Abstract
Aims and objectives
This prospective study was done to evaluate and compare the utility and effectiveness of platelet rich fibrin (PRF) with that of platelet rich plasma (PRP) on soft tissue healing and bone tissue healing of extracted third molar sockets.
Materials and methods
This study included split mouths of 20 patients who underwent bilateral extraction of impacted third molars. During the same appointment, following which PRF and PRP were prepared from patients’ autologous blood and placed in right and left extracted sockets, respectively. The data for soft tissue healing were recorded at end of 1 week, using healing index of Landry et al. and the data for bone tissue healing were recorded at the end of 4 months using digitalized orthopantomogram images on Adobe Photoshop CS; which was then compared between the two sites of the same patient.
Results
The mean values of soft tissue healing collected at 1 week post-operative, for PRF group were significantly higher as compared to PRP group. And the mean values of bone density collected at the end of fourth month post-operative, for PRF group were also significantly higher as compared to PRP group. Both tests showed p value of 0.00.
Conclusion
PRF is significantly better in promoting soft tissue healing and also faster regeneration of bone after third molar extraction, in comparison with PRP. This could be attributed to simpler preparation protocols of PRF over PRP and the ability of PRF to release growth factors in a controlled way.
Keywords: Third molar extraction, Wound healing, PRF, PRP, Growth factors
Introduction
Third molar (M3) extraction is one of the most common procedures performed in oral and maxillofacial surgery units. The literature on complications associated with M3 removal is voluminous. The majority of complications are inflammatory, with alveolitis being the most common. As such, efforts to limit intraoperative or post-operative complications may have a great impact in terms of enhancing patient outcome [1].
The limitation of surgery is that it neither guarantees nor promotes healing. At best, surgeons attempt to remove the known obstacles to healing such as infection, instability, foreign bodies, etc. Use of antibiotics, basic techniques of primary debridement and wound closure, internal fixation was already in prevalence [2].
Then, in 1980s, the three seminal works by Kinghton, Hunt, and Marx et al. identified the pivotal role that oxygen plays in all wound healing; this finding marked a paradigm shift by focusing attention on actively promoting healing than just removing the obstacles to it. Beginning in the 1990s and continuing till today, growths factors have emerged as the “Holy Grail” in wound-healing [2].
Platelet rich plasma (PRP) and the new generation concentrate platelet-rich fibrin (PRF), are biomaterials which are known to contain platelet-derived growth factors (PDGFαα, PDGFββ, and PDGFαβ), 2 of numerous transforming growth factors-β (TGFβ1 and TGFβ2), vascular endothelial growth factor (VEGF), and epithelial growth factor (EGF) [3]. Thus, these biomaterials can be used to promote active wound healing.
However, it is now necessary to look further into platelet and inflammatory features of these biomaterials. Only a perfect understanding of its components and their significance will enable us to comprehend the clinical results obtained and subsequently extend the fields of therapeutic application of these protocols [3].
This study establishes the assessment of role of PRP and PRF in wound healing of extracted M3s. Also, this study compares the effectiveness of these biomaterials in wound healing.
Methodology
This study consisted of 20 patients between ages of 18–28 years having bilateral impacted M3s (Table 1), randomly chosen from the outpatient department of JSS Dental College and Hospital, Mysore, Karnataka, India. Based on the prevalence of bilateral impacted M3s in our institution, sample size was selected using the formula N = (Z2pq)/d2 [where N: sample size, Z = 1.96, p = 1.3 % and q = (1 − p) 1] and the power of the study was set at 95 %.
Table 1.
Patients who underwent bilateral third molar extraction
| Sl. no. | Age in years | Gender | OP no. | Soft tissue healing values (healing index of Landry et al.) | Bone tissue healing values (grey scale intensity as measured on Adobe Photoshop CS) | ||
|---|---|---|---|---|---|---|---|
| PRF side | PRP side | PRF side | PRP side | ||||
| 1 | 21 | Female | 9427/13 | 5 | 5 | 171.23 | 169.02 |
| 2 | 26 | Male | 20103/13 | 5 | 5 | 156.76 | 152.47 |
| 3 | 24 | Male | 11107/13 | 5 | 5 | 149.46 | 148.13 |
| 4 | 27 | Female | 4364/13 | 5 | 5 | 153.87 | 151.74 |
| 5 | 27 | Male | 5146/13 | 4 | 3 | 152.23 | 151.13 |
| 6 | 26 | Male | 4554/13 | 3 | 2 | 169.19 | 152.58 |
| 7 | 25 | Male | 3305/13 | 5 | 4 | 149.81 | 142.62 |
| 8 | 27 | Female | 5215/13 | 5 | 5 | 172.41 | 151.46 |
| 9 | 26 | Male | 4762/13 | 5 | 4 | 158.37 | 144.62 |
| 10 | 25 | Female | 11038/13 | 5 | 5 | 153.64 | 151.69 |
| 11 | 25 | Male | 5622/13 | 4 | 3 | 156.82 | 136.64 |
| 12 | 27 | Female | 4048/13 | 5 | 4 | 166.57 | 131.36 |
| 13 | 25 | Male | 11624/13 | 4 | 3 | 153.41 | 144.37 |
| 14 | 27 | Male | 10015/13 | 5 | 5 | 156.27 | 154.34 |
| 15 | 24 | Male | 6952/13 | 4 | 4 | 147.43 | 139.76 |
| 16 | 27 | Female | 11191/13 | 4 | 3 | 136.48 | 128.83 |
| 17 | 26 | Male | 14851/13 | 4 | 3 | 139.37 | 124.45 |
| 18 | 27 | Female | 13952/13 | 5 | 4 | 150.39 | 140.56 |
| 19 | 23 | Female | 10748/13 | 5 | 5 | 163.34 | 160.18 |
| 20 | 25 | Male | 4248/13 | 5 | 5 | 165.14 | 128.28 |
| Mean values | 4.6 | 4.1 | 156.1095 | 145.2115 | |||
After obtaining history, patients were examined clinically and were explained about the procedure, its complications and the follow-up period involved in the study. The patients who were willing were enrolled for the study.
In Split mouth of 20 patients, evaluations pertaining to classes of third molar impaction were done (Pell and Gregory classification) before placement of PRP on one side and PRF on the other. After obtaining the consent 12 ml of patients’ blood was drawn in a vacutainer and with help of centrifugation PRP and PRF were made in same appointment and placed in extracted third molar sockets, respectively. PRF was placed on the right side and PRP on the left side. The procedure for all the patients was performed by a single operator.
Study was conducted for over a period of 4 months and data for soft tissue healing was collected at end of 1 week, using Healing Index of Landry et al. [9] and data for bone tissue healing was collected at the end of 4 months post-operatively, using digitalized orhtopantomogram (OPG) images on Adobe Photoshop CS. The collected data was then compared between the two sites of the same patient.
Preparation of PRP
Under all aseptic techniques, 6 ml of blood was drawn intravenously from the anticubital region of patient’s forearm using vacutainer needle and BD vacutainers (each 6 ml) containing 3.8 % tri-sodium citrate (0.8 ml each). The vacutainers were thoroughly shaken to ensure mixture of anti-coagulant with the drawn blood. The whole blood was then centrifuged at 2,400 rpm for 10 min. The supernatant formed was platelet poor plasma (PPP) and buffy coat. PPP and buffy coat [upper 1 mm red blood corpuscles (RBC)] layer was collected in a fresh vacutainer and again centrifuged at 3,600 rpm for 10 min. The upper half of the supernatant was discarded and the lower half was mixed thoroughly to yield PRP. Activation of the PRP was done with addition of 10 % calcium gluconate to form PRP gel [4–8] (Fig. 1).
Fig. 1.
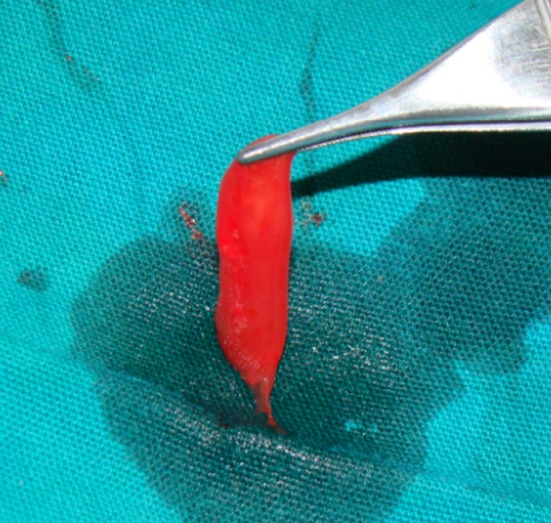
PRP gel
Preparation of PRF
Under all aseptic techniques, 6 ml of blood was drawn intravenously from the anticubital region of patient’s forearm using vacutainer needle and transferred into BD vacutainers without the anticoagulant. The blood sample taken without anticoagulant in tubes was immediately centrifuged at 3,000 rpm for 10 min. The absence of anticoagulant implies the activation in a few minutes of most platelets of the blood sample in contact with the tube walls and the release of the coagulation cascades.
Fibrinogen was initially concentrated in the high part of the tube, before the circulating thrombin transformed it into fibrin. A fibrin clot was then obtained in the middle of the tube, just between the red corpuscles at the bottom and cellular plasma at the top. The fibrin clot was then placed into extracted M3 socket on right side [3] (Fig. 2).
Fig. 2.
PRF gel
Surgical Technique
The patient was asked to rinse the mouth with 0.2 % chlorhexidine for 2 min prior to start of the procedure; the face was prepared with betadine and was draped. Inferior alveolar nerve block, lingual nerve block and long buccal nerve block were administered using 2 % lignocaine hydrochloride with 1: 80,000 adrenaline. Standard Ward’s incision was followed in all the cases. Full thickness mucoperiosteal flap was raised to expose sufficient bone on lateral and distal aspect of the impacted molar. Removal of bone was done with stainless steel bur. Constant irrigation was done with normal saline while removing bone to prevent thermal necrosis. Sectioning of tooth was done as planned preoperatively and surgically removed, in some cases tooth was luxated with the help of straight elevator and then extracted with molar forceps employing minimal force. The surrounding bone was smoothened. The wound was gently irrigated with sterile saline solution and checked for any small detached fragments of bone or tooth pieces. Surgical removal of impacted mandibular third molar was done on both sides in a similar way and PRP was placed on left side and PRF was placed on right side. The preprocessed PRP was taken into the sterile stainless steel bowl and 0.5–1 ml of 10 % calcium gluconate was mixed to obtain the PRP gel, which was then placed into the selected extraction socket and primary wound closure was done. The irregular margins of the wound were trimmed and wound was closed with interrupted sutures. Pressure pack was given.
Post-operative Instructions
Regular post extraction instructions were given. The patient was asked to follow-up as planned at day 7 post-operative for soft tissue healing analysis and fourth month post-operative for bone tissue healing analysis.
Clinical Evaluation of Soft Tissue Healing
Clinical evaluation for soft tissue healing was done on day 7 post-operatively and assessment was done using healing index of Landry et al. [9]. These values were then tabulated, and compared between PRP and PRF sites (Table 2).
Table 2.
Healing index by Landry et al. [9]
| 1. Very poor | Tissue colour: ≥50 % of gingiva red |
| Response to palpation: bleeding | |
| Granulation tissue: present | |
| Incision margin: not epithelialized, with loss of epithelium beyond incision margin | |
| Suppuration present | |
| 2. Poor | Tissue colour: ≥50 % of gingiva red |
| Response to palpation: bleeding | |
| Granulation tissue: present | |
| Incision margin: not epithelialized, with connective tissue exposed | |
| 3. Good | Tissue colour: ≥25 and <50 % of gingiva red |
| Response to palpation: no bleeding | |
| Granulation tissue: none | |
| Incision margin: no connective tissue exposed | |
| 4. Very good | Tissue colour: <25 % of gingiva red |
| Response to palpation: no bleeding | |
| Granulation tissue: none | |
| Incision margin: no connective tissue exposed | |
| 5. Excellent | Tissue colour: all tissues pink |
| Response to palpation: no bleeding | |
| Granulation tissue: none | |
| Incision margin: no connective tissue exposed |
Radiographic Evaluation for Hard Tissue Healing
A digital OPG was taken on Planmeca Promax Version 4, at the end of fourth month post-operatively for every patient. All exposures were standardized at 66 kV and 9 mA.
The images were viewed on a monitor; contrast and brightness were adjusted to enhance the image quality. The first post-operative radiographic image parameters were standardized and recorded and the same were applied for subsequent radiographs at 4 months post-operative for every patient.
The digitalized OPG images of the surgically removed bilateral impacted mandibular third molar sites were measured using Adobe Photoshop CS for grey scale values indicating densities. The data were recorded and stored for statistical analysis.
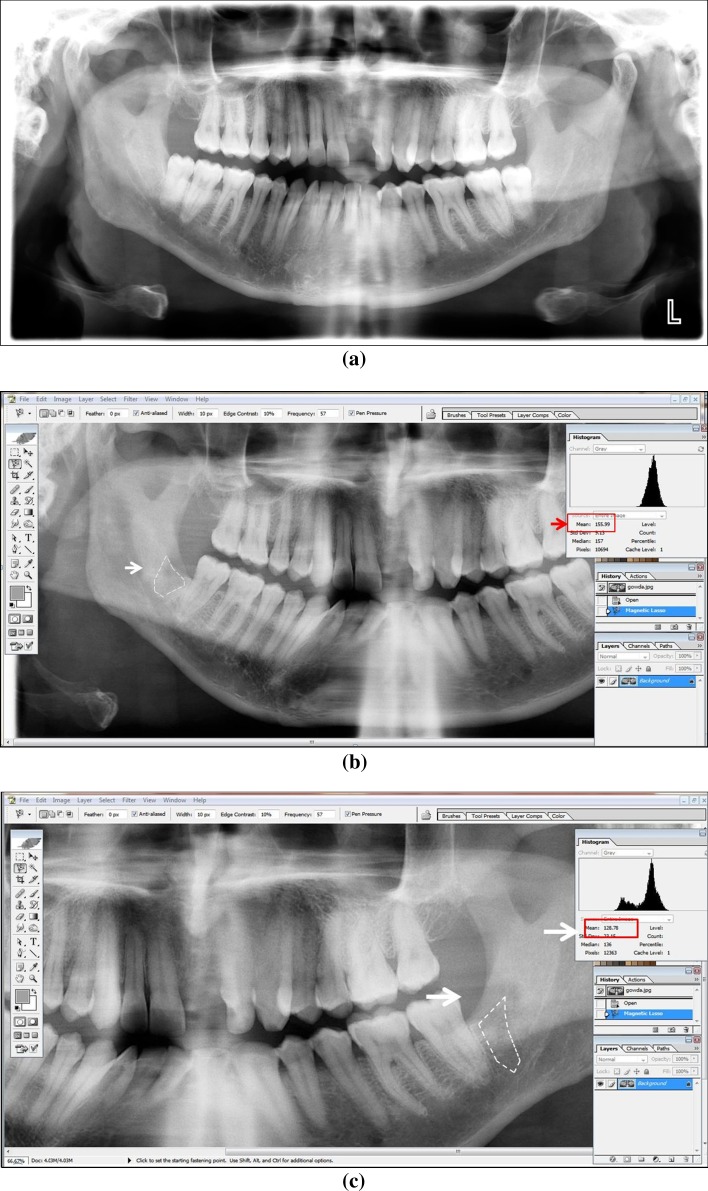
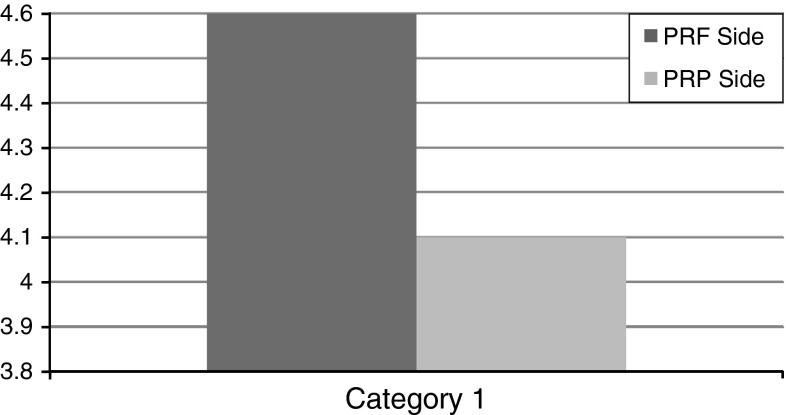
Assessment of Digitalized OPG Images (Fig. 3a–c)
Fig. 3.
a Pre-operative radiograph showing similar class of impaction on both sides. b Assessment of digitalized OPG images PRF side. c Assessment of digitalized OPG images PRP side
The digitalized OPG images were viewed on a screen using Adobe Photoshop CS. Taking the preoperative radiographs as a guide, the area of the extracted third molar socket was marked on each side using the Magnetic Lasso Tool.
The marked area on each side represents the area of bone formation at the end of fourth month post-operatively. The mean grey scale values, of the area bounded within the borders of extracted third molar socket on the PRF and PRP side was then measured using the Histogram Tool and tabulated.
These grey scale values were then recorded in a similar manner for all the 20 patients and 40 sites of PRF and PRP, respectively and tabulated. The mean grey scale values of the two sides were then compared, which represent the bone healing capabilities of PRF and PRP, respectively.
Results
Following statistical methods were employed in the present study. Descriptive statistics, Paired samples ‘t’ test.
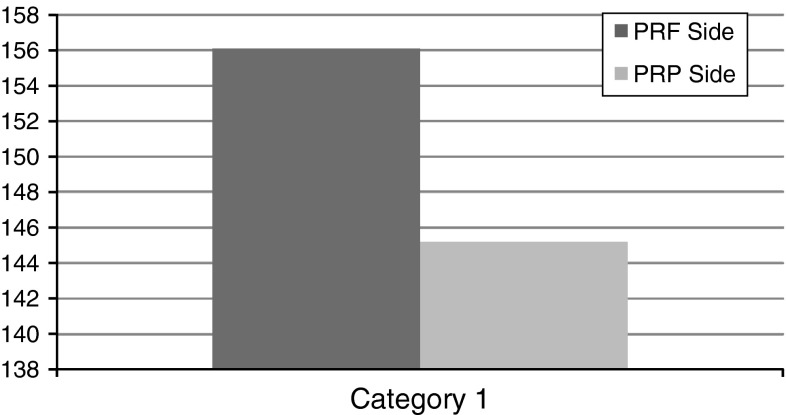
Significant differences were observed in the mean scores of Soft Tissue Healing capabilities of PRF and PRP groups 1 week post-operatively. The mean values of Soft Tissue Healing capability for PRF group were significantly higher as compared to PRP group. The ‘t’ value for 1 week post-operative is 4.359. ‘p’ value 0.000 is significant (Table 3; Fig. 4).
Table 3.
Mean values of soft tissue healing
| Paired T test | ||||
|---|---|---|---|---|
| Mean | N | SD | SEM | |
| PRF side | 4.6000 | 20 | 0.59824 | 0.13377 |
| PRP side | 4.1000 | 20 | 0.96791 | 0.21643 |
| Paired differences mean | t | df | Sig(2-tailed)… | |
|---|---|---|---|---|
| PRF to PRP | 0.5000 | 4.359 | 19 | 0.000 |
Fig. 4.
Mean values of soft tissue healing
Also, significant differences were observed in the mean scores of bone density, measured over digitalized OPG images between PRF and PRP groups fourth month post-operatively. The mean values of bone density for PRF groups were significantly higher as compared to PRP groups. The ‘t’ value for fourth month post-operative is 4.579. ‘p’ value 0.000 is significant (Table 4; Fig. 5).
Table 4.
Mean values of bone tissue healing
| Paired T test | ||||
|---|---|---|---|---|
| Mean | N | SD | SEM | |
| PRF side | 156.1095 | 20 | 9.74862 | 2.17986 |
| PRP side | 145.2115 | 20 | 11.37617 | 2.54379 |
| Paired differences mean | t | df | Sig(2-tailed)… | |
|---|---|---|---|---|
| PRF to PRP | 10.8980 | 4.579 | 19 | 0.000 |
Fig. 5.
Mean values of bone tissue healing
Discussion
We hypothesized that growth factors could be used to promote wound healing, minimize post-operative complications and thereby enhance patient outcome.
Beginning in the 1990s and continuing till today, growths factors have emerged as the “Holy Grail” in wound- healing [2].
Platelet plays a fundamental role in hemostasis and is a natural source of growth factors. These include, PDGF, insulin like growth factor (IGF), platelet derived angiogenic factor (PDAF), VEGF [10].
According to the definition of PRP, it may be assumed that these growth factors are present at increased concentrations in PRP. In addition to growth factors, platelets release numerous other substances (e.g., fibronectin, vitronectin, sphingosine 1-phosphate, etc.…) that are important in wound healing. Recently, the morphologic and molecular configuration of PRP was reported, it showed PRP is a fibrin framework over platelets which have the potential to support regenerative matrix [11, 12].
Fibrin is the activated form of a plasmatic molecule called fibrinogen. In fact, fibrinogen is the final substrate of all coagulation reactions. Being a soluble protein, fibrinogen is transformed into an insoluble fibrin by thrombin while the polymerized fibrin gel constitutes the first cicatricial matrix of the injured site [4]. PRF has to be considered as a fibrin biomaterial. Its molecular structure with low thrombin concentration is an optimal matrix for migration of endothelial cells and fibroblasts. It permits a rapid angiogenesis and an easier remodeling of fibrin in a more resistant connective tissue. Therefore, these PRF membranes can be used for all types of superficial cutaneous and mucous healing [13].
The IGF-1 rates resulting from cPRP or PRP technologies are necessarily low, the greater part of the circulating IGF-I is in the PPP (supernatant) which is conventionally discarded during the initial cPRP or PRP production steps which is not so in case of PRF [14].
Moreover with PRF, a progressive polymerization mode signifies increased incorporation of the circulating cytokines in the fibrin meshes (intrinsic cytokines). Such a configuration implies an increased lifespan for these cytokines, because they will be released and used only at the time of initial cicatricial matrix remodeling (long-term effect). The cytokines thus maintained are available in situ for a convenient period, when the cells start cicatricial matrix remodeling, i.e., when they have to be stimulated to launch injured site reconstruction [14].
A first comparative clinical study done highlights the value of using concentrated platelets for adipocyte grafts. The investigators hypothesized that the PRF would be more effective because the PRF clot forms a strong fibrin matrix with a complex 3-dimensional architecture and, unlike the PRPs, the Choukroun et al. [8] PRF does not dissolve quickly after application; instead, the strong fibrin matrix is remodeled slowly in a manner similar to the formation of a natural blood clot. Platelets and leukocytes are collected with high efficiency with the use of this method, and leukocytes are preserved throughout. The results of this study suggested that the combination of fat and PRF is more effective than the combination of fat and PRP in the context of facial lipostructure surgery [15].
A study was done to evaluate the effect of biologic characteristics of PRP and PRF on proliferation and differentiation of rat osteoblasts. It is demonstrated in this study that PRF experienced controllable and long-term release of growth factors. Levels of released TGF-β1 and PDGF-αβ markedly increased and reached the highest amount at day 14, then decreased mildly. In contrast, PRP experienced uncontrollable and short-term release of TGF-β1 and PDGF-αβ, which reached the highest amount at day 1 and then decreased rapidly. According to the limited data of this study, PRF released autologous growth factors gradually and expressed stronger and more durable effect on proliferation and differentiation of rat osteoblasts than PRP in vitro. The study thus showed that the use of PRF seems to be one of the most promising methods to enhance bone healing in a way that, PRF releases growth factors in more controlled manner and for relatively long-term of time [16].
Platelet-rich fibrin is prepared naturally without addition of thrombin, and it is hypothesized that PRF has a natural fibrin framework and can protect growth factors from proteolysis. Thus, growth factors can keep their activity for a relatively longer period and stimulate bone regeneration effectively [17].
Our results with regard to the enhanced soft tissue healing and increased rate of bone formation with PRF than compared to PRP may be attributed to the above mentioned advantages that PRF possesses over PRP.
Conclusion
The results of this study clearly indicate that: PRF is significantly better in promoting soft tissue healing and also faster regeneration of bone after third molar extraction, in comparison with PRP. Although, both PRF and PRP clinically showed very good soft tissue healing as measured by healing index of Landry et al. [9], further studies with larger sample size are needed to show much convincing effects of these biomaterials in terms of soft tissue healing. Moreover, PRF definitely showed to promote better osseous regeneration over PRP in terms of uniformity and density of regenerated bone which is statistically significant.
Although, the present study was done with a four month follow-up and the osseous regeneration was only measured indirectly over computer aided software (Adobe Photoshop CS), PRF did attribute to be a much simpler and a better platelet concentrate, in promoting soft tissue healing and osseous regeneration over PRP.
However, only a perfect understanding of these components would enable us to comprehend the clinical results obtained and further studies with larger sample size, longer follow-up and better evaluating measures for osseous regeneration will help us understand as to which among PRF or PRP is a better growth promoter.
Contributor Information
Tejesh Yelamali, Phone: +91 9844860995, Phone: +917259827107, Email: tejeshy@gmail.com.
D. Saikrishna, Email: degalasaikrishna@gmail.com
References
- 1.Chi BH, Edward B. Types, frequencies, and risk factors for complications after third molar extraction. Am Assoc Oral Maxillofac Surg. 2003;61(12):1379–1389. doi: 10.1016/j.joms.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE (1999) Chapter 4: platelet rich plasma: a source of multiple autologous growth factors for bone grafts. Tissue engineering. Application in maxillofacial surgery and periodontics. Quintessance publishing Co, Inc., pp 71–82
- 3.David M, Choukroun J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E37–E44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Kim S-G, Kim W-K, Park J-C, Kim H-J. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg. 2002;60:1018–1025. doi: 10.1053/joms.2002.34413. [DOI] [PubMed] [Google Scholar]
- 5.Landesberg R, Glickman RS, Ray M. Quantification of growth factor levels using a simplified method of platelet rich plasma gel preparation. J Oral Maxillofac Surg. 2002;58(3):297–300. doi: 10.1016/S0278-2391(00)90058-2. [DOI] [PubMed] [Google Scholar]
- 6.Gonshor A. Technique for producing platelet rich plasma and platelet concentrate: background and process. Int J Periodontics Restorative Dent. 2002;22:547–557. [PubMed] [Google Scholar]
- 7.How to make autologous platelet gel? Heart Internet. London Perfusion Science Web. http://www.Londonperfusionscience.com/services_platelet_gel_make.html
- 8.Tsay RC, Vo J, Burke A, Eisig S, Lu H, Landesberg R. Differential growth factor retention by platelet rich plasma composites. J Oral Maxillofac Surg. 2005;63:521–528. doi: 10.1016/j.joms.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Landry RG, Turnbell RS, Howley T. Effectiveness of benzydamine HCL in treatment of periodontal post-surgical patients. Res Clin Forums. 1988;10:105–118. [Google Scholar]
- 10.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737. doi: 10.1016/S0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 11.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Barbero JE, Galindo-Moreno P, Avila-Ortiz G, Caba O, Sanchez-Fernandez E, Wang HL. Flow cytometric and morphological characterization of platelet-rich plasma gel. Clin Oral Implants Res. 2006;17:687–693. doi: 10.1111/j.1600-0501.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- 13.David M, Choukroun J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E56–E60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 14.David M, Choukroun J. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E45–E50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Keyban SO, Hemmat S, Badri AA, Abdesbabzadeb A, Kbiabani K. Use of platelet-rich fibrin and platelet-rich plasma in combination with fat graft: which is more effective during facial lipostructure? Am Assoc Oral Maxillofac Surg J Oral Maxillofac Surg. 2013;71:610–621. doi: 10.1016/j.joms.2012.06.176. [DOI] [PubMed] [Google Scholar]
- 16.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–713. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16:356–363. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]