Abstract
This work aims to prepare sustained release buccal mucoadhesive lyophilized chitosan sponges of buspirone hydrochloride (BH) to improve its systemic bioavailability. Chitosan sponges were prepared using simple casting/freeze-drying technique according to 32 factorial design where chitosan grade was set at three levels (low, medium, and high molecular weight), and concentration of chitosan solution at three levels (0.5, 1, and 2%). Mucoadhesion force, ex vivo mucoadhesion time, percent BH released after 8 h (Q8h), and time for release of 50% BH (T50%) were chosen as dependent variables. Additional BH cup and core buccal chitosan sponge were prepared to achieve uni-directional BH release toward the buccal mucosa. Sponges were evaluated in terms of drug content, surface pH, scanning electron microscopy, swelling index, mucoadhesion strength, ex vivo mucoadhesion time, and in vitro drug release. Cup and core sponge (HCH 0.5E) were able to adhere to the buccal mucosa for 8 h. It showed Q8h of 68.89% and exhibited a uni-directional drug release profile following Higuchi diffusion model.
KEY WORDS: buspirone HCL, casting/freeze-drying technique, chitosan, cup and core sponge, mucoadhesive buccal sponges
INTRODUCTION
Chitosan, the second most abundant polymer in nature after cellulose, is prepared by partial N-deacetylation of chitin (1). Chemically, chitosan is a linear polysaccharide consisting of N-acetyl-2-amino-2-deoxy-d-glucopyranose and 2-amino-2-deoxy-d-glucopyranose linked by (1 → 4) β-glycosidic bonds (2). Free amino groups confer a positive charge to chitosan, thus allowing its reaction with negatively charged surfaces and anionic polymers (1). Owing to its cationic nature, chitosan attains good mucoadhesive properties to the anionic sialic and sulfonic acid substructures found in the mucus gel layer by ionic interactions (3,4). Among presently explored mucoadhesive polymers, chitosan is gaining an increasing importance due to its favorable properties such as non-toxicity, biocompatibility, biodegradability (5), and absorption enhancing properties (1).
Sponges may be defined as dispersions of gas (usually air) in a solid matrix to yield a solid porous structure (6). Sponges provide a potential mean of local and systemic drug delivery to the mucosal surfaces. Sponges offer some advantages over other drug delivery systems. Unlike semi-solid polymer gels which flow easily after application, sponges can maintain their swollen gel structure for a longer period allowing longer residence time and effective drug absorption. In addition, sponges have a higher drug loading capacity compared to the thin films due to their porous nature and higher surface area (7,8). Chitosan sponges are prepared by freeze-drying of chitosan solutions generating a porous material due to the removal of ice crystals by the lyophilization process. Factors such as pore size and orientation influence the mechanical properties of chitosan sponges which can be controlled by varying the freezing rate, the ice crystal size, and the geometry of thermal gradients during freezing (9).
Buccal route is an attractive site for local or systemic drug delivery through the buccal mucosal membrane lining of the oral cavity (10). Buccal mucosa is easily accessible and suitable for administration of retentive dosage forms (11). Systemic drug delivery via the buccal route offers some advantages over per oral administration such as bypassing the first-pass effect and avoidance of presystemic elimination within the gastrointestinal tract (4). Other advantages such as low enzymatic activity, easy drug withdrawal, facility to include permeation enhancers, and versatility in designing multidirectional or uni-directional release systems prove that buccal adhesive systems are promising dosage forms (11).
Buspirone hydrochloride (BH) is an anxiolytic drug used in the treatment of generalized anxiety disorder by modulating the serotonergic system (12,13). BH has a very low oral bioavailability (4%) due to extensive first-pass metabolism (14,15). BH low bioavailability and low molecular weight (422 Da) (15), in addition to its short and variable elimination half-life (mean of 2.4 h) (15,16), recommend it a good candidate for sustained release buccal dosage forms.
In our previous work (17), BH mucoadhesive buccal matrix and cup and core tablets were prepared using various mucoadhesive polymers. The selected formula was further evaluated for its in vivo performance in healthy human volunteers, and the results revealed a 5.6-fold increase in bioavailability compared to oral administration of the commercially available BH tablet (Buspar®, 15 mg BH, Glaxo-Smith Kline Co., Cairo, Egypt).
In the present study, BH mucoadhesive sustained release buccal chitosan sponges were developed using simple casting/freeze-drying technique by employing different chitosan grades (low, medium, and high molecular weight) in different concentrations.
MATERIALS AND METHODS
Materials
Buspirone hydrochloride (BH) was kindly supplied by Glaxo-Smith Kline Co. (Cairo, Egypt). Low molecular weight chitosan (L-CH) [3.8–20 kDa, degree of deacetylation 85%, viscosity 30–200 cps], medium molecular weight chitosan (M-CH) [20–190 kDa, degree of deacetylation 85%, viscosity 200–800 cps], high molecular weight chitosan (H-CH) [190–375 kDa, degree of deacetylation 85%, viscosity 800–2000 cps], ethyl cellulose 100 cps (Ethocel), and dibutylphthalate (>98%) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, USA). All other chemicals were of analytical grade and used as received.
Compatibility of BH with Sponge Excipients
Physical mixtures of BH with various excipients, namely L-CH, M-CH, H-CH, and Ethocel, were prepared by mixing in a weight ratio of 1:1. The prepared mixtures were evaluated for possible interactions via differential scanning calorimetry and Fourier transform infrared spectroscopy.
DSC
Differential scanning calorimetry (DSC) analysis was performed using Shimadzu differential scanning calorimeter (DSC-60, Shimadzu, Kyoto, Japan). Samples (3–4 mg) were placed in aluminum pan and heated in the range 10–400°C at a rate of 10°C/min, with indium in the reference pan, in an atmosphere of nitrogen. The DSC studies were performed for the drug, the aforementioned excipients, and the drug-excipient powder mixtures.
FTIR
Fourier transform infrared spectroscopy (FTIR) spectra between 4000 and 500 cm−1 of the drug, the aforementioned excipients, and drug-excipient powder mixtures were determined using FTIR spectrophotometer (Model 22, Bruker, UK) according to the potassium bromide disc technique.
Preparation of BH Buccal Chitosan Sponges Adopting Casting/Freeze-Drying Technique Using Different Chitosan Grades
The composition of BH buccal chitosan sponges was listed in Table I. Accurate quantities of each chitosan grade, namely L-CH, M-CH, and H-CH, were gradually added to 1% v/v acetic acid with constant stirring to prepare 0.5, 1, and 2% w/w chitosan solutions of each grade. An amount of 150 mg BH was dissolved in 5 g of each chitosan solution to give a dose of 15 mg BH per 0.5 g solution. The medicated chitosan solutions were left overnight at room temperature to ensure clear, bubble-free solutions. An amount of 0.5 g of each chitosan solution was poured in a cylindrical plastic mold with a diameter of 13 mm and a thickness of 3 mm. The cylindrical plastic molds were frozen at −20°C for 24 h and then freeze-dried at −45°C under a vacuum of 7 × 10−2 mBAR (Novalyphe-NL 500, Savant, Holbrook, NY, USA). BH buccal chitosan sponges were prepared according to 32 factorial experimental design to investigate the influence of formulation variables on the release profile of the drug and mucoadhesion properties of the formulae. In this design, chitosan grade (X1) and concentration of chitosan solution (X2) were selected as independent variables, whereas mucoadhesion force (Y1), ex vivo mucoadhesion time (Y2), percentage of BH released after 8 h–Q8h–(Y3), and time required for the release of 50% of BH–T50%–(Y4) were chosen as dependent variables. The levels of the chosen independent variables were illustrated in Table II. The objective was to prepare BH buccal sponges that have good mucoadhesion force, sustain the mucoadhesion, and have maximum release extent with a suitable release rate. Once prepared, sponges were stored in a dessicator at 65% relative humidity (provided by saturated sodium nitrite solution) and at room temperature to obtain soft pliable sponges before further investigation (18).
Table I.
Formulae of Different BH Buccal Chitosan Sponges
| Formula | Concentration of different chitosan solutions (% w/w) | Ethocel cup | |||
|---|---|---|---|---|---|
| L-CH | M-CH | H-CH | |||
| Sponges | LCH0.5 | 0.5 | – | – | |
| LCH1 | 1 | – | – | ||
| LCH2 | 2 | – | – | ||
| MCH0.5 | – | 0.5 | – | ||
| MCH1 | – | 1 | – | ||
| MCH2 | – | 2 | – | ||
| HCH0.5 | – | – | 0.5 | ||
| HCH1 | – | – | 1 | ||
| HCH2 | – | – | 2 | ||
| Cup and core sponge | HCH0.5Ea | – | – | 0.5 as core | Ethocel film layer prepared from 0.5 g of 4% Ethocel solution and dibutylphthalate (30% w/w of polymer) |
All formulae contain 15 mg BH
L-CH Low molecular weight chitosan, M-CH medium molecular weight chitosan, H-CH high molecular weight chitosan
Table II.
Levels of Independent Variables
| Factors (independent variables) | Levels of variables |
|---|---|
| X1: Chitosan grade | L-CH |
| M-CH | |
| H-CH | |
| X2: Concentration of chitosan solution (%) | 0.5 |
| 1 | |
| 2 |
L-CH Low molecular weight chitosan, M-CH medium molecular weight chitosan, H-CH high molecular weight chitosan
Preparation of BH Cup and Core Buccal Chitosan Sponges
In an attempt to achieve uni-directional drug release toward the buccal mucosa, additional cup and core buccal chitosan sponge were prepared in which the Ethocel cup served as a backing layer to offer a uni-directional release and the core chitosan sponge contained the drug.
Preparation of the Ethocel Cup Layer
Ethocel solution (4% w/w) was prepared by gradually adding accurate quantity of Ethocel in ethanol containing dibutylphthalate (30% w/w based on polymer) as plasticizer and stirring overnight. An amount of 0.5 g Ethocel solution was poured in the cylindrical plastic molds mentioned before. The molds were kept in 40°C oven (WBT binder E53, Germany) for 24 h to evaporate the solvent leaving an Ethocel film layer acting as the cup.
Preparation of the Cup and Core Sponge
An amount of 0.5 g of the medicated 0.5% H-CH chitosan solution was poured in the plastic molds containing the preformed Ethocel cup layer as mentioned above. The cylindrical plastic molds were frozen at −20°C for 24 h and then freeze-dried at −45°C under a vacuum of 7 × 10−2 mBAR. The prepared sponges were stored in a desiccator under the previously mentioned conditions until further investigations (18). The composition of the prepared BH cup and core buccal chitosan sponge was listed in Table I.
In Vitro Evaluation of BH Buccal Chitosan Sponges
Determination of Drug Content
The amount of BH in the sponge was extracted with 250 ml of simulated saliva fluid (SSF; pH 6.8) at room temperature. After filtration through 0.45 μm millipore filter, BH concentration was determined spectrophotometrically at 240 nm (Shimadzu UV-1601 PC, Kyoto, Japan) and compared to a preconstructed calibration curve (R2 = 0.9998, n = 3) after sufficient dilution with SSF (pH 6.8). The test was done in triplicate, and the mean drug content ± SD was determined.
Surface pH Study
The sponge was allowed to swell by keeping it in contact with 2 ml of SSF (pH 6.8) for 2 h at room temperature. The pH was measured by bringing the electrode of the pH meter in contact with the surface of the sponge and allowing it to equilibrate for 1 min. The surface pH for each sponge was determined in triplicate and the mean ± SD was calculated (19).
SEM
The surface morphology and cross-sections of selected chitosan sponges were examined by scanning electron microscopy (SEM). A thin piece of the sponge was fixed on the SEM sample holder with double-sided adhesive tape and was coated under an argon atmosphere with gold using a sputter coater (Edwards S-105A, England) to achieve a film of 150 Ao thickness. The samples were then examined using SEM (Jeol, JXA-840A, Tokyo, Japan).
Swelling Study
The swelling index (SI) for each sponge was determined in triplicate, and the mean ± SD was calculated. Chitosan sponges were weighed individually (W1), placed separately on 2% agar gel plates, and incubated at 37 ± 1°C. At regular 2 h time intervals until 8 h, the sponge was removed from the petri dish, and excess surface water was removed carefully with filter paper. The swollen sponge was then reweighed (W2), and the swelling index was calculated using the following equation (20):
| 1 |
In Vitro Mucoadhesion Strength Measurement
The modified two-armed physical balance method used in our previous work was used to measure the in vitro bioadhesive force of the prepared chitosan sponges (17), as shown in Fig. 1. Briefly, freshly excised bovine buccal mucosa (B) [obtained from a local slaughterhouse and stored in normal saline at 4°C upon collection] was fixed on the glass stage (C) using cyanoacrylate adhesive. The prepared sponge (D) was attached to the balance pan, and then the glass stage (C) was raised slowly until the sponge surface came in contact with the buccal mucosa. A preload of 50 g (E) was applied over the balance pan above the sponge for 5 min and then removed. The weights (F) were raised until the sponge was detached from the buccal mucosa. The minimum weight, in grams, that detached the sponge from the membrane surface was taken as a measure of the bioadhesive strength. The force of adhesion was deduced using the following equation (19,21):
| 2 |
Fig. 1.
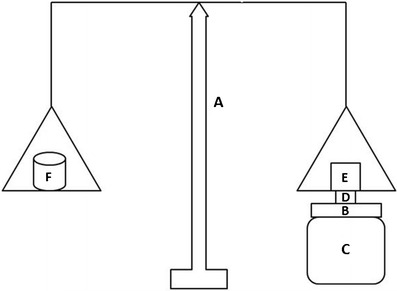
Modified balance method for the measurement of in vitro mucoadhesion strength. (A) modified balance, (B) bovine buccal mucosa, (C) glass stage, (D) buccal chitosan sponge, (E) preload of 50g and (F) weights
Ex Vivo Mucoadhesion Time
A freshly cut bovine buccal mucosa was fixed on the internal side of a beaker with cyanoacrylate adhesive. A side of each sponge was wetted with 50 μl of SSF (pH 6.8) and was attached to the buccal tissue by applying a light force with a fingertip for 20 s. The beaker was filled with 800 ml of SSF and kept at 37 ± 1°C; after 2 min, a stirring rate of 150 rpm was applied to simulate the buccal cavity. Mucoadhesive time was monitored until complete detachment of the sponge occurred. The test was done in triplicate, and the mean mucoadhesion time ± SD was calculated (22).
In Vitro Drug Release Studies
In vitro drug release studies of the prepared BH buccal chitosan sponges as well as the immediate release commercially available Buspar® tablets (Glaxo-Smith Kline Co., Cairo, Egypt) was performed using a modified standard basket apparatus (USP Dissolution Tester, Varian, model VK7000, USA) (17,22,23). One side of the sponge was attached to the bottom flat end of the stirring rod instead of the basket fixture using cyanoacrylate adhesive. This was done to simulate the in vivo conditions. The vessel was filled with 250 ml SSF (pH 6.8) at 37 ± 1°C and stirred at 100 rpm. Aliquots each of 3 ml were withdrawn from the release medium at different time intervals and replaced by equal volume of fresh SSF kept at the same temperature. The concentration of the released drug was measured spectrophotometrically at λmax of 240 nm. The experiments were done in triplicate, and the average ± SD was calculated.
Kinetic Analysis of the Release Data
The release data were kinetically analyzed using Excel 2007 (Microsoft, software) to determine the mechanism and the order of drug release from different formulations. Zero order, first order, Higuchi, and Korsmeyer–Peppas models were used for the analysis of the release kinetics.
Statistical Data Analysis
Analysis of the factorial design was performed using Social Package for Statistical Study software (SPSS 17®, SPSS Inc., Chicago, USA) using a significance of p < 0.05.
RESULTS
Compatibility of BH with Sponge Excipients
DSC
Figure 2 showed the DSC thermograms of BH, each of the aforementioned excipients and the 1:1 w/w drug-excipient physical mixtures. Pure BH exhibited an endothermic peak of 203.23°C corresponding to its melting point as shown in Fig. 2a (24). The DSC peak of BH was preserved in its physical mixtures with each of the aforementioned excipients indicating that there was no interaction between the drug and the used excipients.
Fig. 2.
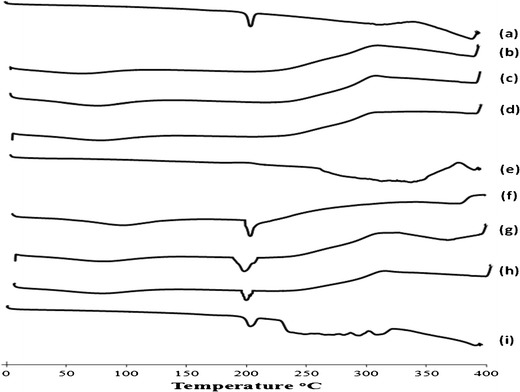
DSC thermograms of (a) BH, (b) L-CH, (c) M-CH, (d) H-CH, (e) Ethocel, (f) physical mixture of BH and L-CH, (g) physical mixture of BH and M-CH, (h) physical mixture of BH and H-CH, (i) physical mixture of BH and Ethocel. All physical mixtures were of (1:1) drug-to-polymer ratio
FTIR
The FTIR spectrum of pure BH showed characteristic peaks at 3032.1, 2886.22 (aromatic C–H stretching), 1724.36, 1678 (−C═O stretching), 1589.34, 1486.19 (aromatic –C═C stretching), and 1273.02 cm−1 (−C–N stretching) (24,25). There were no considerable changes in the IR peaks of BH when mixed with excipients, indicating the absence of chemical interaction with the used excipients.
In Vitro Evaluation of BH Buccal Chitosan Sponges
Determination of Drug Content
The drug content in all prepared chitosan sponges was uniform and did not deviate markedly from the required amount. The average drug content ranged from 14.89 ± 1.29 (HCH0.5) to 16.13 ± 1.14 mg (HCH1).
Surface pH Study
The surface pH of all chitosan sponges ranged from 5.57 ± 0.37 (LCH1) to 6.20 ± 0.33 (MCH2). According to these results, all sponges provided an acceptable pH in the range of salivary pH (5.5–7.0) and they would not produce any local irritation to the mucosal surface upon application.
SEM
SEM photos of L-CH, M-CH, and H-CH sponges, as well as the prepared cup and core sponge (HCH0.5E) were shown in Fig. 3. The micrographs revealed the porous interconnecting polymeric network of the prepared sponges. As observed from the micrographs, L-CH sponges had the smallest pore sizes and lowest pore density compared to M-CH and H-CH sponges. The average pore sizes of the L-CH, M-CH, and H-CH sponges were approximately 27.78 ± 4.57, 41.12 ± 5.53, and 40.34 ± 4.79 μm, respectively. In a previous study, Foda et al. (26) stated that cross-linked L-CH had smaller pore sizes than cross-linked H-CH. The variation in the performance of the different formulae with respect to hydration capacity and release characteristics could be attributed to the differences in pore sizes.
Fig. 3.
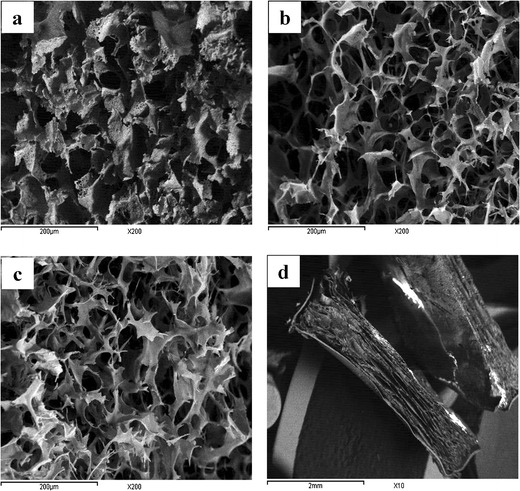
Scanning electron micrographs of a L-CH, b M-CH, and c H-CH in cross-section view (magnification ×200) and d cup and core sponge, HCH0.5E, in cross-section view (magnification ×10)
Regarding the cup and core sponge, the porous structure of the core sponge could be clearly distinguished from the non-porous structure of the Ethocel cup as seen in Fig. 3d.
Swelling Study
As shown in Fig. 4a, sponges prepared from H-CH showed maximum swelling index. Among all formulae, HCH2 showed the highest value of 20.12 ± 0.94 and LCH0.5 showed the lowest value of 5.58 ± 0.64 swelling index at the end of the 8 h. Similar results were obtained by Foda et al. (26) who stated that the dissolution medium uptake capacity of H-CH sponges was higher than that of L-CH sponges. Regarding the cup and core sponge, HCH0.5E, it showed decreased swelling properties compared to the core sponge alone, HCH0.5, as shown in Fig. 4b.
Fig. 4.
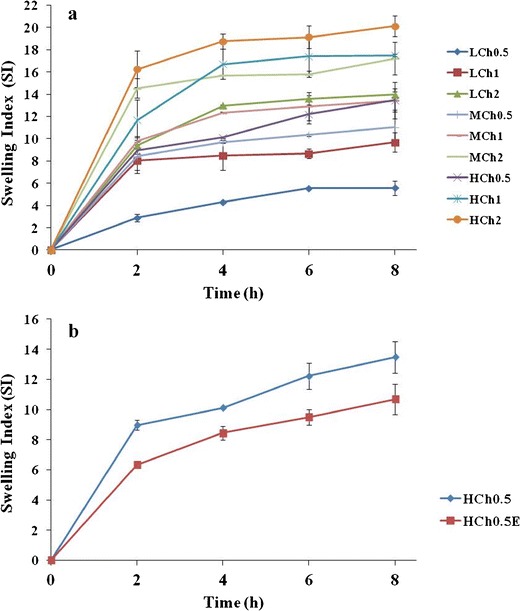
Swelling index of a BH buccal chitosan sponges and b BH cup and core buccal chitosan sponge, HCH0.5E, compared to formula HCH0.5
In Vitro Mucoadhesion Strength Measurement
Table III showed the mucoadhesion force of different buccal chitosan sponges. Formula HCH2 showed the strongest mucoadhesion force (0.36 ± 0.02 N) whereas formula LCH0.5 showed the weakest mucoadhesion force (0.13 ± 0.02 N).
Table III.
Mucoadhesion Force and Ex Vivo Mucoadhesion Time of BH Buccal Chitosan Sponges
| Formula | Mucoadhesion force ± SDa
(N) |
Ex vivo mucoadhesion time ± SDa
(h) |
|---|---|---|
| LCH0.5 | 0.13 ± 0.02 | 5.75 ± 0.35 |
| LCH1 | 0.24 ± 0.01 | 6.00 ± 0.35 |
| LCH2 | 0.26 ± 0.02 | 6.38 ± 0.18 |
| MCH0.5 | 0.20 ± 0.01 | 7.38 ± 0.18 |
| MCH1 | 0.33 ± 0.01 | >8.00 |
| MCH2 | 0.34 ± 0.01 | >8.00 |
| HCH0.5 | 0.22 ± 0.02 | >8.00 |
| HCH1 | 0.34 ± 0.01 | >8.00 |
| HCH2 | 0.36 ± 0.02 | >8.00 |
| HCH0.5Eb | 0.24 ± 0.01 | >8.00 |
SD standard deviation
aMean ± SD, sample size = 3
bBH cup and core buccal chitosan sponge
A full 32 factorial design was applied to evaluate the effect of chitosan grade (X1) and concentration of chitosan solution (X2) on the mucoadhesion force (Y1) (Fig. 5a). Analysis of factorial design demonstrated that the chitosan grade had a significant effect on the mucoadhesion force (p < 0.05). The different chitosan grades could be ranked according to mucoadhesion force as follows: H-CH > M-CH > L-CH. Subsequent least significance difference (LSD) test was performed, and it revealed that the mucoadhesion force of the sponges prepared from L-CH was significantly different from that of the sponges prepared using either M-CH or H-CH. On the other hand, the mucoadhesion forces of the sponges prepared using either M-CH or H-CH were non-significant from each other.
Fig. 5.
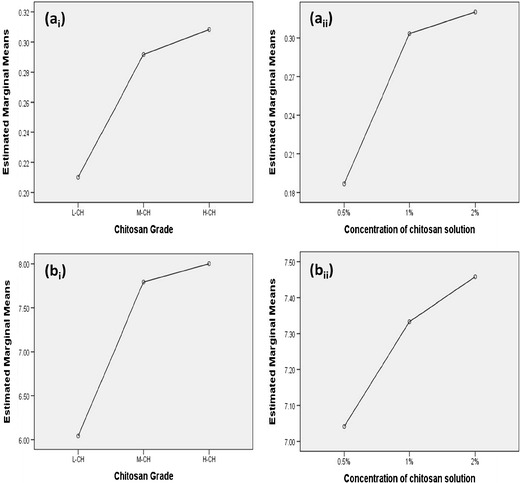
Effect of chitosan grade and concentration of chitosan solution on a the mucoadhesion force and b the ex vivo mucoadhesion time
Results also revealed that the concentration of chitosan solution showed a significant effect on the mucoadhesion force (p < 0.05). Subsequent LSD test revealed that the mucoadhesion force of sponges prepared from 0.5% chitosan solution was significantly different from that of the sponges prepared using either 1 or 2% chitosan solutions. On the other hand, the mucoadhesion forces of the sponges prepared using 1 or 2% chitosan solutions were non-significant from each other.
Cup and core formula (HCH0.5E) attained almost the same mucoadhesion force as formula HCH0.5, as the later served as the core for formula HCH0.5E. Therefore, it was concluded that the Ethocel cup did not interfere with the mucoadhesion power of the cup and core sponge.
Ex Vivo Mucoadhesion Time
Table III showed that M-CH and H-CH buccal sponges attained the longest mucoadhesion time where formulae MCH1, MCH2, HCH0.5, HCH1, and HCH2 acquired adhesion time of 8 h. The cup and core chitosan sponge, HCH0.5E, attained the same mucoadhesion time as formula HCH0.5 which served as the core for the cup and core formula.
A full 32 factorial design was applied to evaluate the effect of chitosan grade (X1) and concentration of chitosan solution (X2) on the ex vivo mucoadhesion time (Y2) (Fig. 5b). Analysis of factorial design demonstrated that the chitosan grade had a significant effect on the mucoadhesion time (p < 0.05). Different chitosan grades could be ranked according to the mucoadhesion time as follows: H-CH ~ M-CH > L-CH. Subsequent LSD test revealed that the mucoadhesion time of the sponges prepared from L-CH was significantly shorter from that of the sponges prepared using either M-CH or H-CH. On the other hand, the mucoadhesion times of the sponges prepared using M-CH and H-CH were non-significantly different from each other.
Results also revealed that the concentration of chitosan solution showed a significant effect on the mucoadhesion time (p < 0.05). Subsequent LSD test revealed that the mucoadhesion time of sponges prepared from 0.5% chitosan solution was significantly different from that of the sponges prepared using either 1 or 2% chitosan solutions. On the other hand, the mucoadhesion times of the sponges prepared using 1 or 2% chitosan solutions were non-significant from each other.
In Vitro Drug Release Studies
The mean equilibrium solubility of BH in SSF (pH 6.8) was determined in our previous work (17) and was found to be 3.73 ± 0.13 mg/ml. Such high solubility ensured sink condition in the volume of dissolution medium used in the in vitro study for the doses of BH loaded in the formulations.
Results of the in vitro release of BH from the different buccal chitosan sponges in comparison to the market product (Buspar®) were shown in Fig. 6. All the chitosan sponges achieved a sustained drug release profile for the duration of 8 h while the market product (Buspar®) released almost 100% of its BH content after 1 h.
Fig. 6.
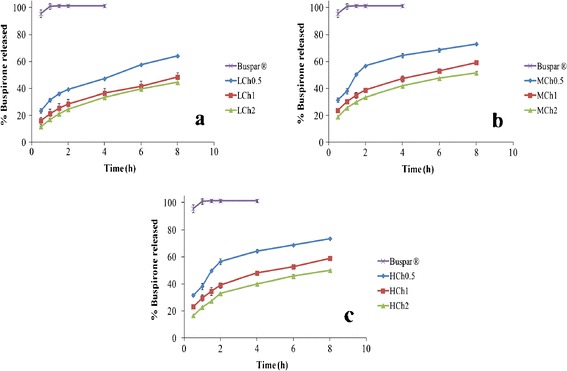
In vitro release profile of BH buccal chitosan sponges prepared using a L-CH, b M-CH, and c H-CH with different concentration of chitosan solution in SSF (pH 6.8) at 37°C in comparison to Buspar® tablet
Initially, the dissolution profiles of all formulae showed burst drug release after 30 min. This burst release ranged from 11.57 ± 0.41% in formula LCH2 to 31.67 ± 1.32% in formula HCH 0.5. After that, the drug release was due to the diffusion process, which was much slower when compared to the initial release. It is also worth noting that none of the formulae showed complete drug release.
A full 32 factorial design was applied to evaluate the effect of chitosan grade (X1) and concentration of chitosan solution (X2) on the Q8h (Y3) and T50% (Y4). The results were shown in Fig. 7a, b. Analysis of factorial design demonstrated that the chitosan grade had a significant effect on the Q8h and T50% (p < 0.05). Subsequent LSD test revealed that L-CH had the significantly lowest Q8h and the longest T50% and the highest retardation of the release rate. On the other hand, the effect of both M-CH and H-CH on the Q8h and T50% was non-significantly different from each other.
Fig. 7.
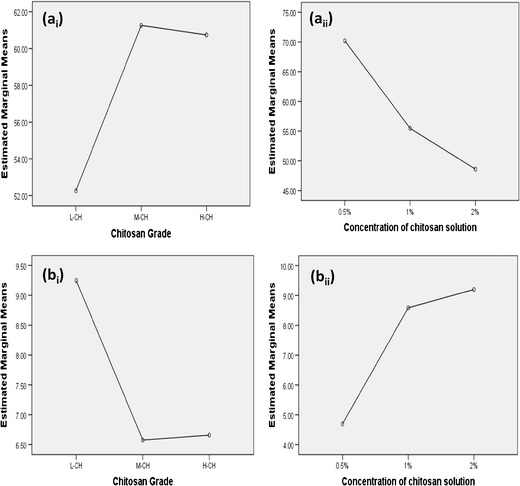
Effect of chitosan grade and concentration of chitosan solution on a the Q8h and b the T50%
Results also revealed that the concentration of chitosan solution showed a significant effect on Q8h and T50% (p < 0.05). It was obvious that increasing the chitosan concentration from 0.5 to 1 and to 2% significantly decreased Q8h and increased T50% and significantly retarded the drug release rate (p < 0.05).
The results of the in vitro release of BH from the prepared cup and core buccal chitosan sponge (HCH0.5E) in comparison to the immediate release market product (Buspar®) as well as the core formula (HCH0.5) were shown in Fig. 8. It was obvious that the prepared cup and core formula (HCH0.5E) showed slightly lower percentages of drug released compared to the core formula (HCH0.5) as well as a slower drug release rate. The effect of adding the cup part to the sponge on the release of the drug was further studied. This was done by statistically analyzing the Q8h and T50% of both the cup and core sponge HCH0.5E and the core formula HCH0.5 using one-way ANOVA to test the significance of difference at p ≤ 0.05. There was a significant difference between the Q8h and T50% of both formulae (p < 0.05).
Fig. 8.
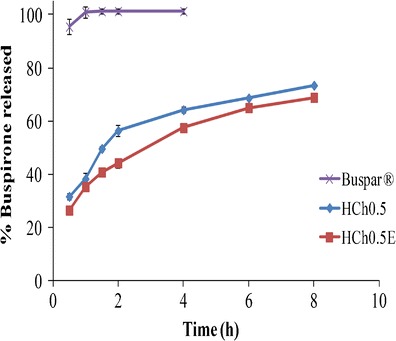
In vitro release profile of BH cup and core buccal chitosan sponge in SSF (pH 6.8) at 37°C in comparison to Buspar® tablet and the core formula, HCH0.5
Kinetic Analysis of the Release Data
The release kinetic data of BH from different chitosan sponges and the corresponding T50% of the drug were presented in Table IV. The in vitro release data were analyzed according to zero, first order, and diffusion-controlled mechanisms according to simplified Higuchi model (27). The selection of a certain mechanism was based on the highest coefficient of determination (r2). Since, in practice, polymeric matrices release the drug through a combination of different mechanisms (28), Korsemeyer–Peppas model was used to analyze the release kinetics. The release data were fitted to the following general equation (29):
| 3 |
Table IV.
Kinetic Parameters of the Release Data from All BH Buccal Chitosan Sponges Prepared
| Formula | Zero | First | Diffusion | Mechanism | Korsemeyer–Peppas model | T50%
(h) |
||
|---|---|---|---|---|---|---|---|---|
| r 2 | r 2 | n | Mechanism | |||||
| LCH0.5 | 0.943 | 0.851 | 0.991 | Diffusion | 0.991 | 0.344 | Diffusion | 6.93 |
| LCH1 | 0.947 | 0.871 | 0.994 | Diffusion | 0.998 | 0.388 | Diffusion | 10.10 |
| LCH2 | 0.962 | 0.863 | 0.996 | Diffusion | 0.998 | 0.443 | Diffusion | 10.69 |
| MCH0.5 | 0.844 | 0.783 | 0.926 | Diffusion | 0.961 | 0.435 | Diffusion | 3.56 |
| MCH1 | 0.928 | 0.861 | 0.986 | Diffusion | 0.997 | 0.323 | Diffusion | 7.77 |
| MCH2 | 0.926 | 0.845 | 0.985 | Diffusion | 0.994 | 0.355 | Diffusion | 8.40 |
| HCH0.5 | 0.852 | 0.792 | 0.932 | Diffusion | 0.966 | 0.423 | Diffusion | 3.60 |
| HCH1 | 0.961 | 0.928 | 0.988 | Diffusion | 0.995 | 0.331 | Diffusion | 7.87 |
| HCH2 | 0.923 | 0.878 | 0.962 | Diffusion | 0.986 | 0.392 | Diffusion | 8.50 |
| HCH0.5Ea | 0.951 | 0.878 | 0.994 | Diffusion | 0.997 | 0.368 | Diffusion | 4.53 |
aBH cup and core buccal chitosan sponge
where Mt/M∞ represents the drug dissolved fraction at time t, K is a kinetic constant, and n is the diffusional exponent. It is worth noting that n ≤ 0.45 corresponds to a Fickian (case I) diffusion, 0.45 < n < 0.89 to an anomalous (non-Fickian) transport (where release is controlled by a combination of diffusion and polymer relaxation), n = 0.89 to a zero order (case II) transport (where the drug release rate is independent of time and involves polymer relaxation), and n > 0.89 to a super case II transport (30). However, this equation is valid only for the early stages (≤60%) of drug release (31).
The release of BH from all buccal chitosan sponges followed diffusion-controlled mechanism according to Higuchi model. It was also observed that in all formulae, the values of n were less than 0.45, indicating a Fickian (case I) diffusion transport.
DISCUSSION
The swelling index was dependent on the chitosan molecular weight and its percentage in each sponge. The higher swelling capacity of M-CH and H-CH sponges compared to L-CH sponges might be attributed to their characteristic structure which showed larger pore sizes and higher pore density, thus allowing the intake of higher amount of water and consequently higher swelling. The decrease in the swelling properties of the cup and core sponge, HCH0.5E, could be due to the presence of the non-porous and non-swellable Ethocel cup, which would hinder the swelling ability of the core sponge.
The mucoadhesion force results were in agreement with the findings of Lehr et al. (32), who stated that the viscosities of the solutions prepared from different chitosan samples were correlated to the measured force of detachment and mucoadhesive performance, so the higher molecular weight of chitosan favored stronger mucoadhesion and subsequent higher force of detachment. The ex vivo mucoadhesion time results were in agreement with the results of the mucoadhesion force test. As expected, the sponges showing stronger force of adhesion remained attached to the mucosa for a longer period of time, i.e., showing longer mucoadhesion time. In addition to the force of adhesion, chitosan sponges showed little erosion at pH 6.8. Park et al. (33) stated that cationic polymers as chitosan possessed good mucoadhesion properties in neutral or slightly alkaline medium, which might be explained by the little solubility of chitosan at this pH leading to little erosion (32). Besides, Lehr et al. (32) reported that weak, short-lasting mucoadhesion of chitosan in an artificial gastric fluid could be explained by chitosan solubility in acidic solutions.
It was obvious that increasing the chitosan concentration from 0.5 to 1 or 2% significantly increased the mucoadhesion force and time (p < 0.05). This could be attributed to the increase in the content of the bioadhesive chitosan in the prepared sponges. This led to greater amount of positively charged amino groups, which interacted with the negatively charged sialic acid and sulfate residues of the mucin glycoprotein and hence leading to a stronger mucoadhesion force (34).
The drug release from chitosan sponges might involve one of three different mechanisms: (a) drug release from the surface of sponges, (b) diffusion through the swollen rubbery matrix, or (c) drug release due to polymer erosion (35). Initially, the dissolution profiles of all formulae showed burst drug release after 30 min which might be attributed to the dissolution of the drug adhering to the surface and not entrapped within the inner matrix of the sponge. After that, the drug release was due to the diffusion process, which was much slower when compared to the initial release. In a previous study, Foda et al. (26) reported that a large percentage of tramadol HCl was released in the first hour for both uncross-linked and cross-linked matrices, and they attributed this to the presence of surface drug which was confirmed by SEM micrographs. It is also worth noting that none of the formulae showed complete drug release as 100% drug release from the matrix occurred only after complete erosion or degradation of the chitosan matrix (35).
Many authors reported that the release of active drugs from different chitosan dosage forms decreased with the increase in chitosan molecular weight. Polk et al. (36) reported that the molecular weight of chitosan was a key variable in the release of albumin from chitosan microspheres, where decreasing the molecular weight increased the release of albumin. Similarly, Ko et al. (37) observed that the release of felodipine from cross-linked chitosan microparticles decreased as the molecular weight of chitosan increased. On the other hand, Genta et al. (38) reported that the fastest ketoprofen release was obtained from the M-CH microspheres. They attributed this to the swelling rate and behavior of chitosan microspheres. They confirmed that M-CH microspheres swelled and dissolved very quickly with respect to L-CH and H-CH microspheres.
In our study, L-CH sponges showed the lowest Q8h, the longest T50%, and the highest retardation of the release rate compared to M-CH and H-CH sponges. This unexpected result could be attributed to the difference in the pore size and pore density between the L-CH sponges and the M-CH and H-CH sponges. As previously observed from the SEM micrographs, L-CH sponges had the smallest pore sizes. In addition, the pore density of L-CH sponges was lower than that of M-CH and H-CH sponges. This might lead to slower dissolution medium influx inside L-CH sponges and slower drug diffusion, leading to more release retardation by L-CH sponges. In a previous study, Foda et al. (26) reported that the release of tramadol HCl from L-CH sponges was slower than from H-CH sponges because smaller pore sizes were evident in L-CH sponges compared to sponges prepared by higher grades of chitosan. In another study, Varshosaz et al. (39) stated that the release of lidocaine from uncross-linked chitosan films increased as the molecular weight of chitosan increased. Lidocaine release from L-CH films was slower than that from M-CH films, and the highest release was obtained from H-CH films. On the contrary, in our study, M-CH and H-CH had non-significant effect on drug release.
The significant decrease in Q8h, increase in T50%, and drug release rate retardation upon increasing the chitosan concentration from 0.5 to 1 and to 2% could be attributed to the increase in the chitosan content leading to increased solution viscosity at higher chitosan concentrations, forming denser chitosan sponges.
Regarding the cup and core sponge, the significant retardation in the release rate manifested by the decrease in the Q8h and the increase in the T50% of the cup and core sponge HCH0.5E in comparison to the core sponge HCH0.5 might be attributed to the presence of the water impermeable Ethocel cup layer. The presence of this Ethocel cup layer led to the release of the drug from only one surface that decreased the surface area available for the diffusion of the drug leading to further release retardation.
CONCLUSION
BH buccal chitosan sponges were effective as mucoadhesive sustained release drug carriers. The analysis of the factorial design revealed that changing the chitosan grade and/or the concentration of chitosan solution had a significant effect on the mucoadhesive properties and the drug release from the prepared sponges. The cup and core sponge, HCH0.5E, sustained the mucoadhesion with the buccal mucosa for 8 h. On the basis of the in vitro release data and the kinetic analysis, HCH0.5E released 68.89% of the drug at the end of the 8 h following Higuchi diffusion model profile. The presence of the Ethocel cup layer led to a slight decrease in the Q8h and more retardation to the release rate compared to the core formula HCH0.5, but this Ethocel cup layer led to uni-directional drug release toward the buccal mucosa, so it could be concluded that a promising sustained release cup and core chitosan sponge were successfully developed for BH delivery via the buccal mucosa. However, further in vivo studies on the BH sustained release cup and core buccal sponge are needed to investigate its clinical efficacy and safety.
References
- 1.Pedro AS, Albuquerque EC, Ferreira D, Sarmento B. Chitosan: an option for development of essential oil delivery systems for oral cavity care? Carbohydr Polym. 2009;76:501–8. doi: 10.1016/j.carbpol.2008.12.016. [DOI] [Google Scholar]
- 2.Dasha M, Chiellini F, Ottenbriteb RM, Chiellini E. Chitosan—a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 2011;36:981–1014. doi: 10.1016/j.progpolymsci.2011.02.001. [DOI] [Google Scholar]
- 3.Bernkop-Schnurch A, Dunnhaupt S. Chitosan-based drug delivery systems. Eur J Pharm Biopharm. 2012;81(3):463–9. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153(2):106–16. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Oungbho K, Müller BW. Chitosan sponges as sustained release drug carriers. Int J Pharm. 1997;156:229–37. doi: 10.1016/S0378-5173(97)00201-9. [DOI] [Google Scholar]
- 6.Lai HL, Abu'Khalil A, Craig DQ. The preparation and characterisation of drug-loaded alginate and chitosan sponges. Int J Pharm. 2003;251(1–2):175–81. doi: 10.1016/S0378-5173(02)00590-2. [DOI] [PubMed] [Google Scholar]
- 7.Ayensu I, Mitchell JC, Boateng JS. Development and physico-mechanical characterisation of lyophilised chitosan wafers as potential protein drug delivery systems via the buccal mucosa. Colloids Surf B: Biointerfaces. 2012;91:258–65. doi: 10.1016/j.colsurfb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Boateng JS, Auffret AD, Matthews KH, Humphrey MJ, Stevens HN, Eccleston GM. Characterisation of freeze-dried wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int J Pharm. 2010;389(1–2):24–31. doi: 10.1016/j.ijpharm.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Shen EC, Wang C, Fu E, Chiang CY, Chen TT, Nieh S. Tetracycline release from tripolyphosphate-chitosan cross-linked sponge: a preliminary in vitro study. J Periodontal Res. 2008;43(6):642–8. doi: 10.1111/j.1600-0765.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- 10.Madhav NV, Shakya AK, Shakya P, Singh K. Orotransmucosal drug delivery systems: a review. J Control Release. 2009;140(1):2–11. doi: 10.1016/j.jconrel.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Shumilov M, Touitou E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int J Pharm. 2010;387(1–2):26–33. doi: 10.1016/j.ijpharm.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Nash JR, Nutt DJ. Pharmacotherapy of anxiety. In: Holsboer F, Ströhle A, editors. Anxiety and anxiolytic drugs. Germany: Springer; 2005. pp. 469–501. [Google Scholar]
- 14.Gannu R, Yamsani SK, Palem CR, Yamsani VV, Kotagiri H, Yamsani MR. Development of high performance liquid chromatography method for buspirone in rabbit serum: application to pharmacokinetic study. Anal Chim Acta. 2009;647(2):226–30. doi: 10.1016/j.aca.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Moffat AC, Osselton MD, Widdop B. Monographs: buspirone. In: Watts J, editor. Clarke’s analysis of drugs and poisons. 4. London: Pharmaceutical Press; 2011. pp. 1014–5. [Google Scholar]
- 16.Sakr A, Andheria M. Pharmacokinetics of buspirone extended-release tablets: a single-dose study. J Clin Pharmacol. 2001;41(7):783–9. doi: 10.1177/00912700122010582. [DOI] [PubMed] [Google Scholar]
- 17.Kassem MA, Elmeshad AN, Fares AR. Enhanced bioavailability of buspirone hydrochloride via cup and core buccal tablets: formulation and in vitro/in vivo evaluation. Int J Pharm. 2014;463(1):68–80. doi: 10.1016/j.ijpharm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Foda NH, El-laithy HM, Tadros MI. Implantable biodegradable sponges: effect of interpolymer complex formation of chitosan with gelatin on the release behavior of tramadol hydrochloride. Drug Dev Ind Pharm. 2007;33(1):7–17. doi: 10.1080/03639040600975188. [DOI] [PubMed] [Google Scholar]
- 19.Darwish MK, Elmeshad AN. Buccal mucoadhesive tablets of flurbiprofen: characterization and optimization. Drug Discov Ther. 2009;3(4):181–9. [PubMed] [Google Scholar]
- 20.Patel VM, Prajapati BG, Patel MM. Formulation, evaluation, and comparison of bilayered and multilayered mucoadhesive buccal devices of propranolol hydrochloride. AAPS PharmSciTech. 2007;8(1):E147–54. doi: 10.1208/pt0801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morsi NM, Abdelbary GA, Ahmed MA. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: development and in vitro/in vivo characterization. Eur J Pharm Biopharm. 2013. [DOI] [PubMed]
- 22.Perioli L, Ambrogi V, Giovagnoli S, Ricci M, Blasi P, Rossi C. Mucoadhesive bilayered tablets for buccal sustained release of flurbiprofen. AAPS PharmSciTech. 2007;8(3):E54. doi: 10.1208/pt0803054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perioli L, Ambrogi V, Pagano C, Scuota S, Rossi C. FG90 chitosan as a new polymer for metronidazole mucoadhesive tablets for vaginal administration. Int J Pharm. 2009;377(1–2):120–7. doi: 10.1016/j.ijpharm.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Al-Zoubi N, Alkhatib HS, Bustanji Y, Aiedeh K, Malamataris S. Sustained-release of buspirone HCl by co spray-drying with aqueous polymeric dispersions. Eur J Pharm Biopharm. 2008;69(2):735–42. doi: 10.1016/j.ejpb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Joshi GV, Kevadiya BD, Bajaj HC. Design and evaluation of controlled drug delivery system of buspirone using inorganic layered clay mineral. Microporous Mesoporous Mater. 2010;132:526–30. doi: 10.1016/j.micromeso.2010.04.003. [DOI] [Google Scholar]
- 26.Foda NH, El-laithy HM, Tadros MI. Optimization of biodegradable sponges as controlled release drug matrices. I. Effect of moisture level on chitosan sponge mechanical properties. Drug Dev Ind Pharm. 2004;30(4):369–79. doi: 10.1081/DDC-120030931. [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–9. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 28.Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm. 1989;57:169–72. doi: 10.1016/0378-5173(89)90306-2. [DOI] [Google Scholar]
- 29.Korsmeyer R, Gumy R, Doelker E, Buri P, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [Google Scholar]
- 30.Harland RS, Gazzaniga A, Sangalli ME, Colombo P, Peppas NA. Drug/polymer matrix swelling and dissolution. Pharm Res. 1988;5(8):488–94. doi: 10.1023/A:1015913207052. [DOI] [PubMed] [Google Scholar]
- 31.Ritger PL, Peppas NA. A simple equation for description of solute release II. fickian and anomalous release from swellable devices. J Control Release. 1987;5:37–42. doi: 10.1016/0168-3659(87)90035-6. [DOI] [Google Scholar]
- 32.Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–8. doi: 10.1016/0378-5173(92)90353-4. [DOI] [Google Scholar]
- 33.Park H, Amiji M, Park K. Mucoadhesive hydrogels effective at neutral pH. Proc Int Symp Control Rel Bioact Mater. 1989;16:217–8. [Google Scholar]
- 34.Abruzzo A, Bigucci F, Cerchiara T, Cruciani F, Vitali B, Luppi B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr Polym. 2011;87:581–8. doi: 10.1016/j.carbpol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Agnihotri SA, Aminabhavi TM. Controlled release of clozapine through chitosan microparticles prepared by a novel method. J Control Release. 2004;96(2):245–59. doi: 10.1016/j.jconrel.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Polk A, Amsden B, De Yao K, Peng T, Goosen MF. Controlled release of albumin from chitosan-alginate microcapsules. J Pharm Sci. 1994;83(2):178–85. doi: 10.1002/jps.2600830213. [DOI] [PubMed] [Google Scholar]
- 37.Ko JA, Park HJ, Hwang SJ, Park JB, Lee JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int J Pharm. 2002;249(1–2):165–74. doi: 10.1016/S0378-5173(02)00487-8. [DOI] [PubMed] [Google Scholar]
- 38.Genta I, Perugini P, Pavanetto F. Different molecular weight chitosan microspheres: influence on drug loading and drug release. Drug Dev Ind Pharm. 1998;24(8):779–84. doi: 10.3109/03639049809082726. [DOI] [PubMed] [Google Scholar]
- 39.Varshosaz J, Karimzadeh S. Development of crosslinked chitosan films for oral mucosal delivery of lidocaine. Res Pharm Sci. 2007;2:43–52. [Google Scholar]


