Abstract
The effective management of leachables in pharmaceutical products is a critical aspect of their development. This can be facilitated if extractables information on the materials used in a packaging or delivery system is available to assist companies in selecting materials that will be compatible with the drug product formulation and suitable for the intended use. The Extractables and Leachables Safety Information Exchange (ELSIE) materials working group developed and executed a comprehensive extraction study protocol that included a number of extraction solvents, extraction techniques, and a variety of analytical techniques. This was performed on two test materials, polyethylene (PE) and polyvinyl chloride (PVC), that were selected due to their common use in pharmaceutical packaging. The purpose of the study was to investigate if the protocol could be simplified such that (i) a reduced number or even a single extraction technique could be used and (ii) a reduced number of solvents could be used to obtain information that is useful for material selection regardless of product type. Results indicate that, at least for the PVC, such reductions are feasible. Additionally, the studies indicate that levels of extractable elemental impurities in the two test materials were low and further confirm the importance of using orthogonal analytical detection techniques to gain adequate understanding of extraction profiles.
KEY WORDS: extractables, extraction technique, polyethylene, polyvinyl chloride
INTRODUCTION
Plastic components used in packaging or delivery systems for pharmaceutical products must be suitable for their intended use. They should function properly: adequately protect the pharmaceutical product over the product’s shelf-life and/or mechanically perform as intended to administer the product to the patient. They should also be compatible with the dosage form and should be composed of materials that are safe for use. Chemical evaluation of plastic materials can be utilized to evaluate safety and compatibility. The modern practice of leachables and extractables evaluation can readily facilitate such an evaluation.
Extractables are chemicals derived from container closure systems (CCS), packaging, and/or device components under laboratory conditions; leachables are chemicals derived from CCS and/or device components that are part of the final drug product under normal storage and patient-use conditions (1). Extractables are potential leachables and thus have only a potential impact on the patient, whereas leachables may have an actual impact on the patient. The effective management of contact material-derived leachables in the packaged product is a critical aspect in the development of many pharmaceutical products, e.g., parenterals, injectables, ophthalmics, and inhalation and nasal products. There is strong impetus from several regulatory agencies, encouraging management of leachables in a drug product, which has resulted in a number of regulatory and industry guidance documents that address expectations for extractables and leachables management (2–13).
Part of an effective process for managing leachables includes obtaining extractables information that can be used to assist in the selection of container closure system and/or delivery system materials to be used for a final drug product (14–16). Extractables are most often associated with the chemical composition of the materials; however, a comprehensive approach also involves understanding of any chemical additives or processing aids that may be used during material or component fabrication. The pharmaceutical industry has pursued several avenues to obtain comprehensive chemical composition information on the ingredients and processes that are used to fabricate materials and components that are employed to construct packaging and delivery systems for pharmaceutical products that include requesting this information from suppliers, asking suppliers if they would perform the testing, or initiating independent extraction studies on materials or leachable studies for products. Most often the burden of acquiring this information has resulted in the pharmaceutical manufacturer sponsoring testing. A collaborative effort to ease this burden was initiated by the member companies in the Extractables and Leachables Safety Information Exchange (ELSIE).
The ELSIE materials working group developed an extraction studies protocol for the purpose of generating extractables data from plastic materials, which could be used by member companies as part of an overall strategy for drug product materials selection (17). General concepts from the efforts of the Product Quality Research Institute (PQRI) orally inhaled and nasal drug products (OINDP) recommendations, specifically those related to chemical analysis best practices, served as a starting point for the protocol (18). The ELSIE protocol therefore included the use of multiple solvents, extraction, and analytical techniques. ELSIE then performed a feasibility study on the protocol by applying it to two materials, polyethylene (PE) and a plasticized polyvinyl chloride (PVC), that were selected due to the material classes’ widespread and common use in pharmaceutical packaging. As part of a broader ELSIE pilot materials program, the ELSIE protocol was also applied to the processed forms of the two materials. Several laboratories that are known providers of extractables and leachables services to the pharmaceutical industry performed the experimental work.
The purpose of the studies discussed here was to investigate if the protocol could be simplified such that (i) a reduced number, or even a single extraction technique, could be used to obtain information useful for materials selection; and (ii) a reduced number of solvents could be used to obtain information useful for materials selection. It was expected that the protocol, when successfully accomplished, would provide qualitative and semiquantitative information on extractables that are consistent with the identity of the material of construction and the material’s additive composition. Generally, material selection, when possible and relevant, would also include information from the supplier regarding processing agents, washing conditions, etc., which would also be analytes investigated in extraction studies. However, the ELSIE pilot program did not use industrially fabricated parts or components, but rather the resin (i.e., the material of construction) and the resin processed via a known protocol (with no processing agents). Therefore, the effect of processing on the extractables profile that resulted from mechanical and thermal stress was evaluated separately using the same testing protocol and has been discussed elsewhere. (19) We describe here the results of the extraction techniques and solvents investigation based on a comparison of known material chemical composition and experimentally observed chemical profiles of the material extracts. Although other such comparisons have been published in the literature, this set of studies describes a minimal set of experiments necessary to obtain the information needed for material selection early in the pharmaceutical development process.
EXPERIMENTAL
Materials
The two materials studied were chosen because they are commonly used, as a class, in pharmaceutical applications. One test material was a commercially available low-density polyethylene (LDPE), specifically Bormed™ LE6601-PH (Borealis AG). This low-density polyethylene is produced additive-free in a high-pressure process and is intended for blow molding of soft and flexible packaging for pharmaceutical products that do not require sterilization at temperatures above 105°C. The other test material was a plasticized polyvinylchloride (PVC) formulation, specifically Teknor 09-X0016A-78NT, Clear (Teknor Apex Company). The general composition of this PVC (including additives) is listed in Table I. The materials were tested as received from the material manufacturer or subjected to processing as described elsewhere. (19)
Table I.
Formulation for PVC—Teknor 09-X0016A-78NT Clear. Information Provided to ELSIE from the Material Supplier
| Chemical name | CAS no. | Percentage (w/w) | Function |
|---|---|---|---|
| Polyvinyl chloride | 9002-86-2 | 61 | Base polymer |
| Di-ethylhexyl phthalate (DEHP); also known as di-octyl phthalate (DOP)a | 117-81-7 | 30 | Plasticizer |
| Epoxidized soybean oil (ESBO) | 8013-07-8 | 7 | Plasticizer; acid scavenger (for HCl from PVC) |
| (Z)-13-Docosenamide (erucamide) | 112-84-5 | 1 | Lubricant, slip agent |
| Ca stearate (octadecanoic acid, calcium salt) | 1592-23-0 | 0.5 | Lubricant, acid scavenger |
| Zn stearate (octadecanoic acid, zinc salt) | 557-05-1 | 0.5 | Lubricant, acid scavenger |
aThere are a range of such phthalates that include diethylhexyl phthalate (DEHP), di-n-octyl phthalate (DOP), and di-isooctyl phthalate. These closely related structural isomers are often referred to generically as di-octyl phthalates
Extraction
Both processed and unprocessed forms of these materials were subject to extraction by a range of different extraction techniques as detailed in Table II with extraction solvents listed in Table III. The extraction techniques and methods used in the protocol included those that are commonly used with traditional lab equipment: sonication, reflux, Soxhlet, and sealed vessel; and others that require specific instrumentation: pressurized solvent extraction, microwave, headspace sampling. In some instances, it was necessary for the performing laboratory to use conditions that were slightly different than those specified in the protocol. Where this was required to maintain consistent practices in the lab, the changes were fully reviewed and their potential impact assessed. Changes were only approved where it was concluded that there was no risk of the change impacting deleteriously on the study outcomes. The actual ranges of extraction conditions used are listed in Table II.
Table II.
Overview of Extraction Techniques and Parameters
| Method | Equipment | Extraction parameters |
|---|---|---|
| Sonication | General ultrasonic bath; calibrated thermometer | Approximately 2–7.5 g of sample sonicated in 20–100 mL of solvent for 1–8 h (the bath temperature was maintained at 40°C or less for organic solvents) |
| Soxhlet | Soxhlet extractor | Approximately 2 g of sample extracted in 100–150 mL of each solvent for 24 h; turnover number of at least 10 volumes |
| Reflux | General reflux apparatus, e.g., round bottom flask (at least 200 mL), condenser, hot plate/mantle | Approximately 1.5–10 g of sample extracted in 15–100 mL of each solvent for 6–24 h with constant stirring |
| Sealed container | Teflon or Pyrex containers, water bath, autoclave | Approximately 1 g per 13–15 mL solvent added to the vessel, mixed and closed tightly. Vessels were then placed in a water bath or autoclave. For IPA solvent mixtures, the bath temperature was at 70°C, for hexane 50°C, for DCM 30°C. Water extractions were performed in an autoclave at 121°C. Incubation times varied from 1.5 to 72 h |
| Microwave | Microwave system, e.g., MARS with Teflon or glass reaction vessels | The amount of 1 g of sample in 15–20 mL of solvent was extracted by microwave for either 1–6 h with temperature set points in the range of 80–110°C. |
| Accelerated solvent extraction (ASE)—a specific form of pressurized solvent extraction | Commercially available instrument, e.g., ASE™ apparatus with 60 mL I-Chem amber collection vials | Approximately 1–2.5 g of sample was weighed into a cellulose thimble, which was placed into a stainless steel extraction cell containing a cellulose filter. The pressure was set at 1500 psi, and temperature was set at 100°C with a total extraction time of 30–90 min. The extract volume was approximately 25–50 mL |
| Headspace sampling | Commercially available headspace unit, e.g., Agilent G1888 Network Headspace Sampler | Approximately 1 g of sample was weighed into a 20-mL headspace autosampler vial, which was then sealed. The headspace oven temperature was set at 80°C with an equilibration time of 30 min |
Table III.
Extraction Solvents and Analytical Techniques Used in the Extraction Studies
| Solvents | Water at pH 2.5 (HCl/KCl mixture) |
| Water at pH 9.5 (phosphate buffer) | |
| Water | |
| Isopropyl alcohol (IPA) to water (1:1) | |
| IPA (spectroscopic grade) | |
| Dichloromethane (DCM) (spectroscopic grade) | |
| Hexane or Iso-hexane (spectroscopic grade) | |
| Analytical techniques | Gas chromatography/mass spectrometry (GC/MS) |
| Gas chromatography/flame ionization detector (GC/FID) | |
| Liquid chromatography/mass spectrometry (LC/MS) | |
| Liquid chromatography/ UV detection (LC/UV) | |
| Inductively coupled plasma/mass spectrometry (ICP/MS) |
Extract Analysis
The resultant extracts and their associated extraction blanks were screened for extractable organic substances by gas chromatography with flame ionization and mass spectrometric detection (GC/FID/MS) and liquid chromatography with ultraviolet absorbance and mass spectrometric detection (LC/UV/MS). The analytical systems and test procedures used were consistent with current practices in extractables and leachables assessment. For the GC analysis, extracts were prepared for analysis, as necessary, by solvent switching and evaporative concentration while the LC analyses were generally performed with no extract processing. ELSIE asked labs to attempt to identify all peaks above 1 ppm (ppm = μg/g) through library searches and to list the top 3–10 closest matches with the quality of match for each peak. The limit of 1 ppm (based on the polymer mass) was chosen as a lower limit to accommodate realistic detection limits and because drug product parameters and configurations such as dosing regimen were not available. All peaks at a concentration of greater than 1 μg of analyte per gram of material (resin or processed material) were to be confidently identified.
Sample Preparation for Elemental Impurities Analysis
After extraction, organic solvents were evaporated, and the nonvolatile residue reconstituted in water with added nitric acid. For aqueous solutions, nitric acid was added directly to the extract preparation. Solutions that appeared turbid were microwave digested before analysis. One CRO used gold to stabilize any mercury that might potentially be present.
Elemental Analyses
Samples were analyzed by inductively coupled mass spectrometry (ICP/MS) using a collision cell which removed interfering diatomics such as argon chloride. Results reported are the ICH Q3D metals as well as for zinc and calcium (potentially present in the form of their stearate salt which have been intentionally added to PVC as lubricants) (20).
Data were collected for some other elements (e.g., Fe, Si, B, Na, K); however, these elements are not of toxicological concern and hence are not reported here. Results reported are for both unprocessed PE and PVC.
RESULTS
Extraction Technique
In order to evaluate the influence of extraction technique specifically, the data presented focuses on two specific solvents, isopropanol (IPA) and hexane, on the PVC material. Additional results (water pH 9.5) are presented where relevant.
A summary of the results for extraction studies performed on PVC are shown in Tables IV, V, and VI. The results are presented in terms of the semiquantitative levels observed as well as the expected (forecast) extractable compounds, based on the known composition of the PVC material, extraction solvent, and analytical technique used.
Table IV.
Extraction Results for PVC in IPA (GC/MS). Concentrations (g/g) are Reported Based on a Response Factor from a Surrogate Standard
| Compound | Forecast | Reflux (lab 1) | Soxhlet (labs 2 and 3) | Microwave (lab 4) | Sealed container (lab 4) |
|---|---|---|---|---|---|
| Di-2-ethylhexyl phthalate (DEHP) | High | >20,000 | >1000 | >400 | >400 |
| (Z)-13-Docosenamide (erucamide) | Moderate | >1000 | >100 | >1000* | >1000* |
| Oleamide, palmitamide, docosanamide, etc. | Low | 1–110 | <10 | ND | ND |
| 2-Ethyl-1-hexanol | Low | <20 | ND | ND | ND |
| Phthalic acid | Low | <2 | ND | ND | ND |
| Mono-2-ethylhexyl phthalate (MEHP) | Low | ND | ND | ND | ND |
| Epoxidized fatty acids | Low | ND | ND | ND | ND |
| Epoxidized soy bean oils | Low | ND | ND | ND | ND |
| Fatty acids | Low | <25 | <400 | ND | ND |
ND not detected
Table V.
Extraction Results for PVC in Hexane (GC/MS). Concentrations (g/g) are Reported Based on a Response Factor from a Surrogate Standard
| Compound | Forecast | Reflux (lab 1) | Soxhlet (labs 2 and 3) | Microwave (lab 4) | Sealed container (lab 4) |
|---|---|---|---|---|---|
| Di-2-ethylhexyl phthalate (DEHP) | High | >400 | >1000 | >200 | >200 |
| (Z)-13-Docosenamide (erucamide) | Moderate | >1000 | >1000 | >1000* | >1000* |
| Oleamide, palmitamide, docosanamide, etc. | Low | 1–110 | <10 | ND | ND |
| 2-Ethyl-1-hexanol | Low | <20 | <20 | >100 | ND |
| Phthalic acid | Low | <2 | ND | ND | ND |
| Mono-2-ethylhexyl phthalate (MEHP) | Low | ND | ND | ND | ND |
| Epoxidized fatty acids | Low | ND | ND | ND | ND |
| Epoxidized soy bean oils | Low | ND | ND | ND | ND |
| Fatty acids | Low | <2 | ND | ND | ND |
ND not detected
Table VI.
Extraction Results for Unprocessed PVC in Water pH 9.5 (GC/MS). Concentrations (g/g) are Reported Based on a Response Factor from a Surrogate Standard
| Compound | Water (pH 9.5) | ||
|---|---|---|---|
| Forecast | Reflux (lab 1) | Soxhlet (lab 3) | |
| Di-2-ethylhexyl phthalate (DEHP) | Moderate | 40 | <5 |
| (Z)-13-Docosenamide (erucamide) | Low | 3.9 | <5 |
| Oleamide, palmitamide, docosanamide, etc. | Low | ND | ND |
| 2-Ethyl-1-hexanol | Low | 4.7 | ND |
| Phthalic acid | Low | 4.3 | ND |
| Mono-2-ethylhexyl phthalate (MEHP) | Low | ND | ND |
| Epoxidized fatty acids | Low | ND | ND |
| Epoxidized fatty acid esters | Low | ND | ND |
| Fatty acids | Low | 0.46–3.7 | <1 |
ND not detected
Detailed Extraction Profile
In addition to the general profiles obtained across the different extraction techniques, detailed extraction profiles were specifically obtained for PVC (both processed and unprocessed) under reflux. These profiles are described in Tables VII and VIII and Figs. 1 and 2 and include data obtained from a range of techniques, including GC/MS, headspace GC/MS, and LC/DAD/MS. Compounds reported from the extracts assessed via LC/DAD/MS were identified as epoxidized soybean oil (ESBO) related via mass spectral information.
Table VII.
Reflux Extractables Levels in Unprocessed PVC (GC/MS). All are Identified Compounds Against Retention Time and MS Library or Analytical Standard, Except for Starred Entries. Concentrations (g/g) are Reported Based on a Response Factor from a Surrogate Standard
| Identified compounds | CAS no. | Reflux with IPA | Reflux with hexane | Reflux with pH 9.5 |
|---|---|---|---|---|
| 6–10 h | 6–10 h | 24 h | ||
| Di-2-ethylhexyl phthalate (DEHP) | 117-81-7 | 67,000 | 56,000 | 40 |
| (Z)-13-Docosenamide (erucamide) | 112-84-5 | 4300 | 820 | 3.9 |
| Tetradecanoic acid (myristic acid) | 544-63-8 | ND | ND | 1.3 |
| Hexadecanoic acid (palmitic acid) | 57-10-3 | 21 | NDc | 3.7 |
| 2-ethyl hexanol | 104-76-7 | 17 | 7.5 | 4.7 |
| Nonanal | 124-19-6 | 1.6 | 1.6 | NDd |
| Phthalic acid | 88-99-3 | 1 | 1.3 | 4.3 |
| 2,6-bis(1,1-dimethylethyl)-4-methyl-phenol (BHT) | 128-37-0 | 17 | 15 | ND |
| Benzoic acid, 2-ethylhexyl estera | 5444-75-7 | 27 | 27 | ND |
| 2-Pentadecanone, 6,10,14-trimethyl- b | 502-69-2 | 1.8 | 1.6 | ND |
| Isopropyl palmitate | 142-91-6 | 51 | ND | ND |
| Methyl palmitate | 112-39-0 | ND | 1.9 | ND |
| iso-Propyl octadecanoatea | 112-10-7 | 21 | ND | ND |
| Tributylacetylcitrate | 77-90-7 | ND | 4.8 | ND |
| Stearic acid, 9,10-epoxy-, isopropyl esterb | 95007-80-0 | 70 | ND | ND |
| Docosanamide | 3061-75-4 | 110 | 23 | ND |
| -Sitosterol acetate | 915-05-9 | 5.1 | 4.6 | ND |
| Tris(2-ethylhexyl) trimellitate | 3319-31-1 | 9.6 | 5.4 | ND |
| Hexanoic acid | 142-62-1 | ND | ND | 0.32 |
| Nonanoic acid | 112-05-0 | ND | ND | 1.2 |
| Dodecanoic acid | 143-07-7 | ND | ND | 0.46 |
| Octadecanoic acid (stearic acid) | 57-11-4 | ND | ND | 0.82 |
aMost probable compound—mass spectrum matches a library or literature spectrum and the presence of the compound is confirmed by data provided by the sponsor or material supplier
bTentatively identified compound—data have been obtained that are consistent with a class of molecule only. Or, the mass spectrum matches a library or literature spectrum
cMethyl palmitate detected in profile
dNonanoic acid detected in profile
ND not detected
Table VIII.
Volatile Compounds—Headspace GC/MS
| Compound | CAS no. | Amount (μg/g) | |
|---|---|---|---|
| Processed PVC | Unprocessed PVC | ||
| Butyraldehyde | 123-72-8 | 0.54 | ND |
| 2-Butanone | 78-93-3 | 0.41 | ND |
| Valeraldehyde | 110-62-3 | 0.52 | ND |
| 2,3-Dihydrofuran | 1191-99-7 | 0.07 | ND |
| Hexanal | 66-25-1 | 1.35 | 0.34 |
| 3-Heptanone | 106-35-4 | 0.54 | 0.09 |
| 5-Ethyl-2,2,3-trimethylheptane | 62199-06-8 | 1.47 | 1.67 |
| Methyl formate | 107-31-3 | 0.90 | 0.93 |
| Pentanal | 110-62-3 | 0.21 | ND |
| Octanal | 124-13-0 | 0.19 | ND |
NR not reported
Fig. 1.
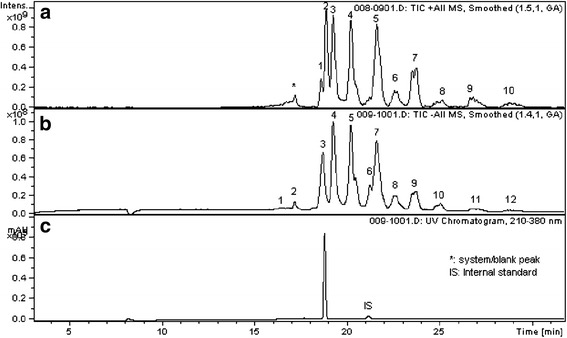
Chromatograms of the LC/DAD/MS analysis of the iso-hexane reflux extract from unprocessed PVC, showing epoxidized soybean oil-related extractables. a APCI positive mode, b APCI negative mode, and c UV 210–380 nm. Internal standard solution: palmitic acid-d31 (499 mg/L) and Tinuvin 327 (51.95 mg/L)
Fig. 2.
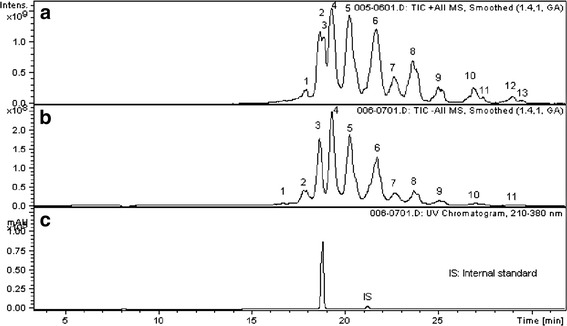
Chromatograms of the LC/DAD/MS analysis of the diluted (20×) IPA reflux sample from unprocessed PVC, showing epoxidized soybean oil-related extractables. a APCI positive mode, b APCI negative mode, c UV 210–380 nm. Internal standard solution: palmitic acid-d31 (499 mg/L) and Tinuvin 327 (51.95 mg/L)
Extraction Solvents
The influence of extraction solvent, in reflux and Soxhlet extractions, on the extractables observed is summarized for PVC and PE in Tables IX, X, and XI.
Table IX.
A Summary of the Reflux and Soxhlet Extractables Data for Unprocessed PVC (μg/g)
| Participating Laboratory | Type of Extractable | Amount measured in the extraction solvent (μg/g) | |||||
|---|---|---|---|---|---|---|---|
| Hexanea | IPA | IPA/water | Water, pH 2.5 | Water | Water, pH 9.5 | ||
| 1 | DEHP | 56,000 | 67,000 | 10,000 | 2 | 38 | 40 |
| Sum of extractables | 57,007 | 71,871 | 10,897 | 2 | 55 | 62 | |
| 5b | DEHP | 331,971 | 261,744 | N/A | 1 | 1 | N/A |
| Sum of Extractables | 345,176 | 277,462 | N/A | 1 | 4 | N/A | |
| 3 | DEHP | 98,325 | 130,537 | 164790c | 1 | 1 | 3 |
| Sum of Extractables | 103,646 | 138,556 | 175,995 | 3 | 4 | 6 | |
aLab 5 used hexane (described as a mixture of isomers); lab 1 and lab 3 used iso-hexane (2-methylpentane)
bExtraction conditions: Soxhlet extraction, various times for different solvents: 23 h iso-hexane, 23 h IPA, 7 h IPA/H2O, 23 h H2O pH 2.5, 23 h H2O pH 7, 23 h H2O pH 9.5
cSoxhlet extraction
N/A not tested
Table X.
A Summary of the Reflux Extractables Data for Unprocessed PE (μg/g)
| Participating laboratory | Extraction technique | Amount of total extractables measured in the extraction solvent (μg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| DCM | Hexanea | IPA | IPA/water | Water, pH 2.5 | Water | Water, pH 9.5 | ||
| 1 | Reflux | 52 | 76 | 37 | NDb | NDb | NDb | NDb |
| 3 | Microwave | NAc | 98 | NDb | NDb | NDb | NDb | NDb |
| 5 | Sonication | 60 | 175 | 4 | 1 | NDb | NDb | NDb |
| Reflux | 394 | 795 | 420 | 131 | NDb | NDb | NDb | |
| ASE | 28 | 189 | 351 | 23 | NDb | NDb | NDb | |
aLab 2 used hexane (described as a mixture of isomers); CRO 1 used isohexane (2-methylpentane)
b ND not detected
cNot applicable as analysis was not performed
Table XI.
Comparison of the Extractables Data for Unprocessed PVC for Various Solvents Compared to the Expected Profile
| Extractable | Formula amount | Amount of individual extractable measured in the extraction solvent (μg/g), reflux extraction | |||
|---|---|---|---|---|---|
| (%) | Hexane | IPA | IPA/water | Water (neutral) | |
| DEHP | 30 | 56,000 | 67,000 | 10,000 | 38 |
| Erucamide | 1 | 820 | 4300 | 490 | 2 |
| 2-Ethyl-1-hexanol | NA | 8 | 17 | 3 | 5 |
| Epoxidized soybean oil | 7 | Detected by LC/MS | Not tested | ||
| Fatty acids | 1 | ND | 21 | 170 | 2 |
Elemental Impurities
The only elements reproducibly extracted at measurable levels (typically <30 ppm) regardless of extraction solvent or technique were Ca (1–468 ppm) and Zn (1–766 ppm) in PVC, where the largest amounts were extracted with IPA/water. Otherwise, no consistent measurable quantities of extracted metals were observed with either aqueous or organic solvent extracts from the PVC and PE materials assessed, despite rigorous extraction. Metals noted in the ICH Q3D step 2b document as being of toxicological concern were not measurable (<0.3 ppm). This result is consistent with the results obtained for the same material analyzed under a different protocol (21).
DISCUSSION
Extraction Technique
A primary objective of the studies was to determine whether the multiple extraction techniques proposed in the protocol were required to generate information suitable to assist a user in material selection or whether the approach could be simplified, perhaps to one or two extraction techniques. For microwave and sealed vessel extraction techniques, the level of detail provided by the testing laboratories was insufficient to give a comprehensive evaluation of the techniques. However, in both cases, two anticipated compounds—the plasticizer (DEHP) and the slip agent (erucamide)—were clearly detected and identified despite the lack of semiquantitative extractable level correlation with the PVC composition level. Additionally, it was clear in the case of the microwave extraction of PVC with hexane that some degradation had taken place due to the significant level of 2-ethyl-1-hexanol that was reported. Since microwave heating efficiency is directly related to the dielectric constant of the solvent, this would mean that for solvents such as hexane, heating is likely to be inefficient. The ability to convert the absorbed energy into heat is also important. The temperature achieved and the energy imparted to the test molecule will be very specific to the solvent/sample itself. The microwave energy frequency is readily absorbed by water-like molecules and not absorbed by non-water-like molecules; hence, a nonpolar solvent such as hexane provides very little shielding effect, and extractables are likely to be exposed in full to the microwave energy, leading to degradation. The potential impact of microwave digestion is illustrated in Fig. 3, showing a comparison of the profile for microwave digestion versus sealed system. The sample obtained through microwave extraction yielded a significant number of unidentified additional peaks. The most probable explanation for these peaks is degradation of the PVC extractables during the microwave extraction process. Degradation by microwave was also confirmed by evidence of charring observed on the PVC material after IPA extraction was complete (results not shown). A data set collected using a sealed vessel with a different protocol (21) showed that there also was not a general quantitative correlation between extracted semiquantitative levels and the ingredient levels of the same PVC evaluated here. Taken together, these results suggest that the use of a sealed vessel or microwave extraction may not produce a profile that would be qualitatively or semiquantatively representative of the PVC material composition. However, sealed vessels have been generally recommended for water extractions (USP <1663>, United States Pharmacopoeia, draft 2013).
Fig. 3.
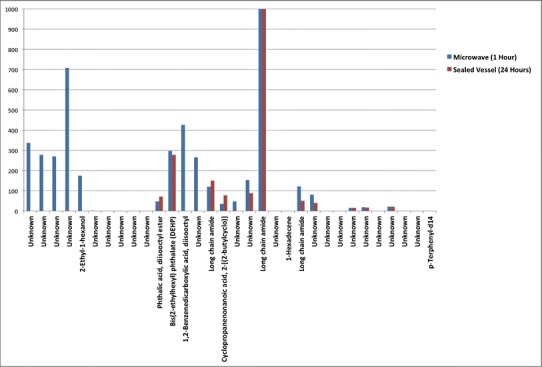
Comparison of the extractables profile obtained from microwave versus sealed vessel extraction (processed PVC; hexane solvent)
The extractable profile concentrations observed for PVC in both isopropanol (IPA) (Table IV) and hexane (Table V) with reflux or Soxhlet are comparable and consistent with the anticipated measureable levels of extractables of the test article. Results obtained using reflux and Soxhlet with water (pH 9.5, Table VI; pH 2.5 or WFI, data not shown) reveal little information relating to the profile suggesting that for this PVC, there are likely few, if any, aqueous soluble extractables. Although there were quantitative differences in the levels observed for the results obtained between labs for the reflux or Soxhlet techniques, the general trend was that the higher the expected level, the higher was the observed level. The correlation between the anticipated profile and those extractables obtained via reflux and Soxhlet indicates that it may be possible to obtain an extractables profile than can adequately represent the material composition. The advantage of Soxhlet is the repeated delivery of fresh pure distilled solvent to the sample, allowing a continual shifting of the transfer equilibrium. A disadvantage, particularly for aqueous extractions, is that because the extraction is performed using the distillate, the actual extraction medium will be water alone and not the acidic/basic system intended. Techniques such as reflux and Soxhlet extraction involve extraction at a temperature limited to the boiling point of the solvent or slightly lower, respectively. As described elsewhere (19), the results from the PE extractions showed that only sonication, reflux, and ASE yielded any measurable extractables from the unprocessed material and no semi-volatile or nonvolatile extractables resulted from Soxhlet extraction of the processed PE. Another study’s results from sealed vessel, reflux, and Soxhlet extraction of a different PE with additives have demonstrated that the greatest number of extractables is consistently obtained by reflux (21). While it is possible that some of these extractables may be degradation products that result from the extraction process itself, they also may indicate a possible degradation product of the material during its shelf life. Reflux in particular seems to be quite capable of extracting the major ingredients from a material as was demonstrated in the case of polypropylene and a peroxide-cured elastomer (4). Although it has been demonstrated that the extractables profile of an ABS material obtained by reflux can be readily reproduced by ASE extraction (22), the use of such specialized equipment may not be readily available at all test sites. For purposes of understanding material composition, the results from this study and several others suggest that reflux could be readily used as a single extraction technique.
Detailed Extraction Profile
The results obtained using IPA in particular (GC/MS detection) reveal the forecasted compounds as well as a range of other extractables. These include the benzoic acid mono-ethylhexanoate ester and 2-ethylhexanol, which originate from the plasticizer (DEHP), as either impurities present in DEHP or degradation products. Also observed at low levels were palmitic and stearic acids (related to the added surfactants Zn and Ca stearate) as well as their corresponding isopropyl esters (most likely formed during the extraction process itself). Docosanamide, the reduced form of erucamide, was also observed.
Of interest is the limited evidence of the presence of extractables associated with epoxidized soybean oil (ESBO), using GC/MS as the detection technique. This is a major formulation ingredient for the tested PVC and is typically added as a scavenger for HCl. However, the results obtained for this class of extractables vary greatly depending on the analytical technique used. On one hand, GC analyses did not reveal the ESBO-related extractables to any great extent. However, LC/MS chromatograms did reveal measurable levels of ESBO-related extractables (Figs. 1 and 2). Both isopropanol and hexane extracts analyzed via LC/MS show high concentrations of a number of individual compounds related to ESBO. These data show compellingly the need to use orthogonal analytical techniques in order to detect volatile or semi-volatile and nonvolatile components.
The results of the headspace GC/MS analysis for both processed and unprocessed PVC are shown in Table VIII. These results reflect the presence of a small number of low-level volatile compounds such as 5-ethyl-2,2,3-trimethylheptane and hexanal. Note that the semiquantitative approach for headspace GC/MS assumes complete thermal desorption of the volatile compounds present in and on the polymer material. It is well known that thermal desorption is strongly temperature dependent and will be more complete at higher temperatures. However, to limit thermal degradation of the polymer material, the headspace vial temperature was restricted to 80°C. Optimization of the incubation time and sample volume can be adjusted to capture all compounds that may volatilize from the material. The reported results might therefore be considered as being at the lower limit of potential concentrations. They nevertheless appear to indicate the absence of substantive levels of highly volatile extractives within this PVC.
Extraction Solvents
Table IX summarizes the absolute amounts of unprocessed PVC reflux extractables, focusing on DEHP individually and total extractables as a composite. The organic solvents IPA and hexane and the IPA/H2O (50/50) mixture showed the highest amounts of extractables for all extraction techniques, both semiquantitatively and qualitatively. The most abundant extractable, DEHP, was present in the organic extracts at the highest concentration. Interestingly, the amount of total IPA extractables was higher than the amount of hexane extractables for the two labs that used iso-hexane, whereas Lab 5, using n-hexane, obtained a higher amount of hexane extractables compared to IPA extractables. The same observation is made for the most abundant extractable DEHP. The extractables propensity of the IPA/water mixture is difficult to evaluate versus the pure organic extraction solvents. Lab 1 obtained a much lower (<20%) amount of extractables in the mixture, whereas lab 3 found a higher amount (>130%) in the mixture, even though the extraction time was less, compared to when extracting with pure organic solvents. One reason might be that lab 1 performed reflux extraction, where the 50:50 mixture (or an even higher concentration of water, as a higher content of IPA was in the vapor) was in direct contact with the PVC sample, whereas lab 3 performed Soxhlet extraction with a higher amount of IPA in the vapor in contact with and extracting the PVC sample.
The pure IPA extracts contain some isopropyl esters, which are not present in the hexane extracts. Those compounds are most likely formed via esterification of the parent extractable acids with the alcoholic extraction medium isopropanol. Such a mechanism might be revealed by applying a second extraction solvent (e.g., hexane), where esterification is not possible. While the extractables levels between water and water at pH 9.5 are similar, water at pH 2.5 provides significantly less extractables. The same trend for water at pH 9.5 and pH 2.5 was observed for the same PVC material analyzed by Jenke et al. (21)
Table X summarizes the absolute amounts of unprocessed PE reflux extractables, expressed as a sum parameter over all extractables as obtained from the three different laboratories (labs 1, 3, and 5). No PE extractables were detected in any of the aqueous media, regardless of pH. This is understandable since only hydrocarbons were extracted from the unprocessed PE. Although different semiquantitative levels of the hydrocarbons obtained by reflux extraction were observed between labs, the trend was the same—the greatest amount was extracted in hexane, which is in line with the hydrophobicity of the PE and its extractables.
The nature of the studies performed, varied extraction conditions and semiquantitative measurements with non-validated methods, was such that there was some observed interlaboratory variability in terms of compounds extracted and their levels. This made the detailed, forensic interpretation of results difficult because differences in the analytical results cannot be uniquely attributed to a specific extraction parameter. As a result, it proved impractical to definitively assess the impact of the solvent system in terms of the level and specific nature of the components extracted; it was however possible to detect trends in the extractables results, allowing for a qualitative evaluation of the extractables propensity of the different extraction solvents and also allowing some general considerations about the choice of extraction solvents for a specific application to be defined. Indeed, using the reflux extraction data for the unprocessed PVC, presented in Table XI, a correlation was observed between the anticipated profile and that observed across the solvents used, leading to a reduction in the number of extraction solvents required being possible. This requires more detailed study both with the materials included within this study and other materials before formal conclusions could be made.
The purely aqueous extraction systems showed a much reduced extractables propensity compared to the organic solvents. Some results suggested that neutral water might be eliminated as a solvent since no new extractables were observed that were not seen in pH 2.5 and pH 9.5 buffered water solvents.
As discussed earlier, Soxhlet extraction might not be appropriate for mixed or buffered solvents, e.g., IPA/water, and buffered water at pH 2.5 and pH 9.5 due to concentration changes caused by distillation—the sample in the Soxhlet thimble is only in contact with the distillation condensate (high IPA in IPA/water and pure water without buffers in buffered water).
As the intent of this study is to consider a solvent or solvent(s) that will provide information relevant to materials selection, the results suggest that the use of organic solvents provide the most information regarding material composition. Evaluation of multiple extraction solvents showed a correlation between the anticipated profile and the profile obtained across the main solvents studied. The study provided evidence that the profiles are different between the organic solvents and aqueous systems (neutral, pH 2.5 and 9.5) and established differences in the aqueous profiles as a function of the pH of the extraction solvent. The differences between the organic solvents studied, IPA versus hexane, were such that neither solvent is recommended versus the other; rather, it is concluded that both solvents could be used in a streamlined protocol for materials selection. For purposes of developing a material screening extraction protocol, the use of isopropanol, hexane, and water could be further explored.
It is understood that not all information collected by an ELSIE streamlined protocol may be useful for material selection for every product in early development. Individual organizations would need to utilize the appropriate and relevant information for their material selection. Typically, final material selection would be based on studies specific to a particular product.
Elemental Impurities
Container closure systems are currently under scrutiny as a potential source of elemental impurities. The draft ICH Q3D elemental impurities step 2B guideline defines the need for a holistic risk assessment of all components that may contribute to the presence of elemental impurities in the final product, including container closure systems (CCS) (20). Concern regarding metal residues in polymers is not new. Indeed, the testing for metals in polymers to be used in pharmaceutical packaging is a pharmacopoeial (US, European, and Japanese pharmacopoeia) requirement (23–25). However, the methods are not harmonized and are a mixture of extraction and digestion with wet chemical and spectroscopic testing. The current USP monograph for containers (USP <661>) stipulates that heavy metal testing (arsenic, cadmium, mercury, lead) should be carried out on water extracts of polyethylene and polypropylene (25). The European Pharmacopoeia (PhEur), in addition to heavy metal testing, requires the analyses of metal catalysts. For polyolefins, polyethylene, polypropylene, polyethylene terephthalate (PET), and non-plasticized PVC, metals are extracted by 0.1 M HCl, while for plasticized PVC and silicone elastomer, total metals are analyzed by digestion or ashing, respectively (23). The Japanese Pharmacopoeia (JP) requires testing for heavy metals and tin by ashing (24). Within ICH Q3D, step 2B limits for 24 elements, including arsenic, cadmium, mercury, and lead, will be based on a permitted daily exposure (PDE) for each element (20). The recommended methodology will be based on ICP-OES or ICP-MS. As part of the control strategy advocated by ICH Q3D, it will be necessary for the manufacturer of the drug product to be aware of potential elemental contamination from plastic components especially if that component is used in products where there is high risk of interaction with the formulation, e.g., liquids or MDIs, and the formulation is intended for parenteral, inhalation, or nasal products.
None of the metals defined in the ICH Q3D document as being of toxicological concern were observed at quantifiable levels in either processed or unprocessed PVC or PE. For PVC, zinc and calcium were expected as zinc and calcium stearates which are part of the formulation composition (see Table I). Zinc was detected at parts per million (ppm) levels when the PVC was extracted with acidic water; however, calcium was not reproducibly detected under any aqueous conditions. Levels of zinc and calcium were high (>100 ppm) from PVC with microwave extraction when IPA or IPA/water was used. The use of ICP-MS as an orthogonal technique is useful for elucidating formulation composition.
CONCLUSION
Findings from this study are based primarily on the PVC as results for the PE showed there to be virtually no extractables, consistent with the supplier claim that this material was additive free. Based on results from this study, we can preliminarily conclude that the broad ELSIE protocol can be refined to include fewer extraction techniques and solvents for the purpose of generating extractables profiles to aid companies in their material selection processes. For example, results from the PVC studies show that extractables profiles generated by reflux or Soxhlet extraction do reflect the organic additive profile including related compounds; and organic extractions, performed with two solvents of varying polarity, e.g., hexane and isopropanol, can provide extractables information needed for materials selection decision making. To assist companies manufacturing aqueous-based products, it would be useful to include a water system also as a solvent. These extraction techniques and three solvents could be evaluated with other materials to determine whether it would be appropriate to include them as part of a streamlined protocol. Of significance with respect to understanding the extractables profile for materials selection purposes is the use of orthogonal analytical detection techniques, i.e., GC/MS, LC/MS, and ICP-MS. Results show the need for such an approach to fully ascertain inorganic and organic volatile, semi-volatile, and nonvolatile species. The information relevant to a specific product type can then be utilized by companies to evaluate materials for a specific application.
The general profiles found via the ELSIE study are similar to that recently published by PQRI, using the same PVC (21). We emphasize that the purpose of the PQRI studies is to obtain information to fully characterize the materials studied, while the purpose of the ELSIE studies is to obtain enough characterization information that would assist in the materials selection process early in development. The ELSIE findings suggest that for material selection purposes, a reduced testing approach, e.g., focused extraction techniques (reflux) with a small number of solvents (IPA, hexane, water), is feasible. Although results from other studies also support this finding, a formal investigation involving other materials could be used to confirm the wider utility of this approach.
ACKNOWLEDGMENTS
The authors thank the laboratories that contributed to this work: Toxikon Corp., PPD, Aspen Research Corp., Hall Analytical Laboratories, Chemic Laboratories, Inc., West Pharmaceutical Services, and Intertek.
Footnotes
Extraction studies on processed and unprocessed polyvinyl chloride and polyethylene were performed to investigate the need for a broad selection of solvents and extraction techniques for materials selection purposes.
REFERENCES
- 1.Norwood DL, Ball DJ, Nagao LM. Overview of leachables and extractables in orally inhaled and nasal drug products. In: Ball DJ, Norwood DL, Stults CLM, Nagao LM, editors. Leachables and extractables handbook: safety evaluation, qualification, and best practices applied to inhalation drug products. Hoboken: Wiley; 2012. p. 6. [Google Scholar]
- 2.Health Canada. Guidance for industry. Pharmaceutical quality of inhalation and nasal products. 2006. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/chem/inhalationnas-eng.php
- 3.European Medicines Agency. Guideline on the pharmaceutical quality of inhalation and nasal products. 2006. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003568.pdf
- 4.Product Quality Research Institute Leachables and Extractables Working Group. Safety thresholds and best practices for extractables and leachables in orally inhaled and nasal drug products. 2006. Available from: http://www.pqri.org/pdfs/LE_Recommendations_to_FDA_09-29-06.pdf
- 5.U.S. Food and Drug Administration Center for Drug Evaluation and Research. Draft guidance for industry. Metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products. Chemistry manufacturing and controls documentation. 1998. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070573.pdf
- 6.U.S. Food and Drug Administration Center for Drug Evaluation and Research. guidance for industry. Container closure Systems for packaging human Drugs and biologics. Chemistry, manufacturing, and controls documentation. 1999. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070551.pdf
- 7.Schroeder AC. Leachables and extractables in OINDP: an FDA perspective. PQRI Leachables and Extractables Workshop. 2005. http://pqri.org/workshops/leach_ext/imagespdfs/presentations/AlanSchroederDay1.pdf
- 8.Markovic I. Challenges associated with extractable and/or leachable substances in therapeutic biologic protein products. Am Pharm Rev. 2006;9(6):20–27. doi: 10.1517/14740338.6.5.487. [DOI] [PubMed] [Google Scholar]
- 9.Ball D, Paskiet D, et al. PQRI research project proposal: reporting and qualification thresholds for leachables in parenteral and ophthalmic drug products. 2007. http://www.pqri.org/commworking/minutes/pdfs/dptc/podpwg/Addl/PQRI_PODP_proposal_04072.pdf
- 10.U.S. Food and Drag Administration Office of Combination Products. Guidance for industry and FDA staff: technical considerations for pen, jet, and related injectors intended for use with drugs and biological products. 2013. Available from: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM147095.pdf
- 11.China Food and Drug Administration. Technical guideline for compatibility studies between chemical drug injections and plastic packaging materials. 2012.
- 12.U.S. Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry nasal spray and inhalation solution, suspension, and spray drug products—chemistry, manufacturing, and controls documentation. 2002. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070575.pdf
- 13.International Organization for Standardization. ISO 10993-1 Biological evaluation of medical devices—Part 1: evaluation and testing within a risk management process. 2009.
- 14.Dohmeier DM, Norwood DL, et al. Use of polymeric materials in orally inhaled and nasal drug products. Med Device Technol. 2009;20(2):32–38. [PubMed] [Google Scholar]
- 15.Ball DJ, Beierschmitt WP, Shaw AJ. Pharmaceutical container closure systems: selection and qualification of materials. In: Ball DJ, Norwood DL, Stults CLM, Nagao LM, editors. Leachables and extractables handbook: safety evaluation, qualification, and best practices applied to inhalation drug products. Hoboken: Wiley; 2012. p. 217. [Google Scholar]
- 16.Tougas T, Roan S, Falco B. Critical component quality control and specification strategies. In: Ball DJ, Norwood DL, Stults CLM, Nagao LM, editors. Leachables and extractables handbook: safety evaluation, qualification, and best practices applied to inhalation drug products. Hoboken: Wiley; 2012. p. 523. [Google Scholar]
- 17.Extractables and Leachables Safety Information Exchange. Controlled extraction studies on materials for ELSIE database: qualitative and semi-quantitative studies. 2010. http://www.elsiedata.org/
- 18.Norwood DL, Paskiet D, Ruberto M, Feinberg T, Schroeder A, Poochikian G, et al. Best practices for extractables and leachables in orally inhaled and nasal drug products: an overview of the PQRI recommendations. Pharm Res. 2008;25(4):727–739. doi: 10.1007/s11095-007-9521-z. [DOI] [PubMed] [Google Scholar]
- 19.Stults CLM, Ansell J, Shaw AJ, Nagao LM. Evaluation of extractables in processed and unprocessed polymer materials used for pharmaceutical applications. AAPS PharmSciTech. 2014 doi: 10.1208/s12249-014-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Conference on Harmonization (ICH). Draft consensus guideline. Guideline for elemental impurities, Q3D. Current step 2b version. 26 July 2013. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q3D/Q3D_Step2b.pdf
- 21.Jenke D, Castner J, Egert T, et al. Extractables characterization of five materials of construction representative of packaging systems used for parenteral and ophthalmic drug products. PDA J Pharm Sci Tech. 2013;67:448–511. doi: 10.5731/pdajpst.2013.00933. [DOI] [PubMed] [Google Scholar]
- 22.Stults CLM, Mikl J, Whelehan O, Morrical B, Duffield W, Nagao LM. A risk-based approach to management of leachables utilizing statistical analysis of extractables. AAPS PharmSciTech. 2014 doi: 10.1208/s12249-014-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Pharmacopoeia. Chapter 3.1. Materials used for the manufacture of containers. 7th ed 7.8 July 2013.
- 24.Japanese Pharmacopoeia. General test 7.02. Test methods for plastic containers. 16th ed, 2011.
- 25.United States Pharmacopeia. <661> Containers—plastics. United States Pharmacopeia. 36; National Formulary 31. 2013


