Abstract
Eutectic mixtures formed between active pharmaceutical ingredients and/or excipients provide vast scope for pharmaceutical applications. This study aimed at the exploration of the crystallization abilities of two eutectic mixtures (EM) i.e., lidocaine-tetracaine and lidocaine-camphor (1:1 w/w). Thermogravimetric analysis (TGA) for degradation behavior whereas modulated temperature differential scanning calorimetry (MTDSC) set in first heating, cooling, and second heating cycles, was used to qualitatively analyze the complex exothermic and endothermic thermal transitions. Raman microspectroscopy characterized vibrational information specific to chemical bonds. Prepared EMs were left at room temperature for 24 h to visually examine their crystallization potentials. The degradation of lidocaine, tetracaine, camphor, lidocaine-tetracaine EM, and lidocaine-camphor EM began at 196.56, 163.82, 76.86, 146.01, and 42.72°C, respectively, which indicated that eutectic mixtures are less thermostable compared to their individual components. The MTDSC showed crystallization peaks for lidocaine, tetracaine, and camphor at 31.86, 29.36, and 174.02°C, respectively (n = 3). When studying the eutectic mixture, no crystallization peak was observed in the lidocaine-tetracaine EM, but a lidocaine-camphor EM crystallization peak was present at 18.81°C. Crystallization occurred in lidocaine-camphor EM after being kept at room temperature for 24 h, but not in lidocaine-tetracaine EM. Certain peak shifts were observed in Raman spectra which indicated possible interactions of eutectic mixture components, when a eutectic mixture was formed. We found that if the components forming a eutectic mixture have crystallization peaks close to each other and have sufficient hydrogen-bonding capability, then their eutectic mixture is least likely to crystallize out (as seen in lidocaine-tetracaine EM) or vice versa (lidocaine-camphor EM).
KEY WORDS: crystallization, degradation, eutectic mixture, Raman spectroscopy, thermal analysis
INTRODUCTION
A eutectic mixture is defined as an intimate mixture of two or more compounds, which melts at a temperature lower than those of individual compounds (1). The formation of eutectic mixture is usually governed by the following factors: (a) the compounds must be miscible in their liquid state and immiscible in their solid state (2), (b) intimate physical interaction between eutectic-forming compounds is necessary for contact-induced melting point depression (3), and (c) the compounds should have chemical groups that can interact to form physical bonds such as intermolecular hydrogen bonding. Eutectic mixtures can be formed between active pharmaceutical ingredients (APIs), APIs and excipients, or between excipients; thereby, providing a vast scope for their application in the pharmaceutical industry (4–8).
The first successful application of a eutectic mixture for topical anesthesia was credited to Jules Aristide Bonain, who discovered that cocaine hydrochloride, phenol, and menthol mixture spontaneously transform into a homogeneous liquid at room temperature (9,10). However, owing to the toxic side effects of cocaine and the caustic properties of phenol, this mixture was seldom used. Later on, an emulsion containing equal amounts of lidocaine and prilocaine, called EMLA (eutectic mixture of local anesthetics) cream, was used for topical anesthesia (11–13). In a study, the EMLA cream was found to be more effective compared to a bonain mixture for eardrum anesthesia (14). However, it was found that prilocaine has a high propensity to cause methemoglobinemia (15–17). Thus, it became important to search for more compounds that could form a eutectic mixture with lidocaine and thereby, improve its effectiveness (18).
In this study, the eutectic mixtures of lidocaine-tetracaine and lidocaine-camphor have been explored. All these drugs are miscible in their liquid state. The selected drugs have varied molecular properties. For instance, lidocaine has one hydrogen donor but camphor has none (19). They have varied thermal properties: lidocaine has a moderate melting point of 68°C (20), tetracaine has a very low melting temperature range of 41–42°C (21), and camphor has sublimation property along with a high melting temperature range of 175–178°C (22). The combination of lidocaine-tetracaine has been known to enhance their transdermal permeability (23–25). Similarly, the combination of lidocaine with a terpene (e.g., camphor) has been reported to enhance its permeability (26). Thermal analytical techniques, such as thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and modulated temperature differential scanning calorimetry (MTDSC), have been extensively used in pharmaceutical development (27–31). Eutectic mixtures too, have been characterized and analyzed using these techniques (32,33). Raman spectroscopy has been used to study eutectic mixtures in chemical industry (34).
In most of the studies on eutectic mixtures, the emphasis has been on the melting points/ranges of individual components of eutectic mixtures. Unlike melting, crystallization is more specific to the lattice structure of compounds and should be used in understating eutectic mixtures (35). This study focuses on the exploration of the relationship between eutectic mixture components, their crystallization behavior, and their stability. We thus hypothesize that the number of hydrogen-bonding groups and crystallization behavior of eutectic mixture components affects eutectic mixture stability.
MATERIALS AND METHODS
Materials
Lidocaine (C14H22N2O) was purchased from PCCA (Houston, TX), tetracaine (C15H24N2O2) was purchased from Sigma-Aldrich, and dl-camphor (C10H16O) was purchased from Gallipot.
METHODS
Sample Preparation
The individual components (i.e., lidocaine, tetracaine, and camphor) were used as received. For eutectic mixture samples: (1) lidocaine-tetracaine were taken in 1:1 w/w ratios and uniformly mixed and (2) lidocaine-camphor were taken in 1:1 w/w ratio, uniformly mixed and heated in a closed container placed on a heating plate. The heating was carried out at 30°C for 5 min.
Thermogravimetric Analysis
Lidocaine, tetracaine, camphor, and their eutectic mixtures were investigated by thermal gravimetric analysis (Shimadzu DTG 60). The samples (approximately 5–15 mg) were taken in an open aluminum pan and heated at a rate of 10°C/min, up to 300°C, with a nitrogen purge.
Modulated Temperature Differential Scanning Calorimetry
The melting and crystallization behaviors of lidocaine, tetracaine, camphor, and their eutectic mixtures were investigated by MTDSC (DSC Q-2000, TA Instrument) [n = 3]. The samples (approximately 5–15 mg) were taken in an aluminum pan. The pan was sealed and subjected to heating and cooling cycles. Lidocaine, tetracaine, and their eutectic mixture were subjected to heating and cooling cycles at a rate of 2°C/min in a temperature range of −40–100°C. Camphor and its eutectic mixture with lidocaine were subjected to heating and cooling cycles at a rate of 2°C/min in a temperature range of −40–200°C. The first MTDSC cycle (first heating cycle) involved heating from room temperature up to 100/200°C; the second MTDSC cycle (cooling cycle) involved cooling from 100/200°C to −40°C, and the third MTDSC cycle (second heating cycle) involved heating from −40°C up to 100/200°C. The upper range of 100°C and 200°C were selected because lidocaine and tetracaine have melting points below 100°C, whereas camphor melts in the range of 175–178°C.
Crystallization Study
A crystallization study was carried out for lidocaine, tetracaine, camphor, and their eutectic mixtures. Lidocaine, tetracaine, and camphor were evenly spread on a Petri dish and their morphology was observed visually. The eutectic mixtures (1:1 w/w) were prepared and mounted evenly on glass slides and their morphology was observed visually. These slides were then left at room temperature for 24 h and analyzed to visually observe the physical changes in the eutectic mixture over a period of time due to crystallization.
Dispersive Raman Microspectroscopy
Lidocaine, tetracaine, camphor, and their eutectic mixtures were subjected to dispersive Raman microspectroscopy (Bruker-Senterra). All the samples were mounted on a glass slide and subjected to Raman light. The aperture of 50 × 100 μm and the object lens of 20× size were used to focus on samples. The samples were subjected to a laser beam having a wavelength of 785 nm and spectral data in the range of 500–1500 cm−1 wavenumber was collected. A resolution level of approximately 3.5 cm−1 was set in the instrument.
RESULTS
Thermogravimetric Analysis
The initiation of degradation for the eutectic mixture of lidocaine-tetracaine was faster i.e., 5% weight loss occurred at 146.01°C, whereas the same for tetracaine and lidocaine occurred at 163.82 and 196.56°C, respectively (Table I). The TGA data of the lidocaine-tetracaine eutectic system revealed that lidocaine was most thermostable, followed by tetracaine; their eutectic mixture was found to be the least thermostable (Fig. 1). The TGA data for lidocaine showed variability. For the lidocaine-camphor eutectic mixture, 25% weight loss occurred at 87.76°C; the same occurred at 121.41 and 231.38°C for camphor and lidocaine, respectively (Table I). The TGA analysis of the lidocaine-camphor eutectic system revealed that lidocaine was more stable than both camphor and their eutectic mixture (Fig. 2). It is important to note that the system undergoes degradation, since there is a weight loss with temperature.
Table I.
Degradation Temperatures of the Three Studied Drugs and Their Respective Eutectic Mixtures at 5, 10, 25, and 50% Weight Loss, Respectively
| Sample | Weight loss | |||
|---|---|---|---|---|
| 5 ± 0.5% | 10 ± 0.5% | 25 ± 0.5% | 50 ± 0.5% | |
| Temperature (°C) | ||||
| Lidocaine | 196.56 | 210.79 | 231.38 | 248.12 |
| Tetracaine | 163.82 | 179.86 | 202.11 | 219.53 |
| Lidocaine-tetracaine EM | 146.01 | 171.05 | 195.46 | 215.94 |
| Camphor | 76.86 | 94.42 | 121.41 | 144.48 |
| Lidocaine-camphor EM | 42.72 | 55.47 | 87.76 | 140.83 |
EM eutectic mixtures
Fig. 1.
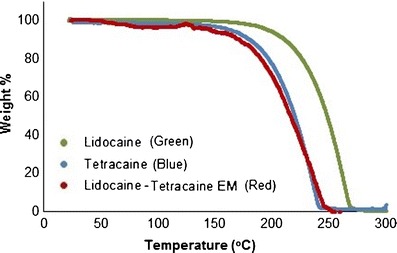
Thermogravimetric analysis (TGA) plot of lidocaine (green), tetracaine (blue) and lidocaine-tetracaine eutectic mixture (red)
Fig. 2.
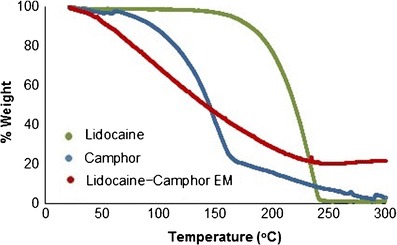
Thermogravimetric analysis (TGA) plot of lidocaine (green), camphor (blue) and lidocaine-camphor eutectic mixture (red)
Figures 3 and 4 exhibited excellent fitting, after weight % in Figs. 1 and 2 was converted into weight loss (%) and plotted against temperature in order to obtain thermal degradation profiles for the two EM systems. Lidocaine, tetracaine, camphor, and the lidocaine-tetracaine EM demonstrated exponential fitting, whereas lidocaine-camphor eutectic mixture revealed linear fitting. The difference in the degradation behavior of these two eutectic mixtures clearly signifies the formation of two distinct systems. Thus, it appears that eutectic mixtures are less thermostable than their individual components.
Fig. 3.
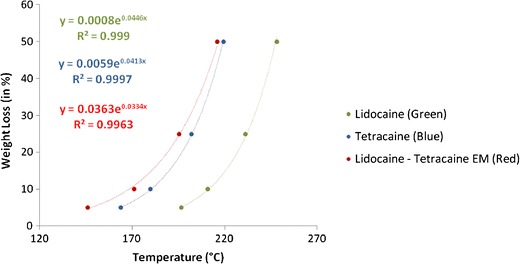
Fitting the thermal degradation profiles of lidocaine, tetracaine, and lidocaine-tetracaine eutectic mixture. Good correlation is present between the experimental data and mathematical equations
Fig. 4.
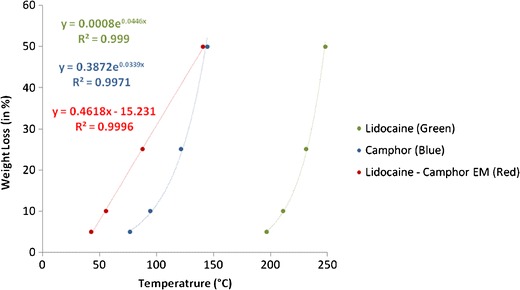
Fitting the thermal degradation profile of lidocaine, camphor, and lidocaine-camphor eutectic mixture. Good correlation is present between the experimental data and mathematical equations
Modulated Temperature Differential Scanning Calorimetry
The MTDSC plot of lidocaine showed a single melting endotherm at 68.17°C during the first heating cycle (Fig. 5a-1) and a similar endotherm at 68.02°C during the second heating cycle (Fig. 5c-1). Lidocaine also showed a crystallization exotherm at 31.86°C (Fig. 5b-1). During the first heating cycle of tetracaine, a single melting endotherm at 42.93°C was seen (Fig. 5a-2); but in the second heating cycle, two melting endotherms at 38.79 and 42.85°C were recorded (Fig. 5c-2). Tetracaine also shows a crystallization exotherm at 29.36°C (Fig. 5b-2). The thermogram of the lidocaine-tetracaine eutectic mixture showed a single broad melting endotherm at 29.95°C (Fig. 5a-3), which can be attributed to the melting-point depression phenomena of the eutectic mixture. The thermogram of the lidocaine-tetracaine eutectic mixture showed no thermal events during the cooling cycle (Fig. 5b-3) and second heating cycle (Fig. 5c-3).
Fig. 5.
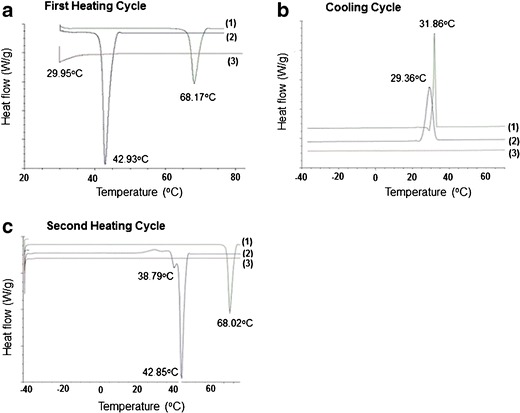
The modulated temperature differential scanning calorimetry (MTDSC) plots of lidocaine (curve 1), tetracaine (curve 2), and lidocaine-tetracaine eutectic mixture (curve 3). a Thermogram of the first heating cycle. b Cooling cycle. c Second heating cycle
The MTDSC plot of camphor showed a melting endotherm at 175.35°C during the first heating cycle (Fig. 6a-2) and a broad melting endotherm at 174.59°C during the second heating cycle (Fig. 6c-2). The endotherms in both heating cycles can be assigned to the melting of camphor. Also, small endothermic events at 188.62 and 190.67°C were observed in both the heating cycles for camphor (Fig. 6a-2 and Fig. 6c-2). Camphor shows a crystallization exotherm at 174.02°C (Fig. 6b-2). The MTDSC plot of the lidocaine-camphor eutectic mixture showed broad endotherms at 32.64 and 165.31°C during the first heating cycle (Fig. 6a-3). Lidocaine-camphor eutectic mixture also shows a crystallization exotherm at 18.81°C during the cooling cycle (Fig. 6b-3). The second heating cycle of the lidocaine-camphor eutectic mixture shows a single endotherm at 67.14°C (Fig. 6c-3).
Fig. 6.
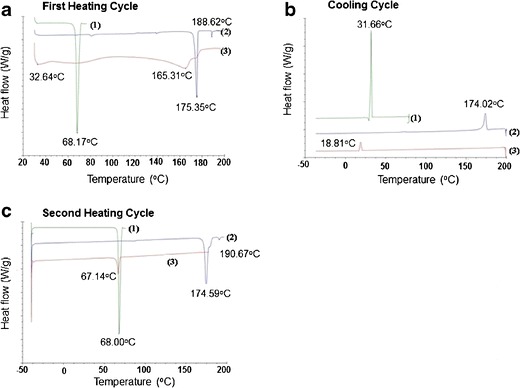
The MTDSC plots of lidocaine (curve 1), camphor (curve 2), and lidocaine-camphor eutectic mixture (curve 3). a Thermogram of the first heating cycle. b Cooling cycle. c Second heating cycle
Crystallization Study
Pure lidocaine appeared like fine white crystalline powder, tetracaine had shiny white flakes, whereas camphor looked like white crystalline agglomerates (Fig. 7a–c). The lidocaine-tetracaine eutectic mixture was formed instantaneously at room temperature (Fig. 7d, left), whereas the lidocaine-camphor eutectic mixture was heated in a closed container at 30°C for 5 min to form the eutectic mixture (Fig. 7e, left). The lidocaine-tetracaine eutectic mixture appeared as a clear, viscous liquid (Fig. 7d, left). No changes were observed in the physical appearance of lidocaine-tetracaine eutectic mixtures for 24 h (Fig. 7d, right). The lidocaine-camphor eutectic mixture appeared as a clear, viscous liquid on its formation but converted into sharp needle/rhombic-like crystals after 24 h (Fig. 7e, right).
Fig. 7.
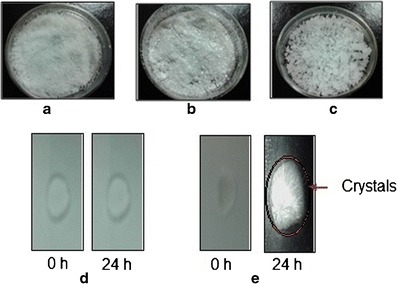
The visual appearance of the three studied drugs and their eutectic mixtures. a Lidocaine. b Tetracaine. c Camphor. d Lidocaine-tetracaine eutectic mixture. e Lidocaine-camphor eutectic mixture
Dispersive Raman Microspectroscopy
The Raman spectra of the lidocaine-tetracaine eutectic mixture showed broad overlapping peaks in the region of 1300–1500 cm−1. The shoulder of peaks at 1377, 705, and 618 cm−1 in the eutectic mixture (Fig. 8a-3 and b-3) correspond to lidocaine although slight shifts are observed in these peaks. Significant peak shifts at 1270, 1174 and 848 cm−1 were observed in the lidocaine-tetracaine eutectic mixture Raman spectra (Fig. 8b-3). Other peaks indicate overlapping areas of lidocaine and tetracaine in the lidocaine-tetracaine eutectic mixture. The Raman spectra of the lidocaine-tetracaine eutectic system confirm the existence of interactions between lidocaine and tetracaine.
Fig. 8.
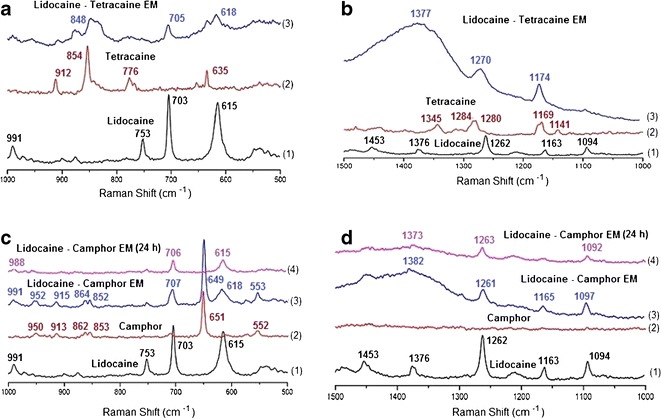
Raman spectra of the eutectic mixture of lidocaine and tetracaine (a-b) and eutectic mixture of lidocaine and camphor (c-d) in comparison to the spectra of each drug. Spectra from wavenumber 500 to 1000 cm−1 (a and c) and from 1000–1500 cm−1 (b and d)
The Raman spectra of the lidocaine-camphor eutectic mixture showed sharp peaks, corresponding to lidocaine and camphor. Characteristic peaks of lidocaine at 1262, 703, and 615 cm−1 and camphor at 862, 853, and 651 cm−1 can be seen in the eutectic mixture spectra (Fig. 8c, d), indicating that the components are in their initial form. The spectra of the lidocaine-camphor eutectic mixture after it turns into solid crystalline form, i.e., after leaving it for 24 h at room temperature, as seen in morphological study (Fig. 7e, right), is similar to the lidocaine spectra (Fig. 8c-4 vs. c-1 and Fig. 8d-4 vs. d-1).
DISCUSSION
Thermogravimetric Analysis
TGA analysis can characterize important structural changes with temperature, and these changes can be used to study the differences between individual components and EM. Ma et al. reported significant weight loss for lidocaine at 130°C, which they attributed to lidocaine’s vaporization (36). However, we did not observe any significant weight loss for lidocaine at 130°C. Shamsudin et al. performed TGA analysis on camphor oil and observed ~50% weight loss at ~145°C, which is similar to our observations (37). In an accelerated stability study on lidocaine-tetracaine cream, it was seen that lidocaine was more stable than tetracaine (38). Similarly, behavior is seen in the TGA curves for lidocaine and tetracaine. Lidocaine, tetracaine, camphor, and the lidocaine-tetracaine EM demonstrated exponential fitting, whereas lidocaine-camphor eutectic mixture revealed linear fitting. The difference in the degradation behavior of these two eutectic mixtures not only clearly signifies the formation of two distinct systems, but also proves that eutectic mixtures are less thermostable than their individual components. This property could be attributed to the melting point depression phenomena of eutectic mixtures.
Modulated Temperature Differential Scanning Calorimetry
Since the initiation and enthalpy of melting endotherms of lidocaine in the first and second heating cycles of the MTDSC plot were similar, 68.17 vs. 68.02°C (Fig. 5a-1 and c-1), it can be inferred that lidocaine undergoes no significant molecular change during its crystallization process. For tetracaine, a single melting endotherm was observed at 42.93°C in the first heating cycle, but there were two melting endotherms observed at 38.79 and 42.85°C in the second heating cycle. Tetracaine has no polymorphic form, as reported by Giron et al. (39). Thus, the first melting endotherm (at 38.79°C) in the second heating cycle may be due to the formation of a metastable state of tetracaine, which is known to cause a decrease in the tetracaine melting point (40,41). Also, Schmidt reported melting points for tetracaine polymorphs I and II at 42 and 37°C, respectively (42). Tetracaine also shows a crystallization exotherm at 29.36°C (Fig. 5b-2). It can be inferred that tetracaine may be undergoing slight molecular change during its crystallization process, resulting in the formation of a metastable hydrate form. The thermogram of the lidocaine-tetracaine eutectic mixture showed a single broad melting endotherm at 29.95°C (Fig. 5a-3), which can be attributed to the melting point depression phenomena of the eutectic mixture. Riga et al. observed a eutectic mixture formation between lidocaine tetracaine at 60:40 w/w compositions, respectively, with a melting point of 15.90°C (43). They also reported a DSC thermogram of lidocaine tetracaine mixture prepared by solvent evaporation at 50:50 w/w compositions, respectively [heating rate 2.50°C/min, showing endotherms at ~16 and ~51°C, respectively (43). The thermogram of the lidocaine-tetracaine eutectic mixture showed no events during the cooling cycle and second heating cycle. This behavior signifies that neither lidocaine nor tetracaine crystallizes out of the eutectic mixture (Fig. 5b-3 and c-3).
Since both initiation and enthalpy of melting for endotherms in both heating cycles of camphor are similar [175.35°C in the first heating cycle (Fig. 6a-2) and at 174.59°C in the second heating cycle (Fig. 6c-2)], we can assume that camphor crystallization has not caused molecular changes in the system and camphor has not sublimed from the system. Small endothermic events at 188.62 and 190.67°C in the same endotherms (Fig. 6a-2 and c-2) were also observed. The reason for these second endotherms is not reported in the literature and needs to be explored. The MTDSC plot of the lidocaine-camphor eutectic mixture showed broad endotherms at 32.64 and 165.31°C during the first heating cycle. The first endotherm can be attributed to the melting point depression phenomena of the eutectic mixture. The second endotherm could be due to the presence of unassociated camphor in the system. Corvis et al. reported that ideal eutectic point temperature for lidocaine-camphor eutectic mixture was 307 K, i.e., 33.85°C, which is closer to the eutectic point temperature of 32.64°C, as observed in our study (26). The MTDSC plot of the lidocaine-camphor eutectic mixture also shows a crystallization exotherm at 18.81°C during the cooling cycle; since this exotherm is at a lower temperature compared to that of the individual components, it can be inferred that this system has undergone significant molecular changes. The second heating cycle of the lidocaine-camphor eutectic mixture shows a single endotherm at 67.14°C, which can be due to lidocaine; this would mean that camphor has sublimed from the eutectic mixture. This finding was not seen during the MTDSC plot of camphor. These changes also signify the difference of behavior between the lidocaine-tetracaine and lidocaine-camphor eutectic mixtures (Figs. 5a-c-3 and 6a-c-3). The fact that lidocaine-tetracaine eutectic mixture does not show any crystallization or melting during cooling cycle and the second heating cycle (Fig. 5b-3 and c-2) clearly suggests the formation of a stable eutectic system. Both lidocaine and tetracaine have significant difference in their melting temperatures but their crystallization exotherms are very close to each other (31.86 vs. 29.36°C, Fig. 5b-1 and b-2) and might be playing an important role in the stabilization of this system. The simultaneous crystallization of pure components in these eutectic mixtures helps in the retention of the weak interactions such as hydrogen bonding at molecular level. Vepuri et al. also reports that the formation of intermolecular interactions such as van der Waal forces and hydrogen bonds are necessary for formation/retention of a eutectic mixture (44). The reverse happens in a system like lidocaine-camphor, wherein the crystallization temperatures are significantly different from each other (Fig. 6b-1 and b-2). Due to the crystallization of pure components at their respective temperatures, disruption of any possible hydrogen bonding is a high possibility which can render the system unstable.
Crystallization Study
The observation of lidocaine-camphor eutectic mixture having to be heated in a closed container at 30°C for 5 min to form the eutectic mixture was contrary to the report made by Corvis et al. (26) in which lidocaine and camphor formed a liquid eutectic mixture instantaneously at room temperature. The change in morphology of the lidocaine-camphor eutectic mixture at room temperature (Fig. 7e, right) was due to the significant difference in the crystallization behavior of the individual components from the eutectic mixtures. The study clearly suggests that lidocaine-tetracaine eutectic mixture is more stable than lidocaine-camphor eutectic mixture (Fig. 7d, right vs. Fig. 7e, right).
Dispersive Raman Microspectroscopy
The Raman spectra of lidocaine, tetracaine, and camphor were similar to those reported in the literature (41,45,46). The Raman spectra of the lidocaine-tetracaine eutectic mixture showed broad peaks which are expected due to the formation of eutectic mixtures and possibly due to the molecular disorder that occurs in the eutectic mixture. The overlapping, yet characteristic, Raman peaks indicate the presence of molecular structure of the individual components. The spectra of the lidocaine-camphor eutectic mixture after it turns to solid crystalline form (i.e., after leaving it for 24 h at room temperature, as seen in morphological study, Fig. 7e-right) is similar to the lidocaine spectra, thereby confirming the MTDSC result of camphor subliming.
Literature search for the hydrogen-bonding groups of lidocaine, tetracaine, and camphor (19) confirmed that the pair of lidocaine and tetracaine offers two hydrogen donors and 7 hydrogen acceptors whereas the pair of lidocaine and camphor only has one hydrogen donor and 4 hydrogen acceptors (Table II). Since both the drugs have hydrogen bond acceptor and donor groups, it is highly likely that hydrogen bonding is playing an important role in the formation of lidocaine-tetracaine eutectic mixtures. (Fig. 8a-3 and b-3). The total number of hydrogen-bonding groups of lidocaine and tetracaine EM is almost doubled that of lidocaine and camphor EM. The absence of hydrogen bond donor in camphor (Table II) may be used to explain the decrease of the hydrogen-bonding ability of lidocaine with camphor, which leads to crystallization from its EM as observed in the 24-h samples. In a study, Schmidt showed the importance of correlation between molecular structure and crystal polymorphism in local anesthetic drugs (42). Thus, it appears that lidocaine and tetracaine on formation of eutectic mixture interact with each other via hydrogen bonding, which retards the crystallization process of lidocaine, tetracaine, and their eutectic mixture. However, for lidocaine and camphor, due to lack of sufficient hydrogen bond-forming groups and their preferential crystallization at significantly different temperatures, their crystallization process is not retarded.
Table II.
The Molecular Structures and Corresponding Hydrogen-Bonding Groups of Three Studied Drugs (19)
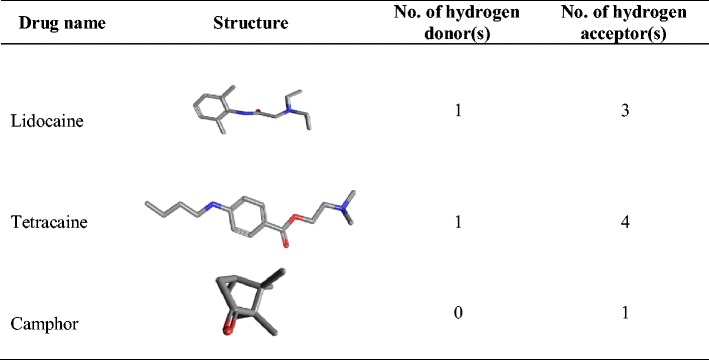
CONCLUSION
There is a good potential to formulate lidocaine-tetracaine eutectic mixture as it is stable and shows no crystallization when the EM was left in room temperature for 24 h. It can be concluded that if the components of a eutectic system have sufficient intermolecular physical interacting groups, and if their crystallization peaks are close to each other, then the eutectic system will tend to remain stable. This finding would be critical when selecting the components for a formulation which is based on a eutectic mixture, so as to ensure that no components crystallize out of the formulation.
Acknowledgments
Conflict of Interest
The authors report no declarations of interest.
References
- 1.Avula SG, Alexander K, Riga A. Predicting eutectic behavior of drugs and excipients by unique calculations. J Therm Anal Calorim. 2010;99:655–658. doi: 10.1007/s10973-009-0595-1. [DOI] [Google Scholar]
- 2.Stott PW, Williams AC, Barry BW. Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J Control Release. 1998;50:297–308. doi: 10.1016/S0168-3659(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 3.Bi M, Sung-Joo H, Kenneth RM. Mechanism of eutectic formation upon compaction and its effects on tablet properties. Thermochim Acta. 2003;404:213–226. doi: 10.1016/S0040-6031(03)00185-0. [DOI] [Google Scholar]
- 4.Ehrenstrom-Reiz GME, Reiz SLA. EMLA—a eutectic mixture of local anaesthetics for topical anaesthesia. Acta Anaesthesiol Scand. 1982;26:596–598. doi: 10.1111/j.1399-6576.1982.tb01822.x. [DOI] [PubMed] [Google Scholar]
- 5.Keiji S, Noboru O. Studies on absorption of eutectic mixture I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem Pharm Bull. 1961;9:866–872. doi: 10.1248/cpb.9.866. [DOI] [Google Scholar]
- 6.Goldberg AH, Gibaldi M, Kanig JL. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures II: experimental evaluation of a eutectic mixture: urea-acetaminophen system. J Pharm Sci. 1966;55:482–487. doi: 10.1002/jps.2600550507. [DOI] [PubMed] [Google Scholar]
- 7.Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA. Preparation and in vitro characterization of a eutectic-based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: mechanism and progress of emulsion formation. Int J Pharm. 2002;235:247–265. doi: 10.1016/S0378-5173(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Gala U, Pham H, Chauhan H. Pharmaceutical applications of eutectic mixtures. J Dev Drug. 2013;2:1–2. [Google Scholar]
- 9.Tainmont J. Dr Bonain, an ENT surgeon with an ocean background. B-ENT. 2007;3:217–230. [PubMed] [Google Scholar]
- 10.Jyvakorpi M. A comparison of topical Emla cream with Bonain’s solution for anesthesia of the tympanic membrane during tympanocentesis. Eur Arch Otorhinolaryngol. 1996;253:234–236. doi: 10.1007/BF00171133. [DOI] [PubMed] [Google Scholar]
- 11.Brodin A, Nyqvist-Mayer A, Wadsten T, Forslund B, Broberg F. Phase diagram and aqueous solubility of the lidocaine-prilocaine binary system. J Pharm Sci. 1984;73:481–484. doi: 10.1002/jps.2600730413. [DOI] [PubMed] [Google Scholar]
- 12.Berndt B, Hans E. Local anesthetic mixture for topical application, and process for its preparation, as well as method for obtaining local anesthesia. US 4562060 (1985).
- 13.Nyqvist-Mayer A, Brodin F, Frank G. Phase distribution studies on an oil–water emulsion based on a eutectic mixture of lidocaine and prilocaine as the dispersed phase. J Pharm Sci. 1985;74:1192–1195. doi: 10.1002/jps.2600741112. [DOI] [PubMed] [Google Scholar]
- 14.Luotonen J, Laitakari K, Karjalainen H, Jokinen K. EMLA in local anaesthesia of the tympanic membrane. Acta Otolaryngol. 1992;492:63–67. doi: 10.3109/00016489209136812. [DOI] [PubMed] [Google Scholar]
- 15.Hahn IH, Hoffman RS, Nelson LS. EMLA-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26:85–88. doi: 10.1016/j.jemermed.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Vasters FG, Eberhart LH, Koch T, Kranke P, Wulf H, Morin AM. Risk factors for prilocaine-induced methaemoglobinaemia following peripheral regional anaesthesia. Eur J Anaesthesiol. 2006;23:760–765. doi: 10.1017/S0265021506000913. [DOI] [PubMed] [Google Scholar]
- 17.Boran P, Tokuc G, Yegin Z. Methemoglobinemia due to application of prilocaine during circumcision and the effect of ascorbic acid. J Pediatr Urol. 2008;4:475–476. doi: 10.1016/j.jpurol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Jun HW, Kang L. Enhanced transdermal anesthesia of local anesthetic agents. WO2000069471 A1 (2001).
- 19.The PubChem Structure Search Database. http://pubchem.ncbi.nlm.nih.gov/search/search.cgi Accessed 13 August 2014.
- 20.Kang L, Jun HW, Mani N. Preparation and characterization of two phase melt systems of lidocaine. Int J Pharm. 2001;222:35–44. doi: 10.1016/S0378-5173(01)00689-5. [DOI] [PubMed] [Google Scholar]
- 21.Woolfson AD, McCafferty DF, Moss GP. Development and characterisation of a moisture-activated bioadhesive drug delivery system for percutaneous local anaesthesia. Int J Pharm. 1998;169:83–94. doi: 10.1016/S0378-5173(98)00109-4. [DOI] [Google Scholar]
- 22.Teng J, Liu S. Re-determination of succinonitrile (SCN)–camphor phase diagram. J Cryst Growth. 2006;290:248–257. doi: 10.1016/j.jcrysgro.2005.12.087. [DOI] [Google Scholar]
- 23.Alster T. A lidocaine 7% and tetracaine 7% peel for induction of local dermal anesthesia for nonablative facial laser resurfacing. J Am Acad Dermatol. 2005;52:44. [Google Scholar]
- 24.Goldberg L. A lidocaine 7% and tetracaine 7% peel for local dermal anesthesia before cryotherapy treatment for actinic keratosis. J Am Acad Dermatol. 2005;52:44. doi: 10.1016/j.jaad.2004.09.024. [DOI] [Google Scholar]
- 25.Fitzpatrick R. A lidocaine 7% and tetracaine 7% peel before pulsed dye laser therapy to treat facial lesions in adults. J Am Acad Dermatol. 2005;52:44. [Google Scholar]
- 26.Corvis Y, Espeau P. Incidence of chirality on the properties of mixtures containing an amide type anesthetic compound. Thermochim Acta. 2012;539:39–43. doi: 10.1016/j.tca.2012.03.027. [DOI] [Google Scholar]
- 27.Giron D. Applications of thermal analysis in the pharmaceutical industry. J Pharm Biomed Anal. 1986;4:755–770. doi: 10.1016/0731-7085(86)80086-3. [DOI] [PubMed] [Google Scholar]
- 28.Coleman J, Craig DQM. Modulated temperature differential scanning calorimetry: a novel approach to thermal analysis. Int J Pharm. 1996;135:13–29. doi: 10.1016/0378-5173(95)04463-9. [DOI] [Google Scholar]
- 29.Giron D. Contribution of thermal methods and related techniques to the rational development of pharmaceuticals—part 1. Pharm Sci Technol Today. 1998;1:191–199. doi: 10.1016/S1461-5347(98)00046-7. [DOI] [Google Scholar]
- 30.Kemp RB. Handbook of thermal analysis and calorimetry: from macromolecules to man. Amsterdam: Elsevier Science; 1999. [Google Scholar]
- 31.Giron D. Thermal analysis, microcalorimetry and combined techniques for study of pharmaceuticals. J Pharm Thermal Anal Calorim. 1999;56:1289–1304. [Google Scholar]
- 32.Clavaguera N, Saurinab J, Lheritierc J, Massec J, Chauvetc A, Clavaguera-Morad MT. Eutectic mixtures for pharmaceutical applications: a thermodynamic and kinetic study. Thermochim Acta. 1997;290:173–180. doi: 10.1016/S0040-6031(96)03077-8. [DOI] [Google Scholar]
- 33.Clas SD, Dalton CR, Hancock BC. Differential scanning calorimetry: applications in drug development. Pharm Sci Technol Today. 1999;2:311–320. doi: 10.1016/S1461-5347(99)00181-9. [DOI] [PubMed] [Google Scholar]
- 34.Ratkje SK, Rytter E. Raman spectra of molten mixtures containing aluminum fluoride. I. Lithium fluoride-trilithium hexafluoroaluminate eutectic mixture. J Phys Chem. 1974;78:1499–1502. doi: 10.1021/j100608a011. [DOI] [Google Scholar]
- 35.Sunol JJ, Berlanga R, Clavaguera-Morad MT, Clavaguera N. Modeling crystallization processes: transformation diagrams. Acta Mater. 2002;50:4783–4790. doi: 10.1016/S1359-6454(02)00321-X. [DOI] [Google Scholar]
- 36.Ma Y, Gill HS. Coating solid dispersions on microneedles via a molten dip-coating method: development and in vitro evaluation for transdermal delivery of a water-insoluble drug. Pharm Drug Del Pharm Technol. 2014 doi: 10.1002/jps.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shamsudin MS, Asli NA, Abdullah S, Yahya SYS, Rusop M. Effect of synthesis temperature on the growth iron-filled carbon nanotubes as evidenced by structural, micro-Raman, and thermogravimetric analyses. Adv Condens Matter Phys. 2012 [Google Scholar]
- 38.Kang L, Jun HW. Formulation and efficacy studies of new topical anesthetic creams. Drug Dev Ind Pharm. 2003;29:505–512. doi: 10.1081/DDC-120018639. [DOI] [PubMed] [Google Scholar]
- 39.Giron D, Draghi M, Goldbronn C, Pfeffer S, Piechon P. Study of the polymorphic behavior of some local anesthetic drugs. J Therm Anal. 1997;49:913–927. doi: 10.1007/BF01996777. [DOI] [Google Scholar]
- 40.Woolfson AD, McCafferty DF. Percutaneous local anaesthesia: drug release characteristics of the amethocaine phase-change system. Int J Pharm. 1993;94:75–80. doi: 10.1016/0378-5173(93)90011-4. [DOI] [Google Scholar]
- 41.Dennis AC, McGarvey JJ, Woolfson AD, McCafferty DF, Moss GP. A Raman spectroscopic investigation of bioadhesive tetracaine local anaesthetic formulations. Int J Pharm. 2004;279:43–50. doi: 10.1016/j.ijpharm.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt AC. The role of molecular structure in the crystal polymorphism of local anesthetic drugs: crystal polymorphism of local anesthetic drugs, part X. Pharm Res. 2005;22:2121–2133. doi: 10.1007/s11095-005-8135-6. [DOI] [PubMed] [Google Scholar]
- 43.Riga AT, Oberoi LM, Alexander KS. Calorimetry as a preformulation tool for studying eutectics relevant in pharmaceuticals. Am Pharm Rev. 2004;7:18–23. [Google Scholar]
- 44.Vepuri SB, Anbazhagan S, Divya D, Padmini D. A review on supramolecular chemistry in drug design and formulation research. Indones J Pharm. 2013;24:131–150. [Google Scholar]
- 45.Kam NWS. Analysis of drug samples using Raman micro-spectroscopy. Physical Sciences & Mathematics. 2001;5. http://legacy.jyi.org/volumes/volume5/issue1/articles/kam.html. Accessed 12 June 2014.
- 46.Daferera DJ, Tarantilis PA, Polissiou MG. Characterization of essential oils from Lamiaceae species by Fourier transform Raman spectroscopy. J Agric Food Chem. 2002;50:5503–5507. doi: 10.1021/jf0203489. [DOI] [PubMed] [Google Scholar]


