Abstract
Introduction
Functional and cosmetic defects in maxillofacial region are caused by various ailments like trauma, neoplasm, developmental, infections and iatrogenic causes. Reconstruction of these defects with free flaps remains the gold standard but demerits like need for surgical expertise and equipment, prolonged duration of surgery, compliance of the patient and increased cost are associated with microvascular reconstruction. Hence reconstruction with nonvascular bone grafts can be considered when defect is nonirradiated and <9 cm and with sufficient soft tissue cover available.
Purpose
To retrospectively evaluate clinical, radiological outcome and complications encountered with mandibular reconstruction using non vascular fibula graft.
Patients and Methods
This retrospective study included 7 patients who were treated in the Department of Oral and Maxillofacial Surgery, Narayana Dental College and Hospital, Nellore, AP between 2011 and 2013 with histologically proven benign osteolytic lesions of mandible that require a segmental mandibulectomy and primary reconstruction using autogenous non-vascularised fibular graft. The clinical case records of the patients and personal patient assessment forms (Quality of Life Assessment Forms) were analysed. They were recalled every 3rd, 6th and 9th month after surgery for evaluation of clinical, radiological outcome of the graft and complications occurring at recipient and donor sites.
Results
In all the 7 patients, the lower border continuity was maintained except in one where the graft was dislodged. Tongue movements in all the patients were unrestricted. Jaw movements were affected in cases of ramus defects with slight deviation to operated side and reduced mouth opening. Radiological observations revealed no significant changes in 3 months except for slight reduction in graft height. The radioopaque bridging with continuity of lower border of mandible was noticed in 6th month indicating the take of the graft. This was achieved in every case except in one where the graft was lost due to dislodged reconstruction plate. In 9th month the edges of the graft i.e., graft to native mandible junction showed more resorption (3 mm) especially where there is >2 mm of gap. Whereas increase in height of graft in other areas especially in graft to graft junction was seen. Significant graft resorption was seen in two cases. There were no major complications associated with the donor site.
Conclusion
Avascular fibula graft although a second choice to vascularised fibula, is a favourable option for mandible defects of 6–10 cm under optimum conditions especially in developing countries where financial and/or surgical resources are limited. An attempt for primary reconstruction with this is never futile as it prevents aesthetic deformity even in the event of failure and thus makes secondary reconstruction easy. However in order to confirm the results a prospective study with large scale of patients is necessary.
Electronic supplementary material
The online version of this article (doi:10.1007/s12663-014-0657-1) contains supplementary material, which is available to authorized users.
Keywords: Mandible reconstruction, Nonvascular fibula graft, Clinical outcome, Radiological evaluation
Introduction
Functional and cosmetic defects in mandible are caused by various ailments like trauma, neoplasm and infections. These need to be addressed according to their extent and severity by ablative surgery of mandible like segmental resection and hemimandibulectomy. These discontinuity defects often severely compromise the mastication, deglutition, speech, protection of airway, and facial aesthetics which makes mandibular reconstruction not only desirable but also essential [1].
The decision to perform a primary reconstruction of mandibular defects as well as specific nature of the technique to be employed is based on defect related factors like, size and location of the mandibular bone defect, distribution and quality of the remaining native dentition. In turn the procedure should be simple with least possible donor site morbidity so as to return the patient to previous state of function [2].
Reconstruction options for mandible range from metallic reconstruction plate to vascularised bone flaps [3–10]. Non vascular bone grafts could be used judiciously for reconstruction of selective mandibular defects with not much of soft tissue loss provided the defect is <9 cm [11], stable fixation to the native mandible and a 2-layer watertight closure both intraorally and extraorally.
Early attempts of primary mandibular reconstruction with nonvascularised bone grafts were fraught with suboptimal results and an unacceptable incidence of complications, especially when the patients were subjected to adjuvant post-operative radiation therapy in malignant tumors of jaw. Any nonvascularised bone graft will be taken when 100 % ideal conditions are provided [11]. They give good contour and aesthetics but are most successful in non-irradiated patients who have adequate soft tissue and where the defect is shorter.
Though reconstruction with free flaps remains the gold standard, factors like need for surgical expertise and equipment, increased intra-operative time, post operative stay, economic reasons, increased age and compromised medical condition of the patient are against micro vascular grafting. Thus nonvascularised bone grafts are still a reasonable option for mandibular reconstruction in the developing world and can be used for primary reconstruction of mandibular defects due to benign pathologies [1]. Further functional rehabilitation with implants and removable prosthesis can be done after 6 weeks of reconstruction.
Thus our present study is focused on evaluation of nonvascular fibula for reconstruction of segmental defects of mandible after benign tumor excision.
Materials and Methods
A retrospective study was done from 2011 to 2013 in the Department of Oral and Maxillofacial Surgery, Narayana Dental College and Hospital, Nellore, AP. Seven patients with histologically proven benign osteolytic lesions of mandible who underwent segmental mandibulectomy and primary reconstruction using autogenous non-vascularised fibular graft were taken for study. Patients were recalled every 3rd, 6th and 9th month after surgery for evaluation.
Inclusion Criteria:
Patients with histologically proven benign osteolytic lesions of mandible that would require segmental mandibulectomy and who were medically fit for surgery.
Exclusion Criteria
(1) Patients who were medically compromised and not fit for surgery. (2) Patients with evidence of osteoporotic changes in fibula. (3) Patients who had other systemic conditions associated with bone health like hyperparathyroidism etc. (4) Patients with composite defects of mandible (5) Patients with osteomyelitis and infected lesions of mandible (6) Patients who denied grafting.
All the patients’ case records and periodical personal assessment forms were analysed. Serial panoramic radiographs of mandible were used for radiological examination of fibula.
Surgical Technique
Under general anesthesia, nasoendotracheal intubation was done. Standard surgical painting and draping was done under strict aseptic conditions. Two team approach was carried out, ablative team for removal of tumour and reconstructive team for harvesting fibula. Sub mandibular curvilinear incision was placed in the first crease of neck from angle to 1 cm short of mentum (except in one case with intra oral approach) subplatysmal dissection was done till lower border of mandible. After preserving marginal mandibular nerve, facial vein and artery were identified and ligated. The tumour was exposed and periosteum stripped along with pterygomasseteric sling. Lingual mucoperiosteal flap was raised and segmental resection done. Inferior alveolar bundle was ligated and cauterized to achieve hemostasis.
Right leg was selected as donor site in all the cases. Leg was elevated for 10 min for venous drainage, Esmarch’s tourniquet applied at midthigh region, sand bag placed under the hip, standard draping and painting was done under strict aseptic conditions. Fibular head and lateral malleolus were marked with upper limit of incision marking placed 8 cm away from fibular head till 7 cm above lateral malleolus. Vertical incision was placed through skin and subcutaneous tissue. Intermuscular septum was exposed and incised, dissection plane between peroneus longus and soleu gastronemus complex was identified to reach lateral border of fibula, extensor muscles were reflected superiorly, flexor and soleus gastronimeus complex muscles were retracted inferiorly (Fig. 1). The fibula was dissected from its muscles in upward direction. Lower osteotomy cut was made 7 cm above the ankle and superior osteotomy cut 10 cm below the knee joint(depending on the defect size) was made taking care not to damage the medial structures to harvest nonvascular fibula. Tourniquet was released after one and half hour. Drain was inserted. Closure of muscular layers was done with 3–0 vicryl and skin closed with 2–0 prolene by subcuticular suturing.
Fig. 1.
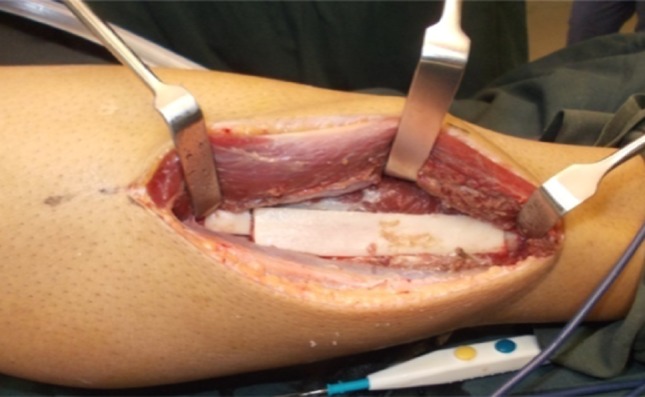
Lateral surface of fibula with extensor muscle
The harvested fibula (Fig. 2) was placed into the previously created mandibular defect. Contouring osteotomies were done on table and fixed with reconstruction plate based on predrilled holes with 2.5 × 8 mm titanium screws in 5 cases (Figs. 3, 4) and by means of miniplates in 2 cases. In defects crossing midline hitching of genioglossus muscles to the plate was done to prevent airway compromise in immediate postoperative period. Adequate care was taken to cover reconstruction plate and graft by watertight closure with drains in order to maintain the vitality of the graft.
Fig. 2.
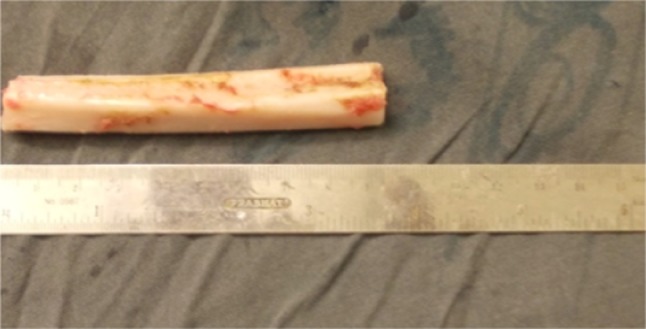
Harvested nonvascular fibula graft for reconstruction
Fig. 3.

Contoured graft for the formed defect along with reconstruction plate
Fig. 4.
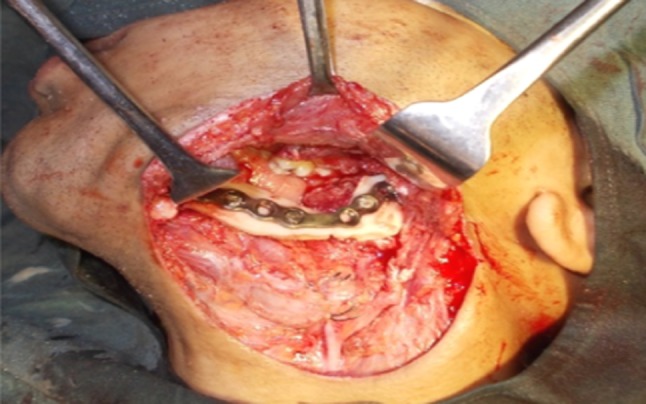
Contoured graft fixed in the resulted defect
Post-operative Follow-up and Evaluation
Patients’ case records were analysed for immediate post-operative orthopantomograms and discharge summaries and they were recalled every 3rd, 6th and 9th month after surgery to evaluate mouth opening, contour, jaw and tongue movements. Recipient and donor sites were examined during every visit.
Clinical Evaluation
Patients were given self assessment forms which were based on modified quality of life assessment scale (UW-QOL) by David d. Vu et al. (for assessment in patients receiving vascularised and nonvascular bone grafts in 2008) during every visit which included assessment of psychological, aesthetic (contour, jaw deviation etc.,) and functional issues (swallowing, chewing, tongue movements etc.,) after the surgery.
In addition we measured mouth opening and compared it with pre-op values.
Radiologic Evaluation
Serial orthopantomograms of all the patients were assessed for formation of radioopaque bridge between the native mandible and the graft, the height of the graft and changes in radiodensity of the graft.
The height of the graft was measured in four intervals of time using Planmeca Digital Sofware in serial orthopantomograms. All X-rays were taken with equal exposure and magnification. A line was drawn from lower border to upper border of the graft in 5 equal intervals maintaining the angle at 90°. All measurements were recorded by a single observer.
Ethical committee approval was obtained from institutional review board and structural informed consent was taken from all patients included in the study.
Results
Totally 7 patients were included in the study. Among them 3 were diagnosed with primary Ossifying Fibroma, 3 cases of Ameloblastoma (granular, follicular and one both granular and follicular variant) and one case of Keratocystic Odontogenic Tumour. Of the 7 patients 5 were females and 2 were males with age ranging from 13 to 62 years (Table 1). Through extraoral submandibular incision the resection of mandible was carried out in all the cases except in one case strict intraoral approach was used (case 6). The size of the defect following mandibulectomy was measured using a tape. The amount of graft to be harvested was based on these values and type of the defect (as per HCL classification) [12]. For a ramus, angle defect (H & L) a graft more than 2 cm of the actual defect was sufficient (cases 2 & 4) except in case 7 where a hemimandibulectomy was done. While the body and parasymphysis defect (CL) which need to be contoured to achieve the natural contour of mandible required a graft of more than 3–4 cm than the defect size (cases 3 & 4). For the defect crossing midline(LCL) as the defect was large the grafted bone was contoured and fixed in retruded position compared to preexisting mandible hence equal or more than 2 mm of graft than the defect size was sufficient (case 6) (Table 2).
Table 1.
General data of the 7 patients included in the study
| S. No | Age (year) | Sex | Diagnosis | Site of invovement in Mandible |
|---|---|---|---|---|
| Case 1 | 42 | F | Ossifying fibroma |
|
| Case 2 | 38 | F | Ameloblastoma |
|
| Case 3 | 13 | F | Ossifying fibroma |
|
| Case 4 | 63 | M | KCOT |
|
| Case 5 | 32 | F | Ossifying fibroma |
|
| Case 6 | 61 | F | Ameloblastoma |
|
| Case 7 | 21 | M | Ameloblastoma |
|
Table 2.
Type and size of defect and graft harvested
| S. No | Age (year) | Sex | Diagnosis | Site | Type of defect | Size of the graft harvested (cm) |
|---|---|---|---|---|---|---|
| Case 1 | 42 | F | Ossifying fibroma |
|
CRLR (10 cm) | 12 |
| Case 2 | 38 | F | Ameloblastoma |
|
LL(11 cm) | 13 |
| Case 3 | 13 | F | Ossifying fibroma |
|
C RLR (6 cm) | 10 |
| Case 4 | 63 | M | KCOT |
|
LL (8.5 cm) | 11 |
| Case 5 | 32 | F | Ossifying fibroma |
|
C RLR (7 cm) | 11 |
| Case 6 | 61 | F | Ameloblastoma |
|
L RC LL (15 cm) | 16 |
| Case 7 | 21 | M | Ameloblastoma |
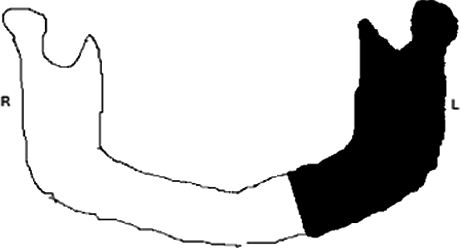
|
HL (8 cm) | 10 |
The extraoral scar in recipient site showed a satisfactory and uneventful healing in all the cases. Intraorally two patients had pus discharge, at proximal end of graft in case 4 and at junction of graft and native mandible on left side in case 6. Routine antibiotics and local debridement measures were done, but as the discharge was persistent and the radiolucent changes were observed in the graft (Fig. 5) the site was exposed in local anesthesia and necrosed part of graft was removed 8 months after the surgery. Presently patient is doing fine and has no compliant. The lower border continuity was maintained in all the patients (Fig. 6) except in case 4 where the graft was lost due to dislodgement of reconstruction plate. Tongue movements in all the patients were unrestricted. Jaw movements were affected in cases of ramus defects with slight deviation to operated side and reduced mouth opening (Table 3).
Fig. 5.
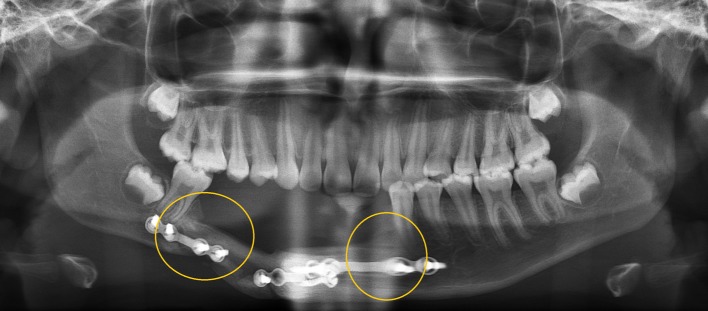
Late post-operative radiograph of case 3—consolidation of graft (two circles)
Fig. 6.
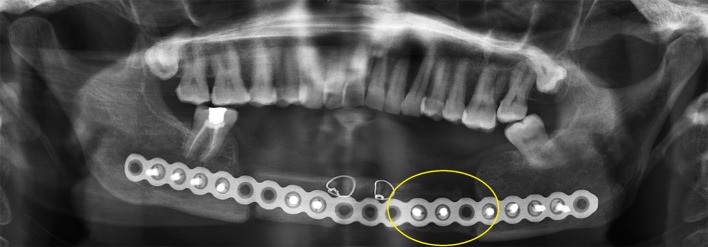
Late post-operative radiograph of case 6-necrosis of the graft (one circle on right of radiograph)
Table 3.
Clinical evaluation results of 7 cases
| S. No | Esthetic contour | Tongue movements | Jaw movements | Mouth opening | |
|---|---|---|---|---|---|
| Post-op (mm) | Pre-op (mm) | ||||
| Case 1 | Satisfied | Unrestricted | Non-restricted | 38 | 32 |
| Case 2 | Good | Non-restricted | 38 | 35 | |
| Case 3 | Satisfied | Slight deviation to right side | 40 | 43a | |
| Case 4 | Good | Deviation to right side | 38 | 26 | |
| Case 5 | Good | No gross change | 40 | 36 | |
| Case 6 | Good | Non-restricted | 35 | 38a | |
| Case 7 | Good | Slight deviation to right side | 42 | 32 | |
aPre-operatively interincisal distance taken, post-operatively lower teeth were missing replaced by midline of lower reconstructed ridge as measurement landmark
There were no major complications associated with the donor site except for slight infection and compression necrosis of skin. All the patients were comfortable with no complaint regarding donor site. There was no foot drop and patients were ambulated on the first postoperative day by means of walker.
There were no significant changes at 3 months except for slight reduction in graft height. The radioopaque bridging with continuity of lower border of mandible was noticed at 6th month indicating the take of the graft. This was achieved in every case except in case 4 where the graft was lost due to dislodged reconstruction plate. At 9th month the edges of the graft i.e., graft to native mandible junction showed more resorption (3 mm) especially where there was >2 mm of gap. Whereas increase in height of graft in other areas especially in graft to graft junction was seen. Significant graft resorption was seen in two cases. In case 4, the total graft showed resorptive changes, whereas only a part of graft adjacent to the screws was necrosed in case 6 (Table 4).
Table 4.
Results of graft height measurement from immediate to 9 months post operative period
| S. No | Immediate post-op | 3rd month (mm) | 6th month(mm) | 9th month (mm) |
|---|---|---|---|---|
| Case 1 | 15.03, 14.87, 14.05, 14.50, 14.52 (ave-14.59) | 15.01, 14.72.14.03, 14.50, 14.52 (ave-14.55) | 14.38, 14.03, 13.42, 14.02, 13.34 (ave-13.83) | 13.07, 14.01, 15.08, 15.45, 13.01 (ave-14.12) |
| Case 2 | 15.33, 14.35, 14.28, 14.34, 14.57 (ave-14.57) | 15.32, 14.32, 14.27, 14.31, 14.54 (ave-14.55) | 14.93, 14.23, 14.09, 14.07, 13.43 (ave-14.15) | 14.88, 14.57, 14.27, 12.31, 10.27 (ave-13.26) |
| Case 3 | 14.37, 14.38, 14.24, 14.04, 13.62 (ave-14.23) | 14.32, 14.34, 14.23, 14.01, 13.54 (ave-14.08) | 14.23, 14.03, 13.29, 14.04, 13.43 (ave-13.80) | 11.15, 13.37, 15.62, 15.61, 16.35 (ave-14.42) |
| Case 4 | 14.88, 13.64, 15.36, 14.87, 13.63 (ave-14.47) | 14.88, 13.62, 15.35, 14.85, 13.61 (ave-14.46) | 13.75, 13.02, 14.54, 14.05, 12.86 (ave-13.64) | 11.02, 8.54, 10.09, 10.34, 9.54 (ave-9.90) |
| Case 5 | 15.45, 14.92, 14.03, 15.29, 14.61 (ave-14.86) | 15.43, 14.88.14.03, 15.27, 14.57 (ave-14.83) | 14.38, 14.76, 13.45, 15.04, 13.34 (ave-14.19) | 13.07, 14.08, 15.98, 15.45, 14.01 (ave-13.15) |
| Case 6 | 15.89, 14.63, 15.35, 14.90, 13.87 (ave-14.92) | 15.88, 14.62, 15.35, 14.85, 13.61 (ave-14.86) | 14.37, 14.85, 15.59, 14.61, 12.63 (ave-14.41) | 13.57, 14.32, 15.10, 11.88.10.89 (ave-13.15) |
| Case 7 | 13.90, 13.43, 13.65, 14.67, 12.45 (ave-13.62) | 13.86, 13.37, 13.63, 14.64, 12.40 (ave-13.58) | 12.90, 13.03, 13.05, 13.54, 12.32 (ave-12.96) | 11.88, 12.87, 13.34, 12.82, 11.23 (12.42) |
Discussion
Reconstruction of mandibular defects has been an area of interest since nineteenth century. From that time various options ranging from alloplastic bone substitutes to free vascularised flaps have been proposed and studied [2]. These defects are often addressed by using vascularised bone flaps and still stand as a gold standard for reconstruction [2–9]. The choice for reconstruction depends on various factors like availability of surgical expertise and equipment, medical fitness of patients and financial factors. In developing countries such as India most of the patients with oral benign lesions are from lower socioeconomic strata, who are less educated and undernourished. Their ability to report at an early stage of the disease and for frequent follow-up is limited [1].
Other factors like, increased intra-operative time and post operative stay, increased age and compromised medical condition of the patient are against micro vascular grafting. Thus, Non Vascularised Bone Grafts (NVBG) is still an accepted method of reconstruction in developing and underdeveloped countries. Use of Non Vascularised Bone Grafts for reconstruction of mandible is in use since 1900s [10]. The various sources for harvesting NVBG include iliac crest, fibula, calvarium, rib and tibia. Fibula which is situated posteriolateral to tibia with its tubular structure similar to mandible, dense cortical plates, and presence of endosteal blood supply allows for multiple graft osteotomies to contour without much compromise in bone viability. Additionally convenience for two-team approach, availability of 20–25 cm of bone for harvest and least donor site morbidity makes it an ideal choice for reconstruction of mandibular segmental defects [13–16]. Since it is not the prime weight bearing bone its removal will not affect the function of the leg. Thus avascular fibula graft is the reconstruction choice in our study for segmental defects of mandible.
The suitability of different bone grafts for mandibular reconstruction depends on bone availability, thickness, shape of obtainable bone segments and possibility of contouring these grafts according to mandibular shape [17]. In our study fibula graft seem to satisfy all the criteria by providing 25 cm of bone of which 10–15 cm were harvested for usage as Non Vascularised Graft. Required number of osteotomies was done to achieve the contour in various areas of defect which resulted in satisfactory esthetic outcome.
The confusion regarding the size of the defect that can be reconstructed with nonvascular bone graft successfully was solved by Pogrel’s comparison study of vascularised and nonvascularized fibula for mandibular reconstruction. He suggested that any defect <9 cm with adequate soft tissue closure can be successfully restored by means of avascular fibula graft [11]. Defect size ranging from 6 cm to maximum of 15 cm were restored by means of avascular graft in our study. Graft of more than 2–3 cm of the defect size was sufficient. Hence grafts ranging from 10 to 16 cm were harvested according to the need.
The common recipient-site complications are dehiscence of intra oral wound, resulting in graft failure. The reasons attributed to this are symphyseal involvement of the tumour and contamination of the wound with oral microorganisms. These are managed by immediate reconstruction, and <10 days of antibiotic therapy [18]. The source of contamination of wound postoperatively is by leakage of saliva into the grafted area, especially if rigorous suction wound drainage is used [19]. Added to this, presence of dead space and prolonged surgical procedure may also increase the risk of wound infection and dehiscence. In our study we have done immediate reconstruction and 7 days of antibiotic therapy for every patient which resulted in optimum results. Gadre et al. [1] suggested using a stay suture of nonresorbable material to fix the soft tissue to the graft or hardware reduces the dead space, hematoma formation and prevents development of additional weight beneath the reconstruction. This aids in reduction of pull and drag on the intraoral suture line and thus the dehiscence rate decreases.
The site of the reconstructed defect also influences the rate of success. In cases of defects crossing the midline, the muscles of floor of the mouth and tongue lose their insertion to the mandible and may result in plate exposure [20]. In addition the mobility of muscle and mucosa in this region due to tongue movement is another probable cause. In our study the patient with the defect crossing midline presented with persistent pus discharge which subsided after removing the necrosed bone graft after the failure of oral antibiotics and local measures to control it over a period of 5 months post reconstruction. However there was no mucosal dehiscence. A 2-layer watertight intraoral closure, thorough irrigation with povidone-iodine and saline solution before graft placement, and use of suction drain both on buccal and lingual sides of the graft have been suggested as a mode of prevention [1].
The role of rigid fixation in success of graft is important, as healing is impaired by micro movements at the graft and native bone interface leading to pseudoarthrosis or infection. Studies on comparison of small plates and reconstruction plates showed advantages of small plates over reconstruction plates for graft fixation, as removal of the former is not always necessary, inturn small plates due to their lower profile interfere less with dentures and implant placement [20, 21]. Further maxillomandibular fixation post operatively will help in minimizing movement of soft tissue over the graft and the hardware. Our study showed no difference in outcome with use of reconstruction plate or miniplates as all the patients were kept under intermaxillary fixation for a period of 1 week post operatively except in one patient where there was loosening of the screw over reconstruction plate leading to subsequent loss of graft (case 4).
Quality of life (QOL) may be described as the “gap between one’s actual functional level and one’s ideal standard,” but it is dynamic, changing over time and situations [22–31]. Rogers et al. [26] stated that with long-term survivors, long-term results of quality of life are similar to that determined after 1 year of surgery. Hence we analyzed the quality of life by means of the questionnaire along with mouth opening measurement in immediate post-op, 3rd, 6th, and 9th month after the surgery resulting in a satisfactory outcome. Tongue movements of all the patients were unrestricted. Jaw movements were affected in cases of ramus defects with slight deviation to operated side and reduced mouth opening (average-10 mm).
Contour restoration was satisfactory in segmental reconstructions of the mandible with minor differences between non-vascularised fibula grafts and grafts from iliac crest and scapula. The latter grafts were more difficult to shape [17]. In our study the contour and esthetic restoration was satisfactory in all the patients.
The healing process of NVBGs is by creeping substitution. The graft acts as supporting structure and is replaced by newly formed bone arising by the process of osteoconduction and osteoinduction after resorption of the mineral matrix [32]. Phase I includes formation of new osteoid that is laid out in the framework of the graft (approximately 4 weeks) and determines the ultimate size of the bone graft. There is no new bone formation in second phase but bone morphogenic protein (BMP) mediates the transformation of the pluripotential host cells into osteoblastic cells that remodel phase I bone and organize the graft. Phase II bone formation starts at about 2 weeks but peaks at 6 weeks and wanes around 6 months. In this phase if the host tissue cannot support the osteogenesis due to hypovascularity and hypocellularity, delayed resorption of the graft will occur often resulting in its total loss [33–38]. High concentrations of BMP in cortical bone was demonstrated by a study by Urist [36]. This further supports the use of avascular fibula with its high cortical plate as a reconstructive option.
The durability of the reconstruction with NVBG was measured by radiographic analysis. Hidalgo, one of the pioneers in this field used revascularised bone grafts for reconstruction of mandible and followed it for 10 years. He used consecutive conventional orthopantomogram of patients in subsequent follow-ups taking the hardware as the stable point [39–41]. He concluded that the graft volume proved uniformly stable with only small losses occurring over a span of 10 years and more than 90 % of fibular height was maintained. It is hypothesized that fibula with its high cortical bone content is less liable for resorption when compared with iliac transplant [16]. Our study has shown resorption rate of 11 % over a period of 9 months (Table 5).
Table 5.
Results of donor site morbidity in 7 cases
| S. No | Age (year) | Gender | Gait disturbance/foot drop | Any other complication/healing |
|---|---|---|---|---|
| Case 1 | 42 | Female | Absent | |
| Case 2 | 38 | Female | ||
| Case 3 | 13 | Female | Infection | |
| Case 4 | 63 | Male | ||
| Case 5 | 32 | Female | Pressure necrosis | |
| Case 6 | 61 | Female | ||
| Case 7 | 21 | Male | Hypertrophic scar |
Fibula graft is known for its least donor site morbidity chiefly because it is a non weight-bearing bone in the leg and allows for two team approach with a good access and hence a clean surgery to harvest the bone with minimal trauma to the adjacent muscles that help in gait. Studies show that preserving at least 5–6 cm of distal fibula is essential for maintaining ankle mortise stability [7, 8, 42]. In our study we preserved more than 7 cm of distal and proximal segments of fibula. Post-operatively the leg was supported by splint of plaster of paris in elderly patients. Rest of the cases crape bandage with drain inserted showed excellent results in healing. All the patients were ambulated after 24 h of surgery. Movements of the ankle were evaluated during every post operative visit. None of the patients showed difficulty in movements of foot right from the immediate post-operative visit. The ankle and hind foot were stable. The patients with teeth present on either side of defect were rehabilitated with fixed partial dentures.
Thus avascular fibula graft with its high bone density, ease of access to harvest, and tendency to show less resorption helps to achieve satisfactory esthetic contour and function in reconstruction of segmental defects of mandible. The feasibility for a two team approach adds in reducing the intraoperative time and post-operative stay. Least donor site morbidity, less need for expertise and equipment makes it a judicious option for mandible defects of 6–10 cm under optimum conditions especially in developing countries.
Conclusion
Nonvascularized bone grafts could be used judiciously for reconstruction of selective mandibular defects caused by benign pathologies involving body and ramus of mandible where there is no need of post-operative radiotherapy provided the defect is in the range of 6–10 cm under optimum conditions especially in developing countries. Sterile conditions, minimum osteotomies (2 segments), stable fixation, 7–10 days of intermaxillary fixation in a healthy host are the primary prerequisites for a successful reconstruction with avascular fibula. In larger defects (>10 cm) crossing midline, reducing the number of osteotomies with stable fixation to the native mandible followed by 10 days of intermaxillary fixation and avoiding an intraoral approach can aid in take up of the graft.
Avascular fibula graft although a second choice to vascularised fibula, is a good option where financial and/or surgical resources are limited. An attempt for primary reconstruction with this is never futile as it surely prevents aesthetic deformity even in the event of failure and thus makes secondary reconstruction easy. However in order to confirm the results a prospective study with large scale of patients is necessary.
Electronic supplementary material
References
- 1.Gadre PK, et al. Nonvascularized bone grafting for mandibular reconstruction: myth or reality? J Craniofac Surg. 2011;22:5. doi: 10.1097/SCS.0b013e31822e633b. [DOI] [PubMed] [Google Scholar]
- 2.Urken ML, Weinberg H, Vickery C, et al. Oromandibular reconstruction using microvascular composite free flaps. Arch Otolaryngol Head Neck Surg. 1991;117:733–744. doi: 10.1001/archotol.1991.01870190045010. [DOI] [PubMed] [Google Scholar]
- 3.Urken L (1991) Composite free flaps in oromandibular reconstruction: review of the literature. Arch Otolaryngol Head Neck Surg 117:724 [DOI] [PubMed]
- 4.Posnick JC. Use of the free fibular flap in the immediate reconstruction of pediatric mandibular tumours: report of cases. J Oral Maxillofac Surg. 1993;51:189–196. doi: 10.1016/S0278-2391(10)80021-7. [DOI] [PubMed] [Google Scholar]
- 5.Ferri J, et al. Advantages and limitations of the fibula free flap in mandibular reconstruction. J Oral Maxillofac Surg. 1997;55:440–448. doi: 10.1016/S0278-2391(97)90685-6. [DOI] [PubMed] [Google Scholar]
- 6.Boyne PJ, et al. A comparison of vascularised and nonvascular bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1206. doi: 10.1016/S0278-2391(97)90166-X. [DOI] [PubMed] [Google Scholar]
- 7.Talyor GI, Miller GD, Ham FJ. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast Reconstr Surg. 1975;55:533–544. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Weiland AJ, Moore JR, Daniel RK. Vascularised bone autografts. Experience with 41 cases. Clin Orthop. 1983;174:87–95. [PubMed] [Google Scholar]
- 9.Gonzalez-Garcia R, et al. Vascularised free fibula flap for the reconstruction of mandibular defects: clinical experience in 42 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:191–202. doi: 10.1016/j.tripleo.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Day TA, Girod, DA (2006) Textbook of oral cavity reconstruction. Taylor & Francis Group, New York
- 11.Pogrel MA, Podlesh S, Anthony JP, et al. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55:1200–1206. doi: 10.1016/S0278-2391(97)90165-8. [DOI] [PubMed] [Google Scholar]
- 12.Jewer DD, Boyd JB, et al. Orofacial and mandibular reconstruction with the iliac crest free flap: a review of 60 cases and a new method of classification. Plast Reconstr Surg. 1989;84:391–403. doi: 10.1097/00006534-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Rana et al Reconstruction of mandibular defects- clinical retrospective research over a 10-year period. Head Neck Oncol. 2011;3:23. doi: 10.1186/1758-3284-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kademani D, Keller E. Iliac crest grafting for mandibular reconstruction. Atlas Oral Maxillofac Surg N Am. 2006;14:161–170. doi: 10.1016/j.cxom.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Foster RD, Anthony JP, Sharma A, et al. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck. 1999;21:66–71. doi: 10.1002/(SICI)1097-0347(199901)21:1<66::AID-HED9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 16.Li Lei, et al. Long term evaluation after mandibular reconstruction with fibular grafts versus microsurgical fibular flaps. J Oral Maxillofac Surg. 2007;65:281–286. doi: 10.1016/j.joms.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Schliephake T, et al. Comparision of late results of mandibular reconstruction using nonvascularised or vascularised grafts and dental implants. J Oral Maxillofac Surg. 1999;57:944–950. doi: 10.1016/S0278-2391(99)90015-0. [DOI] [PubMed] [Google Scholar]
- 18.Egyedi P (1986) Wound infection after mandibular reconstruction with autogenous graft. Ann Acad Med Singapore 15:340–345 [PubMed]
- 19.Koole R, Egyedi P. Postoperative contamination of mandibular osteotomy sites with saliva. Int J Oral Maxillofac Surg. 1987;16:554–558. doi: 10.1016/S0901-5027(87)80105-4. [DOI] [PubMed] [Google Scholar]
- 20.Boyd JB, Mulholland RS, Davidson J, et al. The free flap and plate in oromandibular reconstruction: long-term review and indications. Plast Reconstr Surg. 1995;95:1018–1028. doi: 10.1097/00006534-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Merkx MAW, Fennis JPM, Verhagen CM, et al. Reconstruction of the mandible using preshaped 2.3 mm titanium plates, autogenous particulate cortico-cancellous bone grafts and platelet rich plasma. Int J Oral Maxillofac Surg. 2004;33:733–739. doi: 10.1016/j.ijom.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Vu DD, Schmidt BL, et al. Quality of life evaluation for patients receiving vascularized versus nonvascularized bone graft reconstruction of segmental mandibular defects. J Oral Maxillofac Surg. 2008;66:1856–1863. doi: 10.1016/j.joms.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Hundepool AC, et al. Rehabilitation after mandibular reconstruction with fibula free flap: clinical outcome and quality of life assessment. Int J Oral Maxillofac Surg. 2008;37:1009–1013. doi: 10.1016/j.ijom.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Holzle F, et al. Clinical outcome and patient satisfaction after mandibular reconstruction with free fibula flap. Int J Oral Maxillofac Surg. 2007;36:802–806. doi: 10.1016/j.ijom.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Cella DF, et al. Quality of life: concepts and definition. J Pain Symptom Manage. 1994;9:186. doi: 10.1016/0885-3924(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 26.Rogers SN, Lowe D, Fisher SE, et al. Health-related quality of life and clinical function after primary surgery for oral cancer. Br J Oral Maxillofac Surg. 2002;40:11. doi: 10.1054/bjom.2001.0706. [DOI] [PubMed] [Google Scholar]
- 27.de Graeff A, de Leeuw JR, Ros WJ, et al. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98. doi: 10.1097/00005537-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Rogers SN, Lowe D, Brown JS, et al. The University of Washington head and neck cancer measure as a predictor of outcome following primary surgery for oral cancer. Head Neck. 1999;21:394. doi: 10.1002/(SICI)1097-0347(199908)21:5<394::AID-HED3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 29.Rogers SN, Hannah L, Lowe D, et al. Quality of life 5–10 years after primary surgery for oral and oro-pharyngeal cancer. J Craniomaxillofac Surg. 1999;27:187. doi: 10.1016/S1010-5182(99)80049-3. [DOI] [PubMed] [Google Scholar]
- 30.Nelson Katja, et al. Clinical evaluation of endosseous implants in nonvascularised fibula bone grafts for reconstruction of the severly atrophied mandibular bone. J Oral Maxillofac Surg. 2006;64:1427–1432. doi: 10.1016/j.joms.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Wedler V, et al. Retrospective analysis and clinical evaluation of mandible reconstruction with free fibula flap. Eur J Plast Surg. 2007;29:285–291. doi: 10.1007/s00238-006-0081-y. [DOI] [Google Scholar]
- 32.Booth PW (2006) Textbook of oral and maxillofacial surgery, vol 1. Elsevier, Churchill Livingstone, p 432
- 33.Marx RE. Mandibular reconstruction. J Oral Maxillofac Surg. 1993;51:466–479. doi: 10.1016/S0278-2391(10)80501-4. [DOI] [PubMed] [Google Scholar]
- 34.Abbott LE, Schottstaedt ER, Sannders JB, et al. The evaluation of cortical and cancellous bone as grafting materials: a clinical and experimental study. J Bone Joint Surg. 1947;29:381–414. [PubMed] [Google Scholar]
- 35.Axhausen W. The osteogenetic phases of regeneration of bone, a historical and experimental study. J Bone Joint Surg Am. 1956;38-A:593. [PubMed] [Google Scholar]
- 36.Urist MR. The substratum for bone morphogenesis. Dev Biol. 1970;4(suppl):125. [PubMed] [Google Scholar]
- 37.August M, Tompach P, Chang Y, et al. Factors influencing the long-term outcome of mandibular reconstruction. J Oral Maxillofac Surg. 2000;58:731–737. doi: 10.1053/joms.2000.7255. [DOI] [PubMed] [Google Scholar]
- 38.Carlson ER, Marx RE. Mandibular reconstruction using cancellous cellular bone grafts. J Oral Maxillofac Surg. 1999;54:889–897. doi: 10.1016/S0278-2391(96)90543-1. [DOI] [PubMed] [Google Scholar]
- 39.Disa JJ, Hidalgo DA, et al. Evaluation of bone height in osseous free flap mandible reconstruction: an indirect measure of bone mass. Plast Reconstr Surg. 1999;103:1371–1377. doi: 10.1097/00006534-199904020-00005. [DOI] [PubMed] [Google Scholar]
- 40.Hidalgo DA, Rekow AL. Free flap mandible reconstruction: a 10-year follow-up study. Plast Reconstr Surg. 2002;110:438. doi: 10.1097/00006534-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Hidalgo DA (1989) Fibula free flap, A new method of mandible reconstruction. Plastic and Reconstructive Surgery 84:71–9 [PubMed]
- 42.Garrett A, et al. Evaluation of fibula free flap donor site morbidity. Am J Otolaryngol Head Neck Med Surg. 2006;27:29–32. doi: 10.1016/j.amjoto.2005.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


