Abstract
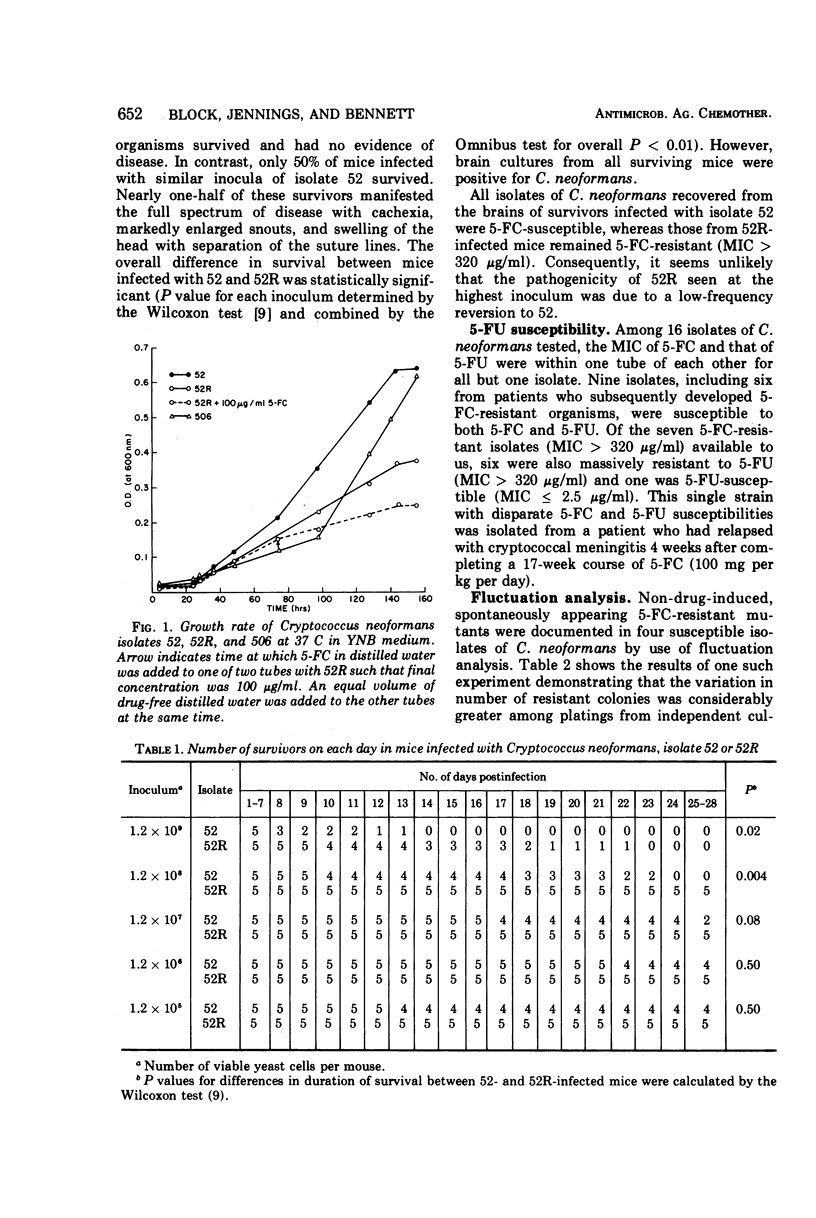
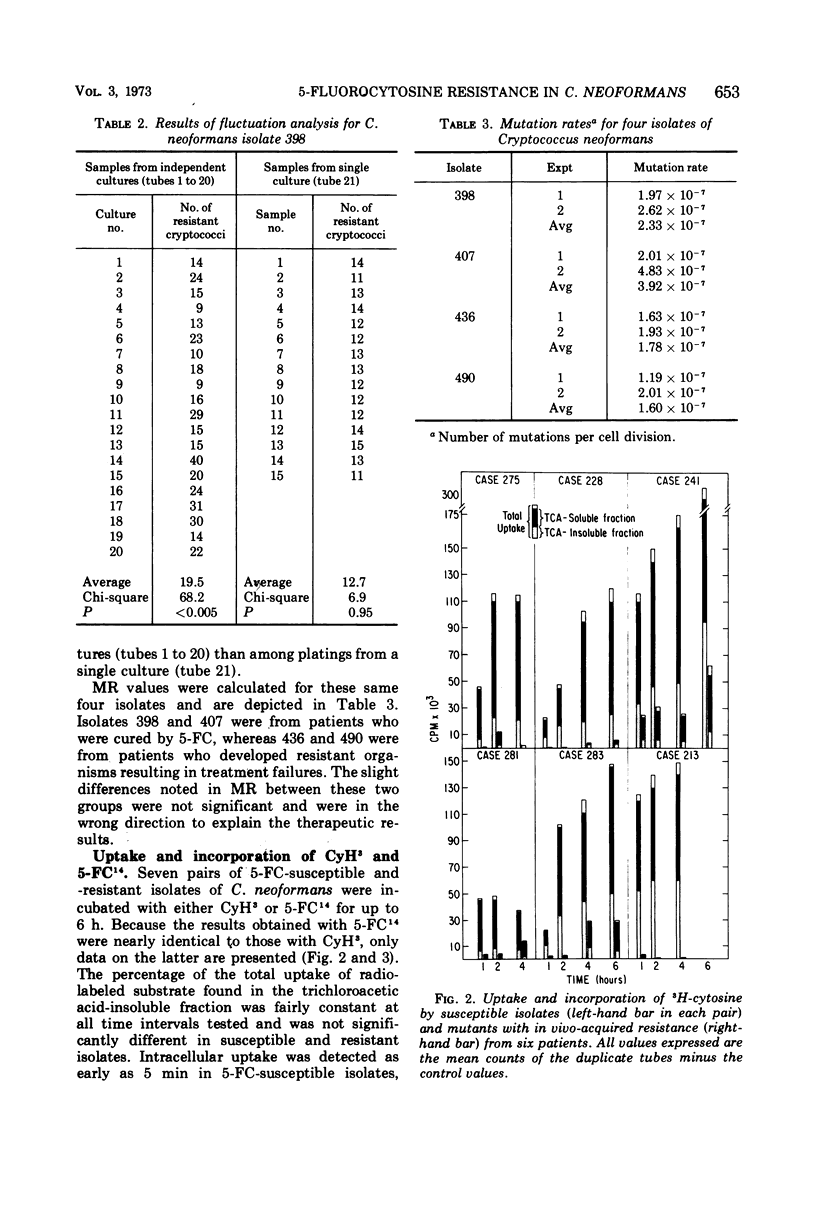
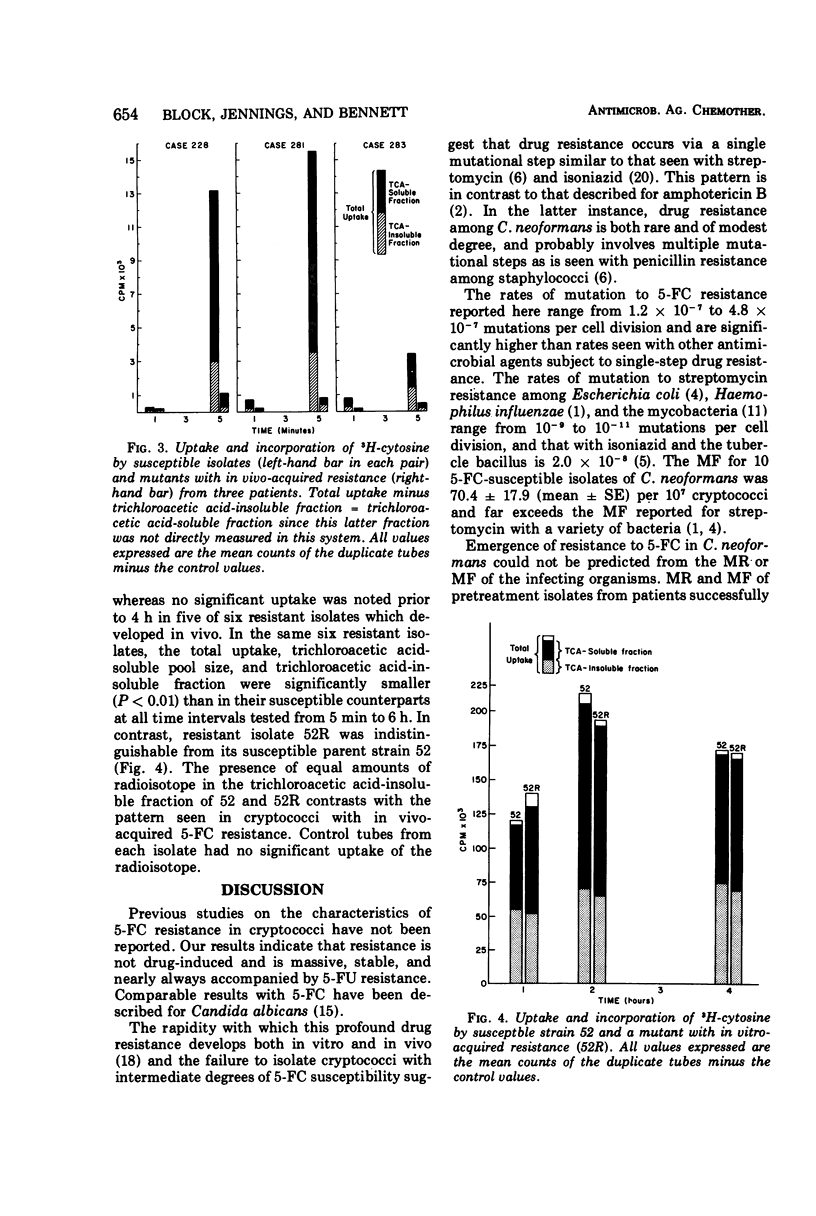
Isolates of Cryptococcus neoformans from six patients were obtained before and after unsuccessful therapy with 5-fluorocytosine (5-FC). Post-therapy isolates exhibited massive and stable 5-FC resistance. The frequency of drug-resistant mutants in susceptible isolates of C. neoformans was <0.001% (70.4 ± 17.9 per 107 cryptococci), whereas mutant frequencies in resistant isolates approached 100%. Non-drug-induced, spontaneously appearing 5-FC resistant mutants were documented in four susceptible isolates of C. neoformans by use of the statistical method of fluctuation analysis. Mutation rates on these same four isolates ranged from 1.2 × 10−7 to 4.8 × 10−7. Total intracellular uptake and incorporation of cytosine-5-3H (CyH3) and 5-fluorocytosine-2-14C (5-FC14) into a trichloroacetic acid-insoluble fraction were markedly reduced in six isolates with in vivo-acquired resistance when compared with susceptible pretreatment strains from the same patients. Five of these six isolates also had acquired massive resistance to 5-fluorouracil (5-FU), suggesting that a mutation in the uridine-5′-monophosphate pyrophosphorylase was responsible for drug resistance. The sixth isolate, which remained susceptible to 5-FU, appeared to have a defect in a cytosine-specific permease accounting for 5-FC resistance. A single isolate with in vitro-acquired 5-FC and 5-FU resistance had no reduction in uptake or incorporation of CyH3 or 5-FC14. The mechanism of resistance in this isolate is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. E. Susceptibility of Cryptococcus neoformans to amphotericin B. Antimicrob Agents Chemother (Bethesda) 1966;6:405–410. doi: 10.1128/AAC.6.4.405. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L L, Lederberg J. Isolation of Pre-Adaptive Mutants in Bacteria by Sib Selection. Genetics. 1956 May;41(3):367–381. doi: 10.1093/genetics/41.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970 Nov;20(5):810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M. Origin of Bacterial Resistance to Antibiotics. J Bacteriol. 1948 Jul;56(1):63–74. doi: 10.1128/jb.56.1.63-74.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON M. P., STAEHELIN M. Studies on the incorporation of 5-fluorouracil into a virus nucleic acid. Biochim Biophys Acta. 1959 Dec;36:351–361. doi: 10.1016/0006-3002(59)90177-5. [DOI] [PubMed] [Google Scholar]
- Grenson M. The utilization of exogenous pyrimidines and the recycling of uridine-5'-phosphate derivatives in Saccharomyces cerevisiae, as studied by means of mutants affected in pyrimidine uptake and metabolism. Eur J Biochem. 1969 Dec;11(2):249–260. doi: 10.1111/j.1432-1033.1969.tb00767.x. [DOI] [PubMed] [Google Scholar]
- HSIE J. Y., BRYSON V. Genetic studies on the development of resistance to neomycin and dihydrostreptomycin in Myobacterium ranae. Am Rev Tuberc. 1950 Sep;62(3):286–299. doi: 10.1164/art.1950.62.3.286. [DOI] [PubMed] [Google Scholar]
- Jund R., Lacroute F. Genetic and physiological aspects of resistance to 5-fluoropyrimidines in Saccharomyces cerevisiae. J Bacteriol. 1970 Jun;102(3):607–615. doi: 10.1128/jb.102.3.607-615.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Schönebeck J. In vitro studies of 5-fluorocytosine resistance in Candida albicans and Torulopsis glabrata. Antimicrob Agents Chemother. 1972 Sep;2(3):114–121. doi: 10.1128/aac.2.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Bacterial resistance studies with derivatives of isonicotinic acid. Am Rev Tuberc. 1952 Jun;65(6):768–770. [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S. 5-Fluoro-2'-deoxyuridylate: covalent complex with thymidylate synthetase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1855–1857. doi: 10.1073/pnas.69.7.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadomy S. Further in vitro studies with 5-fluorocytosine. Infect Immun. 1970 Oct;2(4):484–488. doi: 10.1128/iai.2.4.484-488.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadomy S., Shadomy H. J., McCay J. A., Utz J. P. In vitro susceptibility of Cryptococcus neoformans to amphotericin B, hamycin, and 5-fluorocytosine. Antimicrob Agents Chemother (Bethesda) 1968;8:452–460. [PubMed] [Google Scholar]
- Steer P. L., Marks M. I., Klite P. D., Eickhoff T. C. 5-fluorocytosine: an oral antifungal compound. A report on clinical and laboratory experience. Ann Intern Med. 1972 Jan;76(1):15–22. doi: 10.7326/0003-4819-76-1-15. [DOI] [PubMed] [Google Scholar]
- Utz J. P., Tynes B. S., Shadomy H. J., Duma R. J., Kannan M. M., Mason K. N. 5-Fluorocytosine in human cryptococcosis. Antimicrob Agents Chemother (Bethesda) 1968;8:344–346. doi: 10.1128/AAC.8.3.344. [DOI] [PubMed] [Google Scholar]
- Vandevelde A. G., Mauceri A. A., Johnson J. E., 3rd 5-fluorocytosine in the treatment of mycotic infections. Ann Intern Med. 1972 Jul;77(1):43–51. doi: 10.7326/0003-4819-77-1-43. [DOI] [PubMed] [Google Scholar]



