Abstract
An osteoma is a benign, slow growing osteogenic tumor characterized by proliferation of either compact or cancellous bone. It can be central, peripheral or extraskeletal. Clinically osteomas are usually asymptomatic. These lesions often go undetected unless incidentally found on radiographic survey or until they have extended to such an extent that they cause facial asymmetry or functional impairment. The most common site of osteoma to develop in maxillofacial region is skull. Giant osteomas in mandible are rare. We present and discuss a case of giant osteoma of right mandible which was surgically excised.
Keywords: Giant peripheral osteoma, Mandibular tumor, Gardner’s syndrome, Odontogenic tumors
Introduction
An osteoma is a benign, slow growing osteogenic tumour characterized by proliferation of either compact or cancellous bone [1, 2]. It can be central, peripheral or extraskeletal. Central osteomas arise from endosteum, peripheral osteoma from the periosteum; and extraskeletal osteoma within soft tissues or muscles [3–5]. Clinically osteomas are usually asymptomatic [2, 6]. These lesions often go undetected unless incidentally found on radiographic survey or until they have extended to such an extent that they cause facial asymmetry or functional impairment [2]. The most common site of osteoma to develop in maxillofacial region is skull [2, 7]. Giant osteomas are rarely found in the mandible. We present a rare case of giant osteoma of right mandible with review of literature.
Case Report
A 45 year old female reported to the department of Oral and Maxillofacial Surgery with a history of slowly progressive painless swelling on right submandibular region since 13 years. The patient denied any signs and symptoms like dysphagia, limited mouth opening, inability to masticate, and paraesthesia. There was no history of trauma. Her medical, family and personal history was unremarkable.
On extraoral examination, patient had an obvious facial asymmetry with swelling on right side of face. There was a well- circumscribed mass approximately 5 × 4 cm in size in the submandibular area arising from the inferior border of the mandible. It extends from the angle of the mandible to almost up to the midsymphysis region (Fig. 1). The Worm’s view revealed the swelling to extended up to the midline. However, there was no displacement of the structures to the contralateral side (Fig. 2). The swelling was non tender, hard, immobile and multilobular. The overlying soft tissue was normal and the local temperature was not raised. There was no oedema or erythema associated with the swelling. There was no blanching, pulsation, bruit or thrill detected.
Fig. 1.
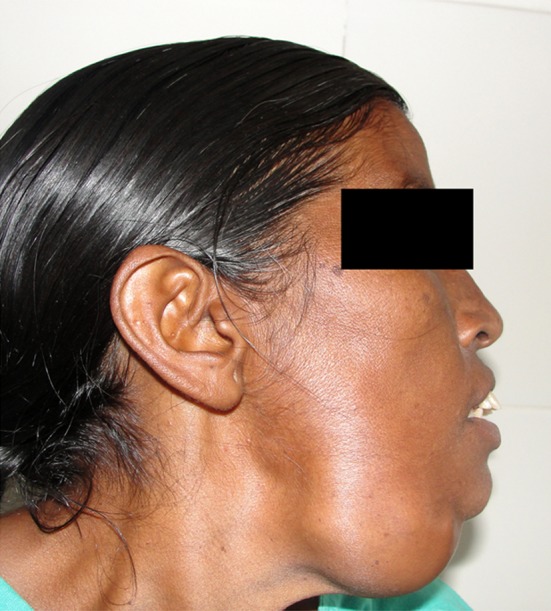
Profile of the patient
Fig. 2.
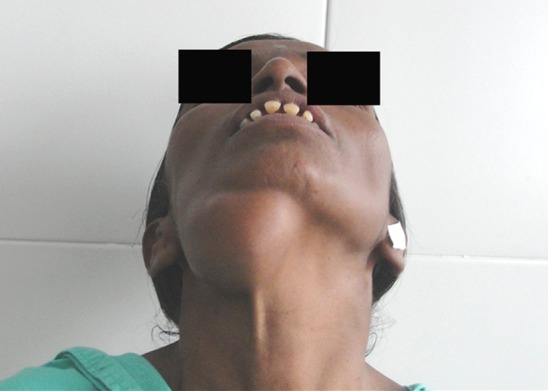
Submental profile view
The examination of the neck revealed no lymphadenopathy or thyromegaly. Carotid pulses were normal. There was no evidence of dermatological lesions like sebaceous cyst, additional osteomas of the cranium or facial bones, any skeletal abnormality or intestinal polyposis, which would have been consistent with Gardner’s syndrome.
Intraorally submucosal hard mass was palpable in the right lower buccal vestibule in the molar region with normal overlying mucosa. The first and second molars were missing and the third molar was carious but was nontender. There was no lingual cortical plate expansion.
There were no supernumerary teeth seen on OPG. A CT scan revealed a large bony mass attached to the buccal and lingual surface of the body, ramus, angle and the inferior border of right mandible. The mass protrudes towards inferolateral surface of the mandible and was causing medial displacement of the right submandibular gland. Superiorly the mass extended up to the mandibular notch and was very close to the lateral pterygoid muscle (Fig. 3).
Fig. 3.
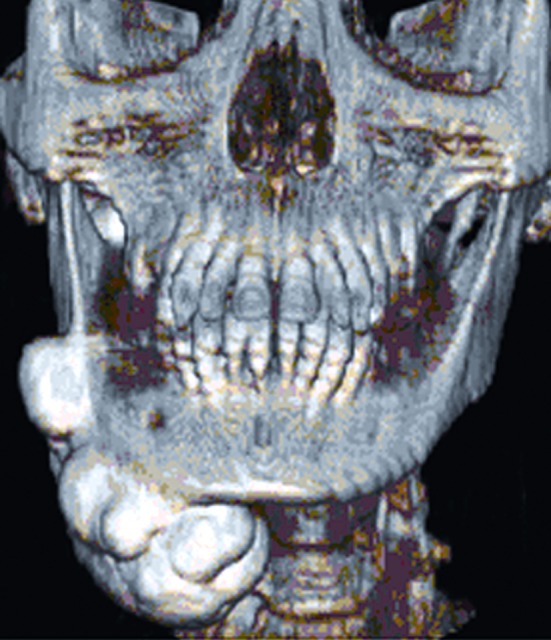
3 D Computed tomography image
A transoral incisional biopsy was performed under local anesthesia and the histopathological report revealed the diagnosis as benign osteoma.
The lesion was exposed through an extraoral appron incision under general anaesthesia (Fig. 4). The large lobe was found to be attached to the lower border of the mandible and extended medially, displacing the right submandibular gland. The smaller lobule was situated posteriorly at the angle of the mandible. Using a fissure bur, a groove was made along its base and the anterior and posterior attachment. With gentle chiseling the mass was removed without difficulty. The inferior alveolar neurovascular bundle was not affected by the mass. All the sharp margins were smoothened. Suction drain was placed and wound was closed in layers (Fig. 5). The post operative course was uneventful.
Fig. 4.
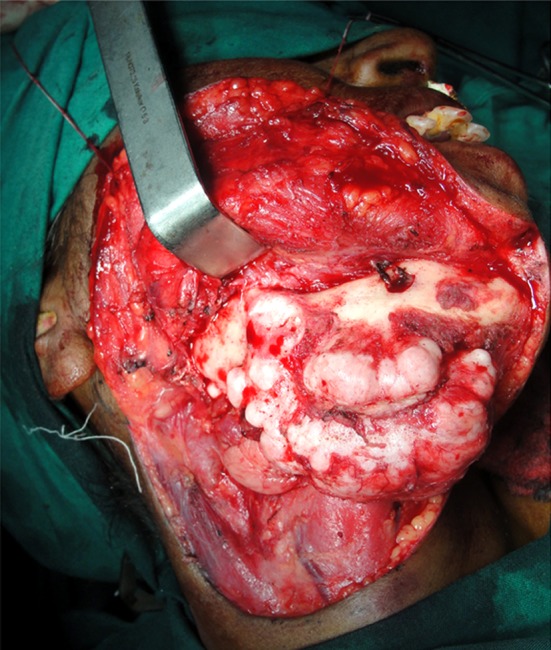
Exposure of the peripheral osteoma during surgery
Fig. 5.
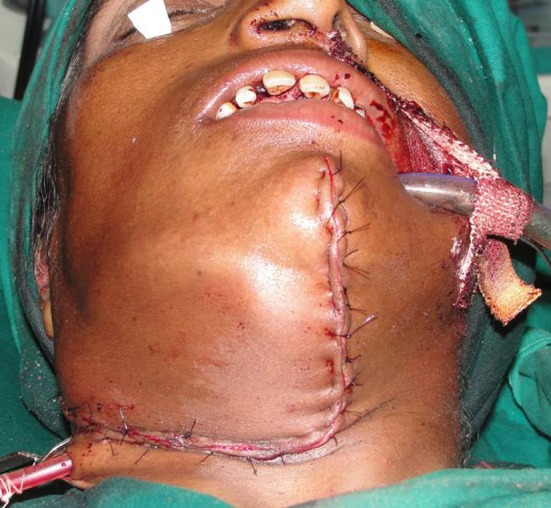
Surgical closure
The gross specimen of the large lobule was hard, irregular, lobulated mass measuring 6.9 × 4.8 × 3.2 cm and weighed 65.9 g. The smaller mass measured 2 × 1.5 × 1 cm and weighed 10 g (Fig. 6). Histopathological examination of the specimen revealed mature bone trabeculae with osteoblastic rimming and numerous osteocytes within lacunae which was consistent with osteoma (Fig. 7).
Fig. 6.
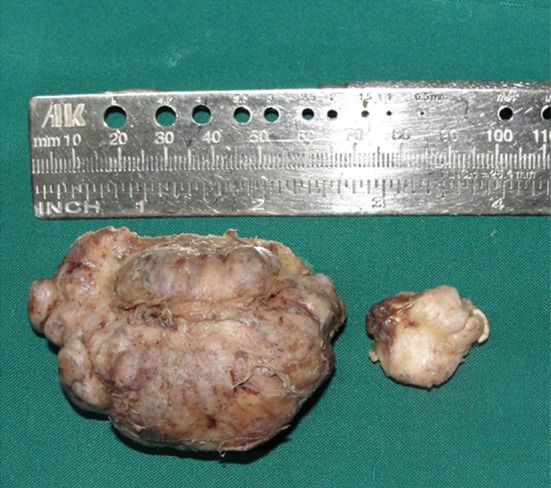
Surgical specimen measure 6.9 × 4.8 × 2.8cm
Fig. 7.
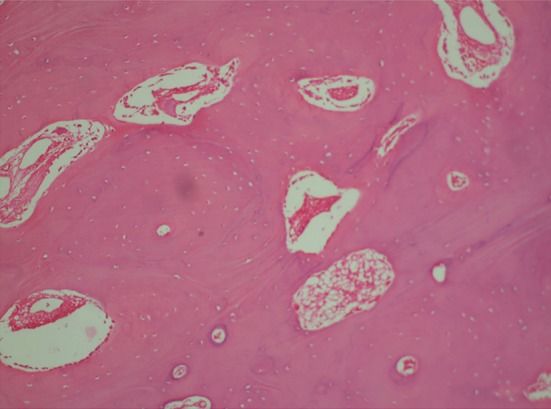
Photomicrograph of osteoma (Hematoxyline-eosin stain section in 100x magnification representing mature bone trabeculae with osteoblastic rimming and numerous osteocytes within lacunae in the represented area.)
Discussion
The exact pathogenesis of osteoma is controversial and unclear [8, 9]. It has been considered to be a true neoplasm, developmental anomaly, or reactive lesion triggered by trauma muscle traction or infection [2, 6, 9]. Endocrine cause has been considered as a possible etiology [9, 10]. Origin from embryonic cartilaginous rest or embryologic periosteum has also been reported [1, 9, 11]. The cause of peripheral osteoma is still debated.
The suggested mechanism that best explains the pathogenesis of peripheral osteoma is a combination of trauma and muscle traction. Trauma (even minor, that is unlikely to be remembered by the patient in later years) may cause subperiosteal bleeding or edema and the muscle traction could locally elevate the periosteum. These two elements might initiate an osteogenic reaction that could be perpetuated by continuous muscle traction in the area. The fact that most peripheral osteomas of the mandible do not reach considerable size also support a reactive rather than neoplastic theory, although reactive process can attain impressive dimensions when stimulus that precipitates the development persists [2, 4, 6].
The lesions of periosteal or peripheral origin (peripheral osteoma) manifest as well circumscribed swelling on the jaw which is asymptomatic until it expands beyond the limit of the surrounding bone producing an obvious asymmetry. The tumour of the endosteal origin (endosteal osteoma) is a slower to present clinical manifestations, because considerable growth must occur before expansion of the cortical plates occurs [2].
Skull is the most common site for an osteoma to develop [2, 7]. The peripheral osteoma occurs mostly in frontal, ethmoidal and maxillary sinus [3–5, 8]. Other documented craniofacial sites include external auditory canal, orbit, temporal bone, zygomatic arch, pterygoid plates and rarely jaws [6, 8, 12, 13]. There is greater occurrence in mandible than maxilla [2, 4, 8]. In the mandible the common locations are the posterior lingual aspect of the body, angle and inferior border of the mandible. The other sites of occurrence are sigmoid notch, coronoid process, genial tubercle, condylar notch [2, 8, 10, 11].
The osteomas can appear at any age but are most commonly found in the younger age group and there is no sex predilection [8].
Peripheral osteomas of the jaw bones are very uncommon [3, 4, 6]. In a review of an English literature over past 76 years, showed 69 well documented cases of peripheral osteoma. The data about location, number of peripheral osteoma, sex and age of the patient were evaluated as shown in the Table 1 [14].
Table 1.
Data from literature concerning 69 cases of peripheral osteoma of the jaw bone
| Location | Cases | Sex | Female:male ratio | Age range (mean in years) |
|---|---|---|---|---|
| Alveolar process | 4 | 3F, 1M | 3:1 | 50 |
| Hard Palate | 2 | 1F, 1M | 1:1 | – |
| Anterior body of the mandible | 4 | 3F, 1M | 3:1 | 9–65 (28.5) |
| Posterior body of the mandible | 19 | 7F, 12M | 1:1.7 | 15–65 (40.9) |
| Angle of the mandible | 9 | 2F, 7M | 1:3.5 | 21–66 (46.2) |
| Ascending ramus of themandible | 7 | 5F, 2M | 2:5.1 | 16–74 (36.5) |
| Condyle | 18 | 10F, 8M | 1.25:1 | 24–85 (43.5) |
| Sigmoid notch | 1 | 0F, 1M | 0:1 | 26 |
| Coronoid process | 5 | 2F, 3M | 1:1.5 | 15.5–26 (34.5) |
| Total | 69 | 33F, 36M | 1.16:1 | 9–85 (36.5) |
F Female, M Male
The peripheral osteomas are usually attached to the bone by a sessile or pedunculated base [11, 14, 15]. They are usually asymptomatic unless their large size results in facial asymmetry [2]. Difficulty in mastication, swallowing and breathing secondary to large osteomas has also been described [8, 15]. Our patient was asymptomatic inspite of the large size of the lesion. Patient had no symptoms such as changes in the vision and balance which have been reported in the large lesion close to the carotid sinus or internal carotid artery [11, 15].
A patient with osteoma needs to be evaluated for Gardner’s syndrome [4, 16, 17]. Patients with Gardner’s syndrome may present with symptoms of rectal bleeding, diarrhoea and abdominal pain. The triad of colorectal polyps, skeletal abnormalities, and multiple impacted or supernumerary teeth are consistent with this syndrome [4, 8]. Onset occurs in the second decade, with malignant transformation of colorectal polyps approaching 100% by the age of 40. The skeletal involvement includes both peripheral and endosteal osteoma, which can occur in any bone but are found most frequently in the skull, ethmoid sinuses, mandible and maxilla. The mandibular osteomas are usually lobulated and located at the angle of the mandible. Additional features of this syndrome include cutaneous fibromas and epidermoid cysts. Fewer than 10% of the patient present with entire triad, but 45% have some characteristics, and 14% have skeletal feature. Gardner’s syndrome was ruled out in our patient with negative clinical symptoms and thus colonoscopy was not advised.
Computed tomography is the best imaging modality for the diagnosis of large osteoma [9]. Radiographically, a large solitary osteoma is similar to parosteal osteosarcoma. It represents as a well circumscribed radiopaque mass that may appear lobulated. Pedunculated nature of lesion is not always evident [15]. So an incisional biopsy is mandatory to confirm the diagnosis.
Histopathologically the peripheral osteoma can be compact osteoma (ivory or eburnated) having sessile base, normally appearing dense bone with minimal marrow spaces and occasional Haversian canals. The size of this type ranges from several millimeters to centimeters. However, a part of the lesion may be in bone masking the true size. The cancellous osteomas are usually pedunculated and resemble bone in origin which contains trabeculae of bone with fibrofatty marrow and osteoblast. The surface can be irregular or smooth with cortical bone at the margin [4, 15].
Peripheral osteoma should be differentiated from exostosis, osteoblastoma, osteoid osteoma, parosteal osteosarcoma and peripheral osteosarcoma [14, 15].
Exostoses are commonly and erroneously termed osteoma [10, 15]. However, exostoses such as tori are bony excrescences that occur on the buccal aspect of the alveolar bone [4]. They are frequently seen and specifically located on mandible (torus mandibularis) and the palate (torus palatinus) [7]. These lesions are hamartomas, reactive or developmental in origin and are not thought to be true neoplasms. They exhibit variable growth and frequently stop growing after puberty [2, 4, 11]. In contrast, the osteoma is a true neoplasm and will continue to increase in size over a time and appears as well circumscribed radiopaque masses that may be lobulated [7].
Osteoblastoma and osteoid osteomas are more frequently painful and may exhibit a more rapid rate of growth than osteoma [8, 15]. Microscopically osteoid osteoma features highly vascular cellular tissue containing osteoid tissue [14] and the osteoblastoma composed is of woven bone with osteoblasts and osteoclast [14].
Parosteal osteosarcoma occurs over a wide age range and peaks at about 39 years. The tumour most commonly involves the distal femoral metaphysis and tends to be large and slow growing. It is more common in females than in males (3:2) when long bones are involved. However, in jaw bones, it is more common in males [18, 24].
Radiographically, the parosteal osteosarcoma is characteristically radio dense and homogenous, more at base than at the periphery [18, 24]. The lesion appear as a lobulated nodule attached to cortical bone by means of short pedicle [19]. There is no radiographic continuity with the underlying marrow cavity. The thin periosteal radiolucency, about 1–3 mm in width which separates the tumour from the sub adjacent cortex is quite characteristic but present only in 30% of cases [20]. New periosteal bone formation is absent [18, 24].
Histologically the parosteal osteosarcoma is well differentiated and characterized by a spindle cell stroma with minimal cellular atypia and rare mitotic figures separating irregular trabeculae of the bone [21]. Occasionally, cells presenting nuclei with pleomorphism are observed [14].
Periosteal osteosarcoma is one-third to one-seventh less common than the parosteal osteosarcoma [22]. It has 2:1 male predominance and a peak occurence at about 20 years. The lesion is usually smaller in size than the parosteal osteosarcoma and commonly involves the upper tibial metaphysis [18, 24]. Only one of twenty-three periosteal osteosarcomas in Dahlin’s series involved the mandible [21].
Radiographically, in periosteal osteosarcoma the cortex is intact and sometimes thickened, and there may be a minimal tumour invasion in the cortex without medullary involvement [23]. Peripheral osteosarcoma is more radiolucent and has more poorly defined periphery. Occasionally, the limits of the lesion may be defined by a periosteal response in the form of a Codman’s triangle [18, 24].
Histologically, periosteal osteosarcoma is composed of lobules of poorly differentiated malignant cartilage which may show central calcification. The diagnostic pattern of fine lace-like osteoid is found in the Chondroid Island and among intervening malignant spindle cells [18].
The management of osteoma is surgical and is only indicated in patient with clinical symptoms [13]. Surgical indications are based on the degree of disfigurement, limitation or loss of function or desired for definitive histopathologic diagnosis [2]. Surgery involves complete removal of the lesion from the base where it unites with the cortical bone [6, 8, 15].
There are no reports of recurrence or undergoing malignant transformation after surgical removal [2, 4]. However, periodical and radiographic follow ups after surgery are advised [14].
Unfortunately our patient was lost for follow up.
Summary
A case of slowly progressive giant osteoma arising from the right posterior-inferior and lingual surface of the mandible in 45 year old female is presented. Patient was asymptomatic despite of such large size and location of the lesion. Following histopathological diagnosis and CT scan evaluation, the osteoma was surgically excised via extraoral approach without complications. The successes of the surgery in cases like presented here require accurate imaging and pre operative planning. Also any patient with solitary osteoma should be evaluated for Gardner’s syndrome.
Acknowledgments
Conflict of interest
None
References
- 1.Schneider LC, Dolinsky HB, Grodijesk JE. Solitary peripheral osteoma of the jaw: report of a case and review of the literature. J Oral Surg. 1980;38:452–455. [PubMed] [Google Scholar]
- 2.Cutilli BJ, Quinn PD. Traumatically induced peripheral osteoma: report of a case. Oral Surg Oral Med Oral Pathol. 1992;73:667–669. doi: 10.1016/0030-4220(92)90006-C. [DOI] [PubMed] [Google Scholar]
- 3.Bodner L, Gatot A, Sino-Vardy N. Peripherial osteoma of the mandibular Ascending Ramus. J Oral Maxillofac Surg. 1998;56:1446–1449. doi: 10.1016/S0278-2391(98)90414-1. [DOI] [PubMed] [Google Scholar]
- 4.Sayan NB, Ucok C, Karasu HA, Gunhan O. Peripherial osteoma of the oral and maxillofacial region: a study of 35 new cases. J Oral Maxillofac Surg. 2002;60:1299–1301. doi: 10.1053/joms.2002.35727. [DOI] [PubMed] [Google Scholar]
- 5.Varboncoeur AP, Vanbelois HJ, Bowen LL. Osteoma of the maxillary sinus. J Oral Maxillofac Surg. 1990;48:882–883. doi: 10.1016/0278-2391(90)90351-2. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan I, Calderon S, Buchner A. Peripherial osteoma of the mandible: a study of 10 new cases and analysis of the literature. J Oral Maxillofac Surg. 1994;52:467–470. doi: 10.1016/0278-2391(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 7.Kerckhaert A, Wolvius E, van der Wal K, Oosterhuis JW. A giant osteoma of the mandible: a case report. J Craniomaxillofac Surg. 2005;33:282–285. doi: 10.1016/j.jcms.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PE, Arendt DM, Fidler JE, Webber CM. Radiopaque mass in the submandibular region. J Oral Maxillofac Surg. 1999;57:709–713. doi: 10.1016/S0278-2391(99)90439-1. [DOI] [PubMed] [Google Scholar]
- 9.Seo-Young An, Chang- Hyeon An, Karp-Shik Choi. Giant osteoma of the mandible causing breathing problem. Korean J Oral Maxillofac Radiol. 2006;36:217–220. [Google Scholar]
- 10.Bessho K, Murakami K, Iizuka T, Ono T. Osteoma in the mandibular condyle. Int J Oral Maxillofac Surg. 1987;16:372–375. doi: 10.1016/S0901-5027(87)80162-5. [DOI] [PubMed] [Google Scholar]
- 11.Richards HE, Strider JW, Short SG, et al. Large peripherial osteoma arising from genial tubercle area. Oral Surg Oral Med Oral Pathol. 1986;61:268–271. doi: 10.1016/0030-4220(86)90373-7. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa T, Yashima S, Hasan H, et al. Osteoma of the lateral pterygoid plate of sphenoid bone. Int J Oral Maxillfac Surg. 1986;15:786. doi: 10.1016/S0300-9785(86)80125-9. [DOI] [PubMed] [Google Scholar]
- 13.Furlaneto EC, Rocha JR, Heitz C. Osteoma of the zygomatic arch. Report of a case. Int J Oral Maxillofac Surg. 2004;33:310–311. doi: 10.1006/ijom.2002.0468. [DOI] [PubMed] [Google Scholar]
- 14.Johann ACBR, Freitas JBd, Aguiar MCFd, Araujo NSd, Mesquita RA. Peripheral osteoma of the mandible: case report and review of literature. J Craniomaxillofac Surg. 2005;33:276–281. doi: 10.1016/j.jcms.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Swanson K, Guttu RL, Miller ME. Gigantic osteoma of the mandible: report of a case. J Oral Maxillofac Surg. 1992;50:635–638. doi: 10.1016/0278-2391(92)90449-A. [DOI] [PubMed] [Google Scholar]
- 16.Lew D, DeWitt A, Hicks RJ, Cavalcanti MG. Osteomas of the condyle associated with Gardner’s syndrome causing limited mandibular movement. J Oral Maxillofac Surg. 1999;57:1004–1009. doi: 10.1016/S0278-2391(99)90026-5. [DOI] [PubMed] [Google Scholar]
- 17.Jones K, Korzcak P. The diagnostic significance, management of Gardner’s syndrome. Br J Oral Maxillofac Surg. 1990;28:80. doi: 10.1016/0266-4356(90)90126-6. [DOI] [PubMed] [Google Scholar]
- 18.Zarbo RJ, Regezi JA, Baker SR. Periosteal osteogenic sarcoma of the mandible. Oral Surg. 1984;57:643–647. doi: 10.1016/0030-4220(84)90287-1. [DOI] [PubMed] [Google Scholar]
- 19.Bianchi SD, Boccadi A, Pomatto E, Valente G. Periosteal osteosarcoma of the mandible. Int J Oral Maxillofac Surg. 1995;24:226–228. doi: 10.1016/S0901-5027(06)80133-5. [DOI] [PubMed] [Google Scholar]
- 20.Shah N, Gupta YK, Safaya R. Juxtacortical osteogenic sarcoma of mandible: a case report. Ind J Dent Res. 2000;11:59–64. [PubMed] [Google Scholar]
- 21.Patterson LA, Greer RO, Howard D. Periosteal osteosarcoma of the maxilla: a case report and review of the literature. J Oral Maxillofac Surg. 1990;48:522–526. doi: 10.1016/0278-2391(90)90246-X. [DOI] [PubMed] [Google Scholar]
- 22.Piattelli A, Favia GF. Periosteal osteosrcoma of the jaw: report of 2 cases. J Perodontol. 2000;71:325–329. doi: 10.1902/jop.2000.71.2.325. [DOI] [PubMed] [Google Scholar]
- 23.Minic AJ. Periosteal osteosarcoma of the mandible. Int J Oral Maxillofac Surg. 1995;24:226–228. doi: 10.1016/S0901-5027(06)80133-5. [DOI] [PubMed] [Google Scholar]
- 24.Balwani SR, Tupkari JV, Barpande SR. Parosteal osteosarcoma of the mandible. J Oral Pathol. 2006;10:10–14. doi: 10.4103/0973-029X.37747. [DOI] [Google Scholar]


