmaceutical Society of Great Britain; 1996. [Google Scholar]
- 4.Mielcarek J. Analytical study of photodegradation of inclusion complexes of nimodipine with alpha-, gamma-cyclodextrin, methyl-beta-cyclodextrin, and hydroxypropyl-beta-cyclodextrin. Drug Dev Ind Pharm. 1998;24(2):197–200. doi: 10.3109/03639049809085608. [DOI] [PubMed] [Google Scholar]
- 5.Eeckhant AV, Robert M, Michotte Y. Separation of neutral dihydropyridines and their enantiomers using electrokinetic chromatography. J Pharmacent Biomed. 2004;36(4):799–805. doi: 10.1016/j.jpba.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Kopecky F, Kopecka B, Kaclik P. Solubility study of nimodipine inclusion complexation with alpha- and beta-cyclodextrin and betasome substituted cyclodextrins. J Incl Phenom Macro. 2001;39(3–4):215–7. doi: 10.1023/A:1011155208944. [DOI] [Google Scholar]
- 7.Vandelli MA, Salvioli G, Mucci A, Panini R, Malmusi L, Forni EF. 2-hydroxypropyl-b-cyclodextrin complexation with ursodeoxycholic acid. Int J Pharm. 1995;118:77–83. doi: 10.1016/0378-5173(94)00342-3. [DOI] [PubMed] [Google Scholar]
- 8.Takayama K, Nambu N, Nagai T. Pharmaceutical interactions in dosage forms and processing. XIX. Dissolution kinetics for coprecipitates of indomethacin with polyvinylpyrrolidone. Chem Pharm Bull. 1980;28:3304–9. doi: 10.1248/cpb.28.3304. [DOI] [Google Scholar]
- 9.Archontaki HA, Vertzoni MV, Athanassiou-Malaki MH. Study on the inclusion complexes of bromazepam with β-and β-hydroxypropyl-cyclodextrins. J Pharm Biomed Anal. 2002;28:761–9. doi: 10.1016/S0731-7085(01)00679-3. [DOI] [PubMed] [Google Scholar]
- 10.Patel RPM, Shah D. Application of cyclodextrin in drug delivery. Int J of Pharm Wor Res. 2010;1(2):1–21. [Google Scholar]
- 11.Jain AS, Date AA, […], Nagarsenker MS. Sulfobutyl Ether7 β-cyclodextrin (SBE7 β-CD) carbamazepine complex: preparation, characterization, molecular modeling, and evaluation of in vivo anti-epileptic activity. AAPS Pharm Sci Tech. 2011; December; 12 (4):1163–1175. [DOI] [PMC free article] [PubMed]
- 12.Lockwood SF, O’Malley S, Mosher GL. Improved aqueous solubility of crystalline astaxanthin (3,3′-dihydroxy-β, β-carotene-4,4′-dione) by captisol® (sulfobutyl ether β-cyclodextrin) J Pharm Sci. 2003;92:922–6. doi: 10.1002/jps.10359. [DOI] [PubMed] [Google Scholar]
- 13.Rajewski RA, Traiger G, Bresnahan J, Jaberaboansari P, Stella VJ, Thompson DO. Preliminary safety evaluation of parenterally administered sulfoalkyl ether cyclodextrin derivatives. J Pharm Sci. 1995;84:927–32. doi: 10.1002/jps.2600840805. [DOI] [PubMed] [Google Scholar]
- 14.Okimoto K, Rajewski RA, Uekama K, Jona JA, Stella VJ. The interaction of charged and uncharged drugs with neutral (HP-beta-CD) and anionically charged (SBE7-beta-CD) beta-cyclodextrins. Pharm Res. 1996;13:256–64. doi: 10.1023/A:1016047215907. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Yang R, Tang X, Zheng L. Part I: characterization of solid dispersions of nimodipine prepared by hot-melt extrusion. Drug Dev Ind Pharm. 2008;34(2):232–3. doi: 10.1080/03639040801938118. [DOI] [PubMed] [Google Scholar]
- 16.Guissi N, Semcheddine F, Wang B. Preparation and characterization of low crystalline and more swollen nimodipine loaded nanostructured lipid carriers and solid lipid nanoparticles in the presence of electrolytes. Int. J. Res. Pharm. Bio. Sci. 2013; 683–691.
- 17.František K, Božena K, Pavol K. Solubility study of nimodipine inclusion complexation with α- and β-cyclodextrin and some substituted cyclodextrins. J Incl Phenom Macro. 2001;39:215–7.
- 18.Yang X, Ke W, Zi P, Liu F, Yu L. Detecting and identifying the complexation of nimodipine with hydroxypropyl-beta-cyclodextrin present in tablets by Raman spectroscopy. J Pharm Sci. 2008;97(7):2702–19. doi: 10.1002/jps.21204. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida A, Yamamoto M, Itoh T, Irie T, Hirayama F, Uekama K. Utility of 2-hydroxypropyl-beta-cyclodextrin in an intramuscular injectable preparation of nimodipine. Chem Pharm Bull (Tokyo) 1990;38(1):176–9. doi: 10.1248/cpb.38.176. [DOI] [PubMed] [Google Scholar]
- 20.Mura P, Faucci MT, Parrini PL, Furlanetto S, Pinzauti S. Influence of the preparation method on the physicochemical properties of ketoprofen-cyclodextrin binary systems. Int J Pharm. 1999;179:117–28. doi: 10.1016/S0378-5173(98)00390-1. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi T, Connors A. Phase-solubility techniques. In: Advances in analytical chemistry instrumentation. New York, NY: Wiley Interscience; 1965; 117–211.
- 22.Zhang H, et al. Molecular modeling-based inclusion mechanism and stability studies of doxycycline and hydroxypropyl-β-cyclodextrin complex for ophthalmic delivery. AAPS Pharm Sci Tech. 2013;14(1):10–8. doi: 10.1208/s12249-012-9877-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jug M, Mennini N, Melani F, Maestrelli F, Mura P. Phase solubility, 1H NMR and molecular modelling studies of bupivacaine hydrochloride complexation with different cyclodextrin derivates. Chem Phys Lett. 2010;500(4–6):347–54. doi: 10.1016/j.cplett.2010.10.046. [DOI] [Google Scholar]
- 24.Fernandes CM, Vieira MT, Veiga FJB. Physicochemical characterization and in vitro dissolution behaviour of nicardipine-cyclodextrins inclusion compounds. Eur J Pharm Sci. 2002;15:79–88. doi: 10.1016/S0928-0987(01)00208-1. [DOI] [PubMed] [Google Scholar]
- 25.Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 26.Carrier RL, Miller LA, Ahmed I. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123:78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed MO. Comparison of impact of the different hydrophilic carriers on the properties of piperazine-containing drug. Eur J Pharm Biopharm. 2001;51:221–6. doi: 10.1016/S0939-6411(01)00128-X. [DOI] [PubMed] [Google Scholar]
- 28.De-Chasteign S, Fessi H, Davissaguet JP, Puisieusx F. Comparative study of the association of Itraconazole with colloidal drug carrier. Drug Dev Res. 1996;38(2):125–33. doi: 10.1002/(SICI)1098-2299(199606)38:2<125::AID-DDR7>3.0.CO;2-L. [DOI] [Google Scholar]
- 29.Nagase Y, Hirata M, Wada K, Arima H, Hirayama F, Irie T, et al. Improvement of some pharmaceutical properties of DY-9760e by sulfobutyl ether β-cyclodextrin. Int J Pharm. 2001;229(1–2):163–72. doi: 10.1016/S0378-5173(01)00851-1. [DOI] [PubMed] [Google Scholar]
- 30.Yanez C, Canete-Rosales P, Castillo JP, Catalan N, Undabeytia T, Morillo E. Cyclodextrin inclusion complex to improve physicochemical properties of herbicide bentazon: exploring better formulations. PLoS One. 2012;7(8):e41072. doi: 10.1371/journal.pone.0041072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y, Haworth IS, Zuo Z, Chow MS, Chow AH. Physicochemical and structural characterization of quercetin-b-cyclodextrin complexes. J Pharm Sci. 2005;94(5):1079–89. doi: 10.1002/jps.20325. [DOI] [PubMed] [Google Scholar]
- 32.Zhou YH, Zhang GM, Wang ZR, Wang HY, Dong C, MinShuang S. The interaction of piroxicam with neutral (HP-β-CD) and anionically charged (SBE-β-CD) β-cyclodextrin. J Incl Phenom Macro Chem. 2006;56:215–20. doi: 10.1007/s10847-006-9086-1. [DOI] [Google Scholar]
- 33.Jiang H, Li D, Shanshan Y. Synthesis and characterization of β-cyclodextrin inclusion complex containing di(8-hydroxyquinoline) magnesium. Spectrochim Acta A. 2008;70:878–83. doi: 10.1016/j.saa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Jain AS, Date AA, Pissurlenkar RR, Coutinho EC, Nagarsenker MS. Sulfobutyl Ether7 β-cyclodextrin (SBE7 β-CD) carbamazepine complex: Preparation, characterization, molecular modeling, and evaluation of in vivo anti-epileptic activity. AAPS Pharm Sci Tech. 2011;12(4):1163–75. doi: 10.1208/s12249-011-9685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiremath SN, Bharti N, Swamy PV, Raju SA. Improved dissolution rate of valdecoxib inclusion complexes with hydroxypropyl-β-cyclodextrin. Ind J Pharm Sci. 2007;69(3):442–5. doi: 10.4103/0250-474X.34559. [DOI] [Google Scholar]
- 36.Danciu C, et al. Genistein in 1:1 inclusion complexes with ramified cyclodextrins: theoretical, physicochemical and biological evaluation. Int J Mol Sci. 2014;15(2):1962–82. doi: 10.3390/ijms15021962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro LSS, Ferreira DC, Veiga FJB. Physicochemical investigation of the effects of water-soluble polymers on vinpocetine complexation with b cyclodextrin and its sulfobutyl ether derivative in solution and solid state. Eur J Pharm Sci. 2003;20:253–66. doi: 10.1016/S0928-0987(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 38.Bayomi MA, Abanumany KA, Al-Angary AA. Effect of inclusion complexation with cyclodextrins on photostability of nifedipine in solid state. Int J Pharm. 2002;243:107–17. doi: 10.1016/S0378-5173(02)00263-6. [DOI] [PubMed] [Google Scholar]
- 39.Manosroi J, Apriyani MG, Foe K, Manosroi A. Enhancement of the release of azaleic acid through the synthetic membranes by inclusion complex formation with hydroxypropyl-b-cyclodextrin. Int J Pharm. 2005;293:235–40. doi: 10.1016/j.ijpharm.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Bekers O, Uijtendaal EV, Beijnen JH, Bult A, Underberg WJM. Cyclodextrins in the pharmaceutical field. Drug Dev Ind Pharm. 1991;17:1503–49. doi: 10.3109/03639049109026630. [DOI] [Google Scholar]
- 41.Williams RO, Mahaguna V, Sriwongjanya M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-b-cyclodextrin. Eur J Pharm Biopharm. 1998;46:355–60. doi: 10.1016/S0939-6411(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 42.Batt DK, Garala KC. Preparation and evaluation of inclusion complexes of diacerein with β-cyclodextrin and hydroxypropyl β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2013;77(1–4):471–81. doi: 10.1007/s10847-012-0268-8. [DOI] [Google Scholar]
- 43.Spamer E, Mullera DG, Wessels PL, Venter JP. Characterization of the complexes of furosemide with 2-hydroxypropyl-b-cyclodextrin and sulfobutyl ether-7-b-cyclodextrin. Eur J Pharm Sci. 2002;16:247–53. doi: 10.1016/S0928-0987(02)00107-0. [DOI] [PubMed] [Google Scholar]
- 44.Cannavà C, Crupi V, Guardo M, Majolino D, Stancanelli R, Tommasini S, et al. Phase-solubility and FTIR-ATR studies of idebenone/sulfobutyl ether beta-cyclodextrin inclusion complex. J Incl Phenom Macrocycl Chem. 2013;75:255–66. doi: 10.1007/s10847-012-0110-3. [DOI] [Google Scholar]
- 45.García-Zubiri I, González-Gaitano G, Sánchez M, Isasi JR. FTIR study of dibenzofuran-2-carboxylic acid and its complexes with β-cyclodextrin. Vib Spectrosc. 2003;33:205–13. doi: 10.1016/j.vibspec.2003.09.002. [DOI] [Google Scholar]
- 46.Zaibunnisa et al. Characterization of cyclodextrin complexes with turmeric oleoresin. Food Chemistry 114 (2009) 459–465.
- 47.Gines JM, Pérez-Martínez JI, Arias MJ, Moyano JR, Morillo E, Ruiz-Conde A, et al. Inclusion of the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) with beta- cyclodextrin by different processing methods. Chemosphere. 1996;33:321–34. doi: 10.1016/0045-6535(96)00175-0. [DOI] [Google Scholar]
- 48.Bongiorno D. et al. Inclusion complexes of cyclomaltooligosaccharides (cyclodextrins) with melatonin in solid phase. ARKIVOC 2005 (xiv) 118–130.
- 49.Corrigan OI, Stanley CT. Mechanism of drug dissolution rate enhancement from beta-cyclodextrin-drug systems. J Pharm Pharmacol. 1982;34:621–6. doi: 10.1111/j.2042-7158.1982.tb04689.x. [DOI] [PubMed] [Google Scholar]
- 50.Dollo G, Corre P, Chollet M, Chevanne F, Bertault M, Burgot J, et al. Improvement in solubility and dissolution rate of 1,2-dithiole-3-thiones upon complexation with b-cyclodextrin and its hydroxypropyl and sulfobutyl ether-7 derivatives. J Pharm Sci. 1999;88:889–95. doi: 10.1021/js990067o. [DOI] [PubMed] [Google Scholar]
- 51.Rawat S, Jain SK. Enhancement of intestinal absorption of few COX-2 inhibitors through interaction with β-cyclodextrin. Ind J Pharm Sci. 2007;69:529–34. doi: 10.4103/0250-474X.36939. [DOI] [Google Scholar]
- 52.Sathigari S, et al. Physicochemical characterization of Efavirenz–cyclodextrin inclusion complexes. AAPS PharmSciTech. 2009;10(1):81–7. doi: 10.1208/s12249-008-9180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dollo G, Corre P, Chollet M, Chevanne F, Bertault M, Burgot JL, et al. Improvement in solubility and dissolution rate of 1, 2-dithiole-3-thiones upon complexation with beta-cyclodextrin and its hydroxypropyl and sulfobutyl ether-7 derivatives. J Pharm Sci. 1999;88:889–95. doi: 10.1021/js990067o. [DOI] [PubMed] [Google Scholar]


