Abstract
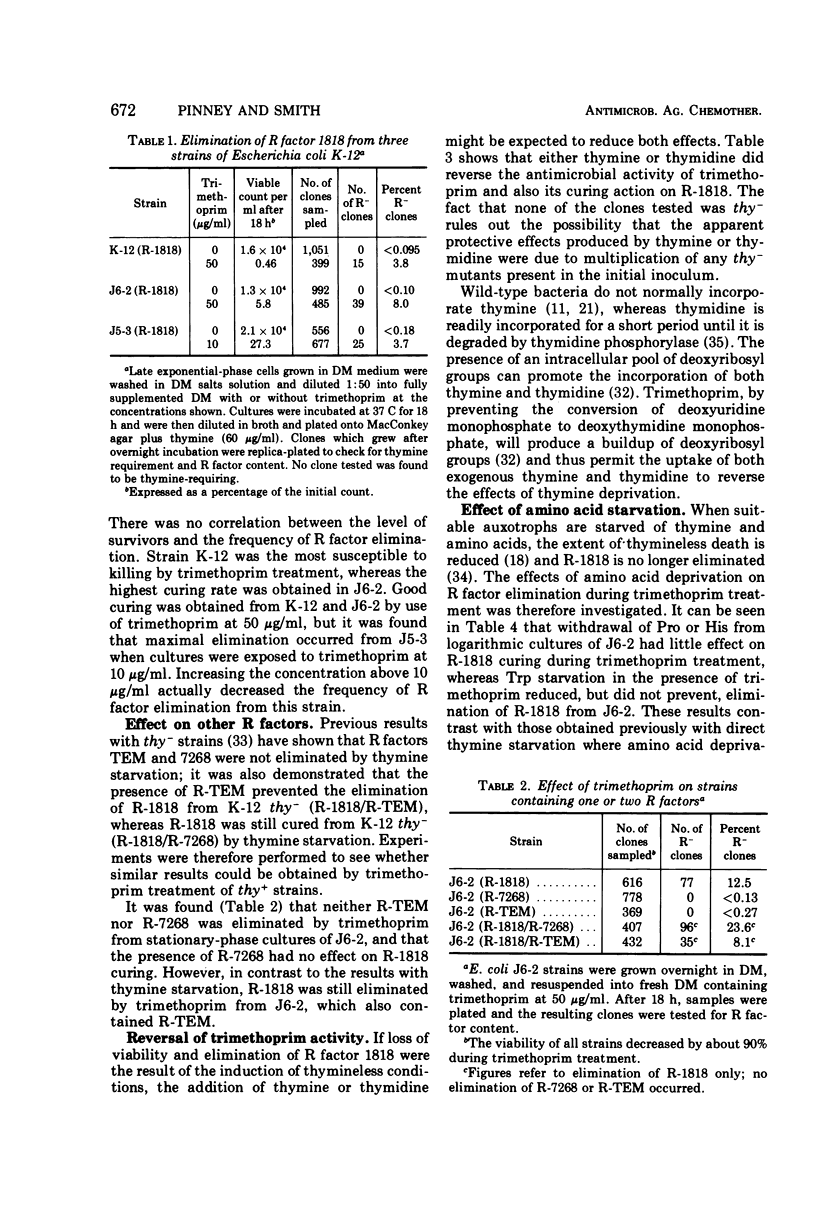
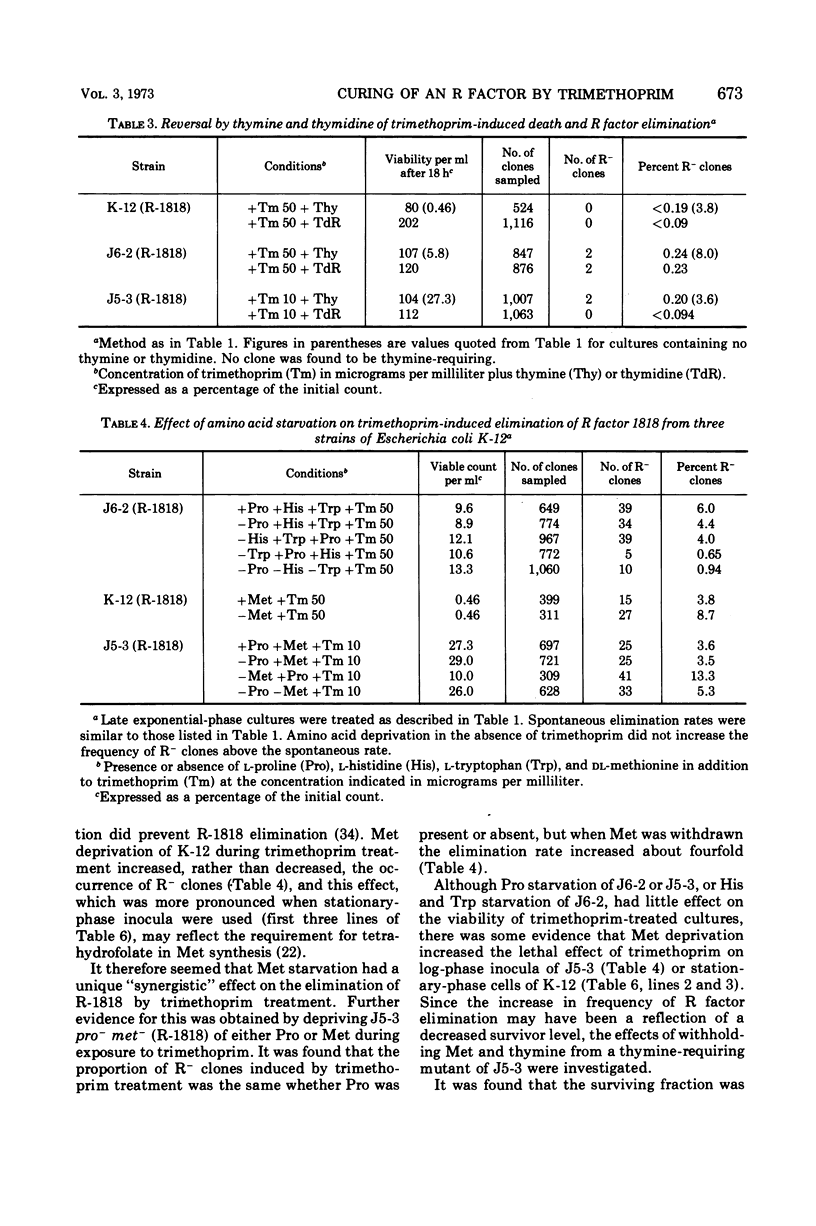
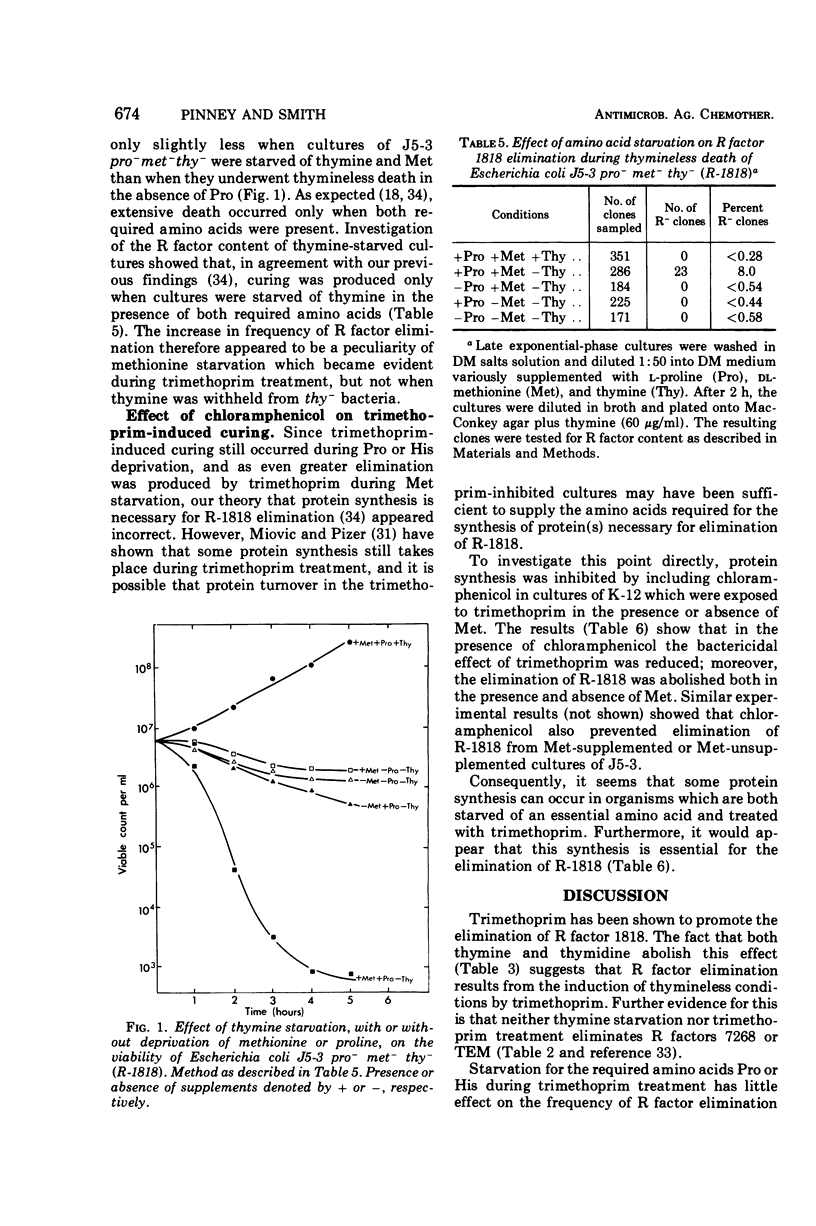
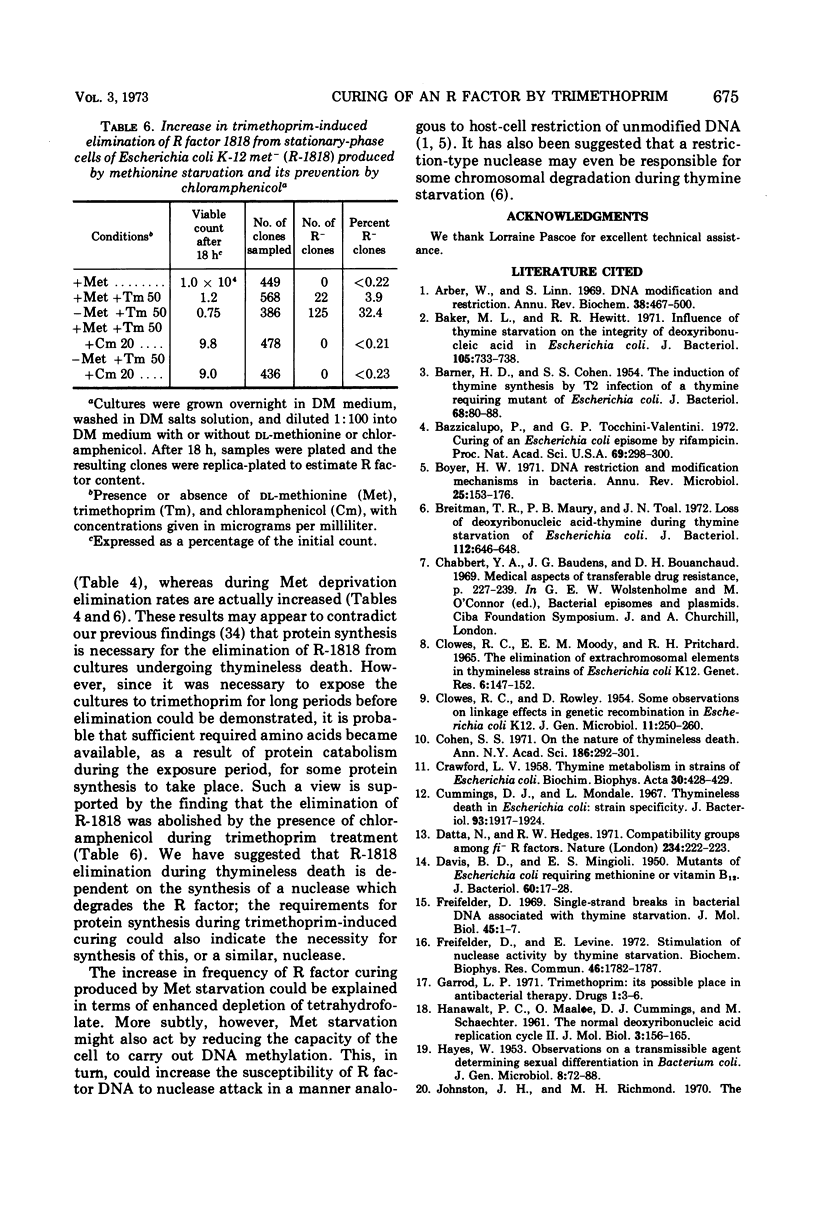
R factor 1818, which we have shown previously to be eliminated by thymine starvation, was cured from three strains of Escherichia coli K-12 by overnight exposure to trimethoprim. Elimination was abolished in the presence of added thymine or thymidine, which suggests that curing is the result of the induction of thymineless conditions by trimethoprim. Starvation of the required amino acids proline and histidine had little effect on elimination, whereas methionine deprivation enhanced it. R factor curing was abolished by the presence of chloramphenicol, and it is concluded that protein synthesis is required for elimination to occur. It is suggested that elimination may result from the activity of a nuclease which is synthesized or induced during both direct thymine starvation and by trimethoprim treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- BARNER H. D., COHEN S. S. The induction of thymine synthesis by T2 infection of a thymine requiring mutant of Escherichia coli. J Bacteriol. 1954 Jul;68(1):80–88. doi: 10.1128/jb.68.1.80-88.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. L., Hewitt R. R. Influence of thymine starvation on the integrity of deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1971 Mar;105(3):733–738. doi: 10.1128/jb.105.3.733-738.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzicalupo P., Tocchini-Valentini G. P. Curing of an Escherichia coli episome by rifampicin (acridine orange-F + -F - -Hfr-lac). Proc Natl Acad Sci U S A. 1972 Feb;69(2):298–300. doi: 10.1073/pnas.69.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Maury P. B., Toal J. N. Loss of deoxyribonucleic acid-thymine during thymine starvation of Escherichia coli. J Bacteriol. 1972 Oct;112(1):646–648. doi: 10.1128/jb.112.1.646-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOWES R. C., ROWLEY D. Some observations on linkage effects in genetic recombination in Escherichia coli K-12. J Gen Microbiol. 1954 Oct;11(2):250–260. doi: 10.1099/00221287-11-2-250. [DOI] [PubMed] [Google Scholar]
- CLWES R. C., MOODY E. E., PRITCHARD R. H. THE ELIMINATION OF EXTRACHROMOSOMAL ELEMENTS IN THYMINELESS STRAINS OF ESCHERICHIA COLI K12. Genet Res. 1965 Feb;6:147–152. doi: 10.1017/s0016672300004018. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. Thymine metabolism in strains of Escherichia coli. Biochim Biophys Acta. 1958 Nov;30(2):428–429. doi: 10.1016/0006-3002(58)90071-4. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Thymineless death in Escherichia coli: strain specificity. J Bacteriol. 1967 Jun;93(6):1917–1924. doi: 10.1128/jb.93.6.1917-1924.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Compatibility groups among fi - R factors. Nature. 1971 Nov 26;234(5326):222–223. doi: 10.1038/234222a0. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Levine E. Stimulation of nuclease activity by thymine starvation. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1782–1787. doi: 10.1016/0006-291x(72)90051-4. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Single-strand breaks in bacterial DNA associated with thymine starvation. J Mol Biol. 1969 Oct 14;45(1):1–7. doi: 10.1016/0022-2836(69)90205-8. [DOI] [PubMed] [Google Scholar]
- Garrod L. P. Trimethoprim: its possible place in antibacterial therapy. Drugs. 1971;1(1):3–6. doi: 10.2165/00003495-197101010-00002. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- HAYES W. [Observations on a transmissible agent determining sexual differentiation in Bacterium coli]. J Gen Microbiol. 1953 Feb;8(1):72–88. doi: 10.1099/00221287-8-1-72. [DOI] [PubMed] [Google Scholar]
- Johnston J. H., Richmond M. H. The increased rate of loss of penicillinase plasmids from Staphylococcus aureus in the presence of rifampicin. J Gen Microbiol. 1970 Jan;60(1):137–139. doi: 10.1099/00221287-60-1-137. [DOI] [PubMed] [Google Scholar]
- KISLIUK R. L., WOODS D. D. Interrelationships between folic acid and cobalamin in the synthesis of methionine by extracts of Escherichia coli. Biochem J. 1960 Jun;75:467–477. doi: 10.1042/bj0750467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN D., WEISSBACH A. Thymineless induction in Escherichia coli K12 (lambda). Biochim Biophys Acta. 1962 Nov 26;61:775–790. doi: 10.1016/0926-6550(62)90060-9. [DOI] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krcméry V., Janousková J. Effect of rifampicin on stability and transfer of R-factors. Z Allg Mikrobiol. 1971;11(2):97–101. doi: 10.1002/jobm.3630110204. [DOI] [PubMed] [Google Scholar]
- MELECHEN N. E., SKAAR P. D. The provocation of an early step of induction by thymine deprivation. Virology. 1962 Jan;16:21–29. doi: 10.1016/0042-6822(62)90198-8. [DOI] [PubMed] [Google Scholar]
- MENNIGMANN H. D., SZYBALSKI W. Molecular mechanism of thymine-less death. Biochem Biophys Res Commun. 1962 Nov 27;9:398–404. doi: 10.1016/0006-291x(62)90023-2. [DOI] [PubMed] [Google Scholar]
- Medoff G., Overholt S. Thymineless death in Escherichia coli 15T- and recombinants of 15T- and Escherichia coli K-12. J Bacteriol. 1970 Apr;102(1):213–216. doi: 10.1128/jb.102.1.213-216.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miovic M., Pizer L. I. Effect of trimethoprim on macromolecular synthesis in Escherichia coli. J Bacteriol. 1971 Jun;106(3):856–862. doi: 10.1128/jb.106.3.856-862.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney R. J., Smith J. T. R factor elimination during thymine starvation: effects of inhibition of protein synthesis and readdition of thymine. J Bacteriol. 1972 Aug;111(2):361–367. doi: 10.1128/jb.111.2.361-367.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney R. J., Smith J. T. R-factor elimination by thymine starvation. Genet Res. 1971 Oct;18(2):173–177. doi: 10.1017/s001667230001257x. [DOI] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- Reichenbach D. L., Schaiberger G. E., Sallman B. The effect of thymine starvation on chromosomal structure of Escherichia coli JG-151. Biochem Biophys Res Commun. 1971 Jan 8;42(1):23–30. doi: 10.1016/0006-291x(71)90356-1. [DOI] [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Bridges B. A. Alkaline sucrose gradient sedimentation of chromosomal deoxyribonucleic acid from Escherichia coli PolA + and PolA - strains during thymine starvation. J Bacteriol. 1971 Dec;108(3):1422–1423. doi: 10.1128/jb.108.3.1422-1423.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T. Production of thymineless mutants in gram-negative bacteria (Aerobacter, Proteus). J Gen Microbiol. 1967 Apr;47(1):131–137. doi: 10.1099/00221287-47-1-131. [DOI] [PubMed] [Google Scholar]
- Smith J. T. R-factor gene expression gram-negative bacteria. J Gen Microbiol. 1969 Jan;55(1):109–120. doi: 10.1099/00221287-55-1-109. [DOI] [PubMed] [Google Scholar]
- Walker J. R. Thymine Starvation and Single-Strand Breaks in Chromosomal Deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1391–1392. doi: 10.1128/jb.104.3.1391-1392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


