Abstract
In the United States, fibrin sealants have been used to achieve hemostasis for nearly two decades. Although their clinical utility was first demonstrated in cardiac surgery, their effectiveness and safety have since been demonstrated to extend to a wide array of procedures. Fibrin sealants typically contain two components—fibrinogen and thrombin—that are combined and delivered simultaneously to a target bleeding site in order to achieve hemostasis. However, many commercial formulations contain other additional components, such as antifibrinolytic agents, that have been associated with adverse outcomes. This subanalysis compares the safety and effectiveness of a fibrin sealant versus an absorbable hemostat for achieving hemostasis during urologic procedures with mild to moderate bleeding.
Key words: Hemostasis, Hemostatics, Fibrin tissue adhesive, Urologic surgical procedures, Surgical technique
In the United States, fibrin sealants have been used to achieve hemostasis for nearly two decades. Although their clinical utility was first demonstrated in cardiac surgery,1 their effectiveness and safety have since been demonstrated to extend to a wide array of procedures, including cardiovascular, gastrointestinal, pneumothoracic, neurologic, urologic, otolaryngologic, dental, and reconstructive surgeries.2,3 Within the field of urology, fibrin sealants have been used to manage bleeding from renal trauma,4 as well as to facilitate hemostasis during renal surgeries, including partial nephrectomies.5–7
Fibrin sealants typically contain two components—fibrinogen and thrombin—that are combined and delivered simultaneously to a target bleeding site (TBS) in order to achieve hemostasis.3 Many commercial formulations contain other additional components, such as antifibrinolytic agents, that have been associated with adverse outcomes. For example, in an observational study (N = 4374), the antifibrinolytic aprotinin was associated with an increased risk of long-term mortality within 5 years following coronary artery bypass graft surgery.8 Furthermore, repeated exposure to aprotinin may lead to allergic or potentially fatal anaphylactic reactions.9–11 For this reason, the US Food and Drug Administration (FDA) issued an alert in 2006 indicating that caution should be used when using aprotinin in patients with a history of previous exposure to the product.12 Tranexamic acid, another antifibrinolytic present in some commercial fibrin sealants, has been associated with alterations in neural tissue growth and adherence.13 Because of the risk of cerebral neurologic toxicity, fibrin sealants containing tranexamic acid are contraindicated for use in neurosurgery or in surgical procedures during which contact with cerebrospinal fluid or dura mater may occur.14
In a phase III, randomized, single-blind, parallel-group, multicenter study,15 the fibrin sealant CROSSEAL™ (Ethicon, Inc., Somerville, NJ) significantly reduced the time to hemostasis during liver resection surgery compared with conventional hemostatic techniques. EVICEL® Fibrin Sealant (Human) (Ethicon, Inc.), the successor of CROSSEAL, requires no antifibrinolytic additive and is therefore both aprotinin and tranexamic acid free, and achieves hemostasis using exclusively human components.16 The effectiveness and safety of this fibrin sealant for hemostasis in soft tissue during elective retroperitoneal or intra-abdominal surgery were compared with an absorbable hemostat (SURGICEL® Absorbable Hemostat; Ethicon, Inc.) in a randomized, active-controlled, multicenter study.17 This article describes a subanalysis of data from the largest patient subgroup from that study, and evaluates the effectiveness and safety of a fibrin sealant versus an absorbable hemostat for patients who underwent urologic surgical procedures.
Materials and Methods
Data for this subanalysis came from a phase III, randomized, active-controlled, multicenter study conducted at 16 sites in the United States. Detailed methods of that study have been described elsewhere, 17 and are summarized here. The study was conducted in accordance with the FDA regulations, International Conference for Harmonization (ICH) Tripartite Guideline for Good Clinical Practice (GCP), and the Declaration of Helsinki. Institutional Review Board (IRB) approval was obtained from each study site prior to the undertaking of any study-related procedures.
Patients scheduled to undergo elective retroperitoneal or intra-abdominal surgery were screened for study eligibility within 21 days prior to their surgery. Women who were pregnant or nursing were excluded from study participation. In addition, the study excluded patients with known intolerance to blood products or to one of the components of the study product, those unwilling to receive blood products, those with the presence of any autoimmune immunodeficiency disease, current alcohol and/ or drug abusers, and anyone who acknowledged recent (within 30 d) participation in another investigational drug or device research study. Prior to undertaking any study-specific procedures, patients were fully informed of all aspects of the study and were asked to sign a consent form.
During the procedure, the surgeon identified an appropriate soft tissue TBS, which was defined as the first soft tissue identified with mild or moderate bleeding for which conventional methods of control (eg, suture, ligature, and cautery) were ineffective or impractical and an adjunct product was required to achieve hemostasis. Bleeding was classified as mild (small areas of capillary, arteriole, or venule oozing in tissues) or moderate (larger areas of bleeding similar to those described as mild, but more challenging because of the larger area involved, an increased volume of blood loss, or flowing or pulsatile bleeding not from a large artery). Patients were excluded from study participation if bleeding sites were parenchymal or anastomotic, or if the surgeon determined that there were intraoperative findings that could have precluded the conduct of the study. Once intraoperative eligibility was confirmed, patients were randomized on a 1:1 basis to receive either fibrin sealant or absorbable hemostat (control group) as an adjunct for hemostasis once conventional surgical techniques were considered impractical or ineffective. The time at which the randomization envelope was opened was recorded as T0. For patients randomized to receive fibrin sealant, the fibrin sealant was dripped or sprayed onto the TBS; for patients randomized to the absorbable hemostat group, absorbable hemostat was directly applied with manual compression.
At 4, 7, and 10 minutes following randomization, bleeding at the TBS was evaluated. The primary effectiveness endpoint was the achievement of hemostasis at 10 minutes. The secondary effectiveness endpoints were the hemostasis outcome at 4 and 7 minutes, the incidence of complications that were potentially related to bleeding, and the incidence of treatment failures (defined as the presence of bleeding at the TBS 10 minutes postrandomization or brisk bleeding that required use of additional hemostatic measures during the 10-minute period). The absolute time to hemostasis was recorded for each patient. Adverse events were also recorded as they occurred.
Statistical Analysis
Analyses of effectiveness measures were conducted using the intent-to-treat population, which included all randomized patients. Analyses of safety measures were conducted using all patients who were randomized and received treatment. Absence of bleeding at the TBS at 4, 7, and 10 minutes following randomization, the incidence of treatment failures, and the incidence of complications that were potentially related to bleeding were analyzed. The proportion of patients in each treatment group who achieved hemostatic success was calculated, and a two-sided 95% confidence interval (CI) was constructed to evaluate the ratio of proportions of success (relative risk [RR]) between the treatment groups (fibrin sealant to absorbable hemostat) using a previously described method.18 If the lower limit of the 95% CI was > 0.80, the fibrin sealant was determined to be noninferior to the absorbable hemostat. If the lower limit was > 1.0, this was considered to be evidence for the superiority of the fibrin sealant in terms of statistical significance at the 5% level (P < .05). In this case, the P value associated with the test of superiority was calculated.
Results
Patients
A total of 124 patients were enrolled and randomized in the original clinical trial. Data from the 53 patients who underwent urologic surgery (fibrin sealant, n = 28; absorbable hemostat, n = 25) were included in this subanalysis. Demographic characteristics were similar between the treatment groups (Table 1). The majority of patients were white (69.8% [37/53]) and men (69.8% [37/53]). The most common surgical procedures were nephrectomy (39.6% [21/53]), which included partial, simple, and radical procedures, and prostatectomy (26.4% [19/53]).
Table 1.
Demographic and Baseline Characteristics of Patients
| Absorbable | |||
|---|---|---|---|
| Fibrin Sealant | Hemostat | Total | |
| Characteristic | (n = 28) | (n = 25) | (n = 53) |
| Mean (SD) age, y | 54.7 (21.6) | 52.5 (19.7) | 53.7 (20.6) |
| Mean (SD) BMI, kg/m2 | 26.0 (5.1) | 28.0 (7.5) | 27.0 (6.4) |
| Sex, n (% male) | 19 (67.9) | 18 (72.0) | 37 (69.8) |
| Race, n (%) | |||
| White | 21 (75.0) | 16 (64.0) | 37 (69.8) |
| Black | 7 (25.0) | 7 (28.0) | 26 (26.4) |
| Hispanic | 0 (0) | 2 (8.0) | 2 (3.8) |
| History of smoking, | 15 (53.6) | 12 (48.0) | 27 (50.9) |
| n (% yes) | |||
| Primary operative procedure, n (%) | |||
| Nephrectomy | 10 (35.7) | 11 (44.0) | 21 (39.6) |
| Prostatectomy | 7 (25.0) | 7 (28.0) | 14 (26.4) |
| Cystectomy | 6 (21.4) | 3 (12.0) | 9 (17.0) |
| Other | 5 (17.9) | 4 (16.0) | 9 (17.0) |
BMI, body mass index; SD, standard deviation.
Hemostasis
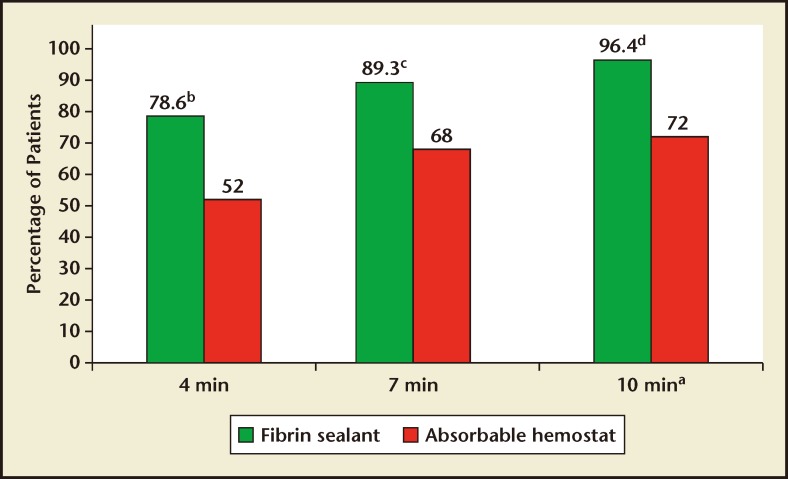
A greater percentage of patients who received fibrin sealant achieved the primary endpoint of hemostasis at 10 minutes compared with patients who received absorbable hemostat (96.4% [27/28] vs 72.0% [18/25], respectively; RR 1.34; 95% CI, 1.07–1.85; P = .013; Figure 1). A greater percentage of patients who received fibrin sealant also achieved the secondary endpoint of hemostasis by 4 minutes compared with patients who received absorbable hemostat (78.6% [22/28] vs 52.0% [13/25], respectively; RR = 1.51; 95% CI, 1.02–2.42; P = .041). At the 7-minute time point, comparison of percentages of the treatment group of patients who achieved hemostasis nearly reached statistical significance (89.3% [25/28] vs 68.0% [17/25], respectively; RR = 1.31; 95% CI, 0.99–1.87; P = .056). A lower overall incidence of treatment failure was observed for patients who received fibrin sealant versus absorbable hemostat (3.6% [1/28] vs 28.0% [7/25], respectively; RR = 0.13; 95% CI, 0.02–0.72; P = .013).
Figures 1.
Percentage of patients in each treatment group that achieved hemostasis at 4, 7, and 10 minutes after randomization. aPrimary endpoint. bP = .041. cP = .056. dP = .013.
Complications Potentially Related to Bleeding
The incidence of complications that were potentially related to bleeding in the first 10 minutes was similar between the two treatment groups (fibrin sealant, 0.0% [0/28]; absorbable hemostat, 4.0% [1/25]; RR = 0; 95% CI, 0–3.33; P = .285). However, the overall percentage of complications potentially related to bleeding was numerically lower for the fibrin sealant group than for the absorbable hemostat group (7.1% [2/28] vs 20.0% [5/25], respectively; RR = 0.36; 95% CI, 0.08–1.45; P = .172.
Achieving Hemostasis for Mild Versus Moderate Bleeding
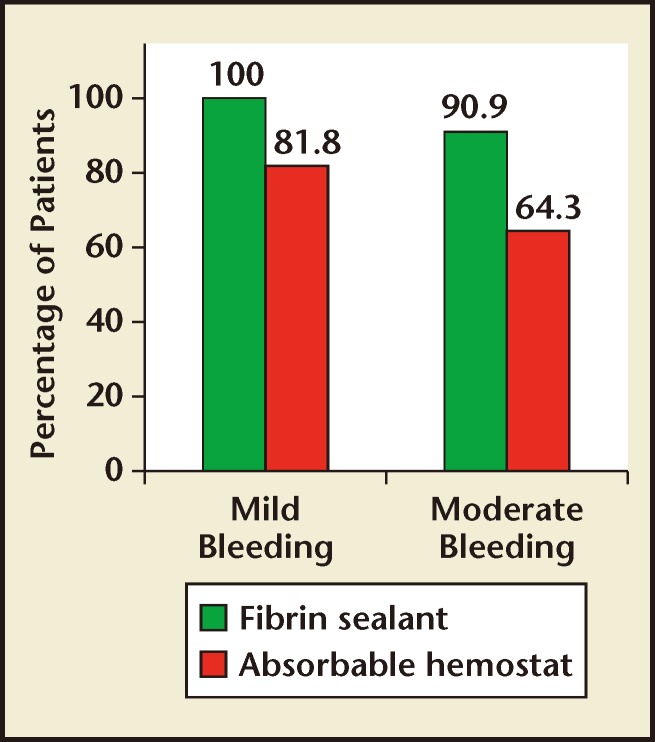
Bleeding at the TBS was classified as mild in 28 patients (fibrin sealant, n = 17; absorbable hemostat, n = 11) and moderate in 25 patients (fibrin sealant, n = 11, absorbable hemostat, n = 14). Regardless of whether patients had mild or moderate bleeding, the percentage of patients achieving hemostasis within 10 minutes was numerically greater with fibrin sealant compared with absorbable hemostat (Figure 2).
Figures 2.
Percentage of patients achieving hemostasis at 10 minutes with mild bleeding or moderate bleeding at the target bleeding site.
Safety
The adverse event profiles were similar between the treatment groups. A comparable number of patients in each group had at least one adverse event (fibrin sealant, 64.3% [18/28]; absorbable hemostat, 68.0% [17/25]). The most frequently reported adverse events (occurring in ≥ 10% of either treatment group) included pyrexia, hypokalemia, hypertension, insomnia, anemia, hypomagnesemia, hypotension, nausea, tachycardia, vomiting, and pruritus (Table 2). These events are consistent with those expected in patients undergoing surgical procedures. Postoperative transfusion rates were not actively collected during the trial and are not available for evaluation.
Table 2.
Adverse Events Occurring in ≥ 10% of Patients
| Absorbable | |||
|---|---|---|---|
| Fibrin Sealant | Hemostat | Total | |
| Adverse Event, n (%) | (n = 28) | (n = 25) | (n = 53) |
| Any event | 18 (64.3) | 17 (68.0) | 35 (66.0) |
| Hypokalemia | 3 (10.7) | 4 (16.0) | 7 (13.2) |
| Pyrexia | 5 (17.9) | 1 (4.0) | 6 (11.3) |
| Insomnia | 4 (14.3) | 2 (8.0) | 6 (11.3) |
| Hypomagnesemia | 3 (10.7) | 2 (8.0) | 5 (9.4) |
| Hypertension | 1 (3.6) | 4 (16.0) | 5 (9.4) |
| Hypotension | 3 (10.7) | 2 (8.0) | 5 (9.4) |
| Anemia | 1 (3.6) | 3 (12.0) | 4 (7.5) |
| Nausea | 3 (10.7) | 1 (4.0) | 4 (7.5) |
| Tachycardia | 3 (10.7) | 0 (0) | 3 (5.7) |
| Vomiting | 3 (10.7) | 0 (0) | 3 (5.7) |
| Pruritus | 3 (10.7) | 0 (0) | 3 (5.7) |
Discussion
This subanalysis demonstrates that the fibrin sealant used in this study is safe and effective as an adjunct for achieving hemostasis for mild to moderate bleeding in soft tissue during urologic surgery. Consistent with the findings of the larger phase III study,17 a greater percentage of patients undergoing urologic procedures who received the fibrin sealant relative to the absorbable hemostat in this subanalysis achieved hemostasis within 10 minutes, the primary efficacy outcome of the original trial. Both treatments were safe and well tolerated.
The safety of the fibrin sealant used in this study reflects a number of its formulation-specific characteristics. Unlike some other commercially available fibrin sealants, 3 the fibrin sealant used in this study was derived exclusively from human components and is tranexamic acid free.16 The absence of these additives makes it appropriate for use in a broader population, including those with previous exposure to aprotinin-containing products and those undergoing surgical procedures during which contact with cerebrospinal fluid or dura mater may occur. In addition, to minimize the risk of inadvertent transmission of blood-borne pathogens, the fibrin sealant used in this study underwent two distinct virus inactivation and/or removal steps during the manufacturing process.16 Such processes, in combination with donor and plasma screening, help ensure that plasma-derived products are free of blood-borne pathogens.19–21
The findings of this subanalysis should be interpreted with mindfulness of the potential limitations of the small sample size (n = 53), the nonblinded study design, and short follow-up period. Although it is not possible to perform a blinded comparison of these two agents (because of differences in application methods), further nonblinded comparisons in larger urologic surgery populations could provide additional insight into the full clinical potential of this fibrin sealant in the urology setting. Finally, subjects had their last follow-up visit 7 to 14 days postsurgery; any lateroccurring complications may not have been observed by this study.
Conclusions
This analysis demonstrates that this fibrin sealant is safe and effective for achieving predictable and rapid hemostasis during urologic surgical procedures with mild to moderate bleeding, consistent with findings from studies that include a wider range of surgical procedures. Fibrin sealants are well suited for achieving intraoperative hemostasis, especially in broad surface area bleeding that is encountered during urologic procedures.
Main Points.
Fibrin sealants, which have been used to achieve hemostasis in the United States for nearly 2 decades, typically contain two components—fibrinogen and thrombin—that are combined and delivered simultaneously to a target bleeding site (TBS) in order to achieve hemostasis. Many commercial formulations contain other additional components, such as antifibrinolytic agents, that have been associated with adverse outcomes.
In a phase III, randomized, active-controlled, multicenter study of patients undergoing elective retroperitoneal or intra-abdominal surgery, 53 patients who underwent urologic surgery were randomized to receive either fibrin sealant or absorbable hemostat as an adjunct to hemostasis. Bleeding was evaluated 4, 7, and 10 minutes after an appropriate bleeding site was identified.
A greater percentage of patients who received fibrin sealant achieved hemostasis at the 4-, 7-, and 10-minute assessment compared with patients who received absorbable hemostat. The primary endpoint of hemostasis at 10 minutes was statistically significant in favor of the subjects who received fibrin sealant compared with those who received absorbable hemostat, and the incidence of complications that were potentially related to bleeding in the first 10 minutes was similar between the two treatment groups.
This subanalysis demonstrates that the fibrin sealant used in this study is safe and effective as an adjunct for achieving hemostasis for mild to moderate bleeding in soft tissue during urologic surgery.
Footnotes
This study was supported by OMRIX Biopharmaceuticals (Kiryat Ono, Israel), and Ethicon, a Johnson & Johnson company (Somerville, NJ). Administrative support for the writing of this manuscript was provided by Ashley O’Dunne, PhD, of MedErgy (Morrisville, PA) and was funded by Ethicon. The authors were not compensated and retained full editorial control over the content of the manuscript.
References
- 1.Rousou J, Levitsky S, Gonzalez-Lavin L, et al. Randomized clinical trial of fibrin sealant in patients undergoing resternotomy or reoperation after cardiac operations. A multicenter study. J Thorac Cardiovasc Surg. 1989;97:194–203. [PubMed] [Google Scholar]
- 2.Jackson MR. Fibrin sealants in surgical practice: an overview. Am J Surg. 2001;182(2 suppl):1S–7S. doi: 10.1016/s0002-9610(01)00770-x. [DOI] [PubMed] [Google Scholar]
- 3.Albala DM. Fibrin sealants in clinical practice. Cardiovasc Surg. 2003;11(suppl 1):5–11. doi: 10.1016/S0967-2109(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 4.Kram HB, Ocampo HP, Yamaguchi MP, et al. Fibrin glue in renal and ureteral trauma. Urology. 1989;33:215–218. doi: 10.1016/0090-4295(89)90395-6. [DOI] [PubMed] [Google Scholar]
- 5.Levinson AK, Swanson DA, Johnson DE, et al. Fibrin glue for partial nephrectomy. Urology. 1991;38:314–316. doi: 10.1016/0090-4295(91)80142-t. [DOI] [PubMed] [Google Scholar]
- 6.Lapini ACM, Serni S, Stefanucci S, et al. The use of fibrin sealant in nephron sparing surgery for renal tumors. In: Schlag G, Melchior H, Wallwiener D, et al., editors. Gynecology and Obstetrics in Urology, vol. 7. New York, NY: Springer-Verlag; 1994. pp. 79–82. [Google Scholar]
- 7.Rouach Y, Delongchamps NB, Patey N, et al. Suture or hemostatic agent during laparoscopic partial nephrectomy? A randomized study using a hypertensive porcine model. Urology. 2009;73:172–177. doi: 10.1016/j.urology.2008.08.477. [DOI] [PubMed] [Google Scholar]
- 8.Mangano DT, Miao Y, Vuylsteke A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. JAMA. 2007;297:471–479. doi: 10.1001/jama.297.5.471. [DOI] [PubMed] [Google Scholar]
- 9.Oswald AM, Joly LM, Gury C, et al. Fatal intraoperative anaphylaxis related to aprotinin after local application of fibrin glue. Anesthesiology. 2003;99:762–763. doi: 10.1097/00000542-200309000-00053. [DOI] [PubMed] [Google Scholar]
- 10.Scheule AM, Beierlein W, Lorenz H, Ziemer G. Repeated anaphylactic reactions to aprotinin in fibrin sealant. Gastrointest Endosc. 1998;48:83–85. doi: 10.1016/s0016-5107(98)70138-5. [DOI] [PubMed] [Google Scholar]
- 11.Kober BJ, Scheule AM, Voth V, et al. Anaphylactic reaction after systemic application of aprotinin triggered by aprotinin-containing fibrin sealant. Anesth Analg. 2008;107:406–409. doi: 10.1213/ane.0b013e31817e6043. [DOI] [PubMed] [Google Scholar]
- 12.Aprotinin Injection (marketed as Trasylol) Information. US Food and Drug Administration website. [Accessed February 6, 2015]. http://www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm142720.htm.
- 13.Cox S, Cole M, Mankarious S, Tawil N. Effect of tranexamic acid incorporated in fibrin sealant clots on the cell behavior of neuronal and nonneuronal cells. J Neurosci Res. 2003;72:734–746. doi: 10.1002/jnr.10623. [DOI] [PubMed] [Google Scholar]
- 14.Quixil [package insert] Rhode-St-Genèse, Belgium: Omrix Biopharmaceuticals S.A.; 2005. [Google Scholar]
- 15.Schwartz M, Madariaga J, Hirose R, et al. Comparison of a new fibrin sealant with standard topical hemostatic agents. Arch Surg. 2004;139:1148–1154. doi: 10.1001/archsurg.139.11.1148. [DOI] [PubMed] [Google Scholar]
- 16.EVICEL [package insert] Somerville, NJ: Johnson & Johnson Wound Management, a division of Ethicon, Inc.; 2005. [Google Scholar]
- 17.Fischer CP, Wood CG, Shen J, et al. A randomized trial of aprotinin-free fibrin sealant versus absorbable hemostat. Clin Appl Thromb Hemost. 2011;17:572–577. doi: 10.1177/1076029611404212. [DOI] [PubMed] [Google Scholar]
- 18.Koopman PAR. Confidence intervals for the ratio of two binomial proportions. Biometrics. 1984;40:513–517. [Google Scholar]
- 19.Burnouf T, Radosevich M. Nanofiltration of plasmaderived biopharmaceutical products. Haemophilia. 2003;9:24–37. doi: 10.1046/j.1365-2516.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz B, Ben-Hur E. Efforts in minimizing risk of viral transmission through viral inactivation. Ann Med. 2000;32:475–484. doi: 10.3109/07853890009002023. [DOI] [PubMed] [Google Scholar]
- 21.Weimer T, Streichert S, Watson C, Gröner A. Hightiter screening PCR: a successful strategy for reducing the parvovirus B19 load in plasma pools for fractionation. Transfusion. 2001;41:1500–1504. doi: 10.1046/j.1537-2995.2001.41121500.x. [DOI] [PubMed] [Google Scholar]