Abstract
Hepatitis C virus (HCV) infection is common among injection drug users (IDUs). There is accumulating evidence that circulating microRNAs (miRNAs) are associated with HCV infection and disease progression. The present study was undertaken to determine the in vivo impact of heroin use on HCV infection and HCV-related circulating miRNA expression. Using the blood specimens from four groups of the study subjects (HCV-infected individuals, heroin users with/without HCV infection, and healthy volunteers), we found that HCV-infected heroin users had significantly higher viral load than HCV-infected non-heroin users (p = 0.0004). Measurement of HCV-related circulating miRNAs in plasma showed that miRs-122, 141, 29a, 29b, and 29c were significantly increased in the heroin users with HCV infection, whereas miR-351, an HCV inhibitory miRNA, was significantly decreased in heroin users as compared to control subjects. Further investigation identified a negative correlation between the plasma levels of miR-29 family members and severity of HCV infection based on aspartate aminotransferase to platelet ratio index (APRI). In addition, heroin use and/or HCV infection also dysregulated a panel of plasma miRNAs. Taken together, these data for the first time revealed in vivo evidence that heroin use and/or HCV infection alter circulating miRNAs, which provides a novel mechanism for the impaired innate anti-HCV immunity among IDUs.
Keywords: Hepatitis C virus (HCV), Injection drug users (IDUs), Circulating microRNAs
Introduction
HCV infection is highly prevalent among injecting drug users (IDUs), as a result of collective use of injecting equipment and drug solutions, as well as through sexual behaviors. Rates of HCV infection among past and current IDUs are extremely high generally ranging from 70 % to over 90 % (antibody positive for HCV) in the United States (Chang et al. 1999; Williams 1999; Edlin et al. 2001). IDUs are frequently involved in the abuse of heroin, one of the most commonly used opiates. Although injection use of heroin contributes significantly to HCV transmission (Backmund et al. 2003; Day et al. 2003; Quaglio et al. 2003; Smyth et al. 2003; Bassani et al. 2004; Garten et al. 2004; Ye et al. 2008), there is limited information available about whether heroin abuse increases HCV replication, or facilitates disease progression. Several lines of evidence have shown that morphine, the primary metabolite of heroin, undermines IFN-mediated anti-HCV innate immunity and enhances HCV replication in vitro (Li et al. 2003; Wang et al. 2005).
Innate immunity is critical in the control of HCV infection/replication. As an important part of host innate immune system, a number of miRNAs have been identified to be involved in regulation of host-virus interactions and antiviral immune responses (Pedersen et al. 2007; Valastyan and Weinberg 2011; Natarajan et al. 2012; Nikitina et al. 2012; Palanisamy et al. 2012; Farazi et al. 2013; Nazarov et al. 2013). Alterations in miRNA expression profiles upon viral infection may either favour or inhibit different stages of the viral life cycle. Although there is no evidence of HCV-encoded miRNAs, cellular miRNA-122, a well-studied liver-specific miRNA, has been shown to promote HCV infection by binding to the 5 prime untranslated region (5’UTR) of HCV genome (Henke et al. 2008; Fehr et al. 2012; Goergen and Niepmann 2012; Jopling 2012; Tsai et al. 2012; Mortimer and Doudna 2013). In contrast, some miRNAs like miR-199a and those (miR-448, miR-196, miR-431, miR-351, and miR-296) modulated by interferon beta (IFN-β) can target the HCV genome and inhibit HCV RNA production (Pedersen et al. 2007; Henke et al. 2008; Goergen and Niepmann 2012; Mortimer and Doudna 2013). MiRNAs are found not only within cells but also in serum/plasma and other body fluids. Although the function of these extracellular circulating miRNAs remains to be determined, their profile changes have been associated with different diseases (Xu et al. 2011; Ding et al. 2012; Kawano et al. 2013). Because circulating miRNAs are stable and can be detected by quantitative PCR in clinical specimens of body fluids, they have become potential biomarkers of diseases.
Given the fact that miRNAs are involved in important biological processes and innate immunity against viral infections, including HCV (Xu et al. 2011; Ding et al. 2012; Kawano et al. 2013), it is of significance to determine whether environmental factors such as heroin abuse, a common practice among IDUs, can dysregulate both cellular and cell-free miRNAs. Although it is known that opiates impair host immunity and enhance HCV replication in vitro (Li et al. 2003; Wang et al. 2005), there is little information about the in vivo impact of heroin on the expression of miRNAs related to innate immunity and HCV infection.
Materials and Methods
Study Subjects
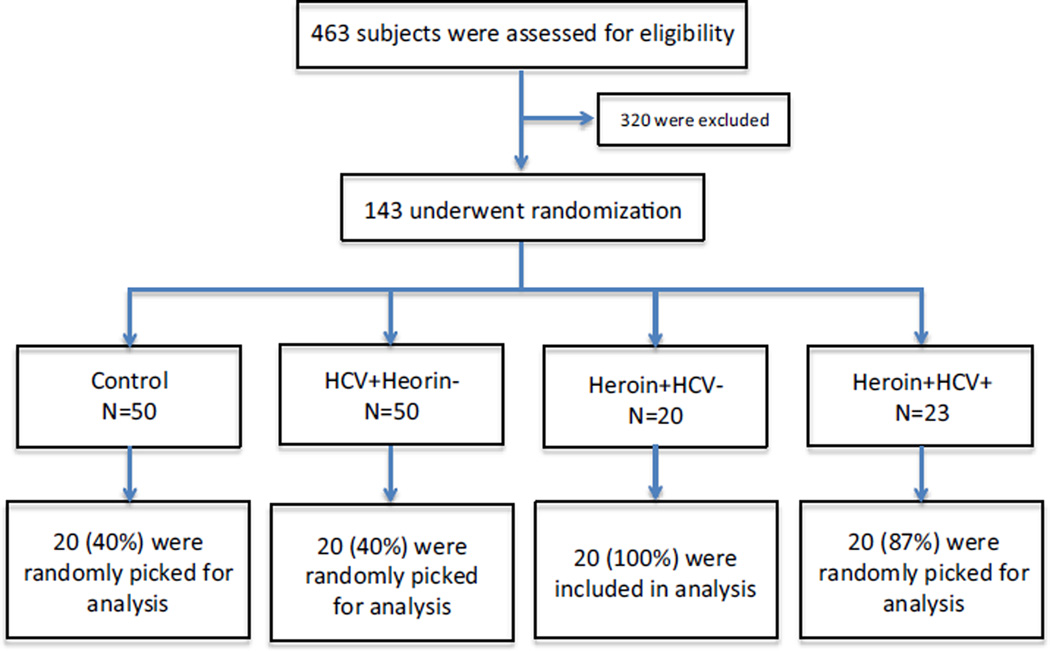
The study subjects addicted to heroin were recruited by Wuhan Centers for Disease Prevention and Control in China, based upon the subject self-report and urine screen for selection. HCV-infected subjects with no history of heroin abuse were recruited by Zhongnan Hospital, Wuhan University School of Medicine. Informed consent was obtained from the study subjects. Institutional Review Boards of the Wuhan Centers for Disease Prevention and Control, Wuhan University and Temple University approved this study. The blood samples from the subjects were identified as HIV antibody negative by anonymous testing on the basis of enzyme-linked immunosorbent assay (Beckman Coulter, Inc, Hialeah, FL). Subjects were excluded if they had a chronic systemic illness (cardiac, renal, pulmonary, hepatic, endocrine, metabolic, or autoimmune disorders) or a major psychiatric disorder or if they were abusing substances other than heroin (as determined by urine drug test result). The control subjects were recruited using convenience sampling from the community in which the study site was located. Control subjects who had major medical or psychiatric disorders were also excluded. The flow of study subject selection is shown in Fig. 1. Out of 463 subjects examined, 320 were excluded and 143 were randomized. Of these, 20 subjects were randomly selected from each group (Control, HCV patients, heroin users with HCV infection, and heroin users without HCV infection). The characteristics of the study subjects were summarized in Table 1.
Fig. 1.
Enrollment and randomization of the study subjects. Subjects were enrolled through Wuhan CDC and Zhongnan Hospital, Wuhan University School of Medicine, China. A total of 463 subjects were initially screened for eligibility by a questionnaire survey. A total of 143 subjects were eligible and randomized into 4 groups: 50 control subjects, 50 HCV-infected patients with no heroin use, 20 heroin abusers with no HCV, 23 heroin abusers with HCV infection. 20 subjects were randomly selected from each group for analysis
Table 1.
Characteristics of study subjects
| Category | Control (N = 20) | HCV (N = 20) | Heroin (N = 20) | HCV + Heroin (N = 20) |
|---|---|---|---|---|
| Age (Yrs mean ± SD) | 43±4 | 43±5 | 40±7 | 43±6 |
| Range of age (Yrs) | 35–46 | 31–56 | 29–60 | 27–53 |
| Gender (male/female) | 20/0 | 20/0 | 20/0 | 20/0 |
| Race (ethnic group) | Asian (Han) | Asian (Han) | Asian (Han) | Asian (Han) |
| Heroin use | −* | −* | +** | +** |
| HCV | − | + | − | + |
| HIV | − | − | − | − |
No history of opiate and other substance abuse
Heroin only
miRNA Real-Time PCR and miRNA Array
Total RNA, including miRNA, was extracted from plasma using miRNeasy Mini Kit (QIAGEN) in accordance with the manufacturer’s instructions. RNA (1 µg) was reverse-transcribed with miScript Reverse Transcription Kit (QIAGEN). Real-time reverse-transcription polymerase chain reaction (RT-PCR) for the quantification of a subset of miRNAs (miRNA-29a, miRNA-29b, miRNA-29c, miRNA-122, miRNA-141, and miRNA-351) was carried out with miScript Primer Assays and miScript SYBR Green PCR Kit from QIAGEN. MiRNA arrays were performed using Serum & Plasma miRNA PCR Array Kit (MIHS-106Z, QIAGEN), and data were analyzed as directed by the manufacturer.
HCV Load in Plasma
Total RNA from plasma was extracted with Tri-Reagent-BD (Molecular Research Center) in accordance with the manufacturer’s instructions. The real time RT-PCR assay that we have developed (Li et al. 2003) with minor modifications was used for the quantification of HCV RNA. HCV genome primers used in this study are: 5′-RAYCACTCCCCTGTGA GGAAC-3′ (Forward), and 5′-AGAAAAATAATGCAGAGC CAAATT-3′ (Reverse). Real time RT-PCR was performed with iQ SYBR Green Supermix. A standard curve of HCV was generated with 10-fold dilutions of HCV 5’non-coding region RNA control that had been pre-quantified by a spectrophotometer (Eppendorf Scientific, Inc., Westbury, NY). Thermal cycling conditions were designed as follows: initial denaturation at 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 1 min.
Statistical Analysis
Where appropriate, data are expressed as mean ± SD of each group. For comparison of the mean of two groups (HCV vs Heroin combined HCV), statistical significance was assessed by Student’s t-test. If there were more than two groups (Control vs Heroin, HCV, or Heroin combined HCV), one-way repeated measures of analysis of variance (ANOVA) with Tukey-Kramer adjustment for multiple comparisons were used. For the correlation analysis, linear regression was used to assess the positive or negative correlation. Statistical analyses were performed with Graphpad Instat Statistical Software (GraphPad Software Inc., San Diego, CA, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
In vivo Effect of Heroin Use on HCV
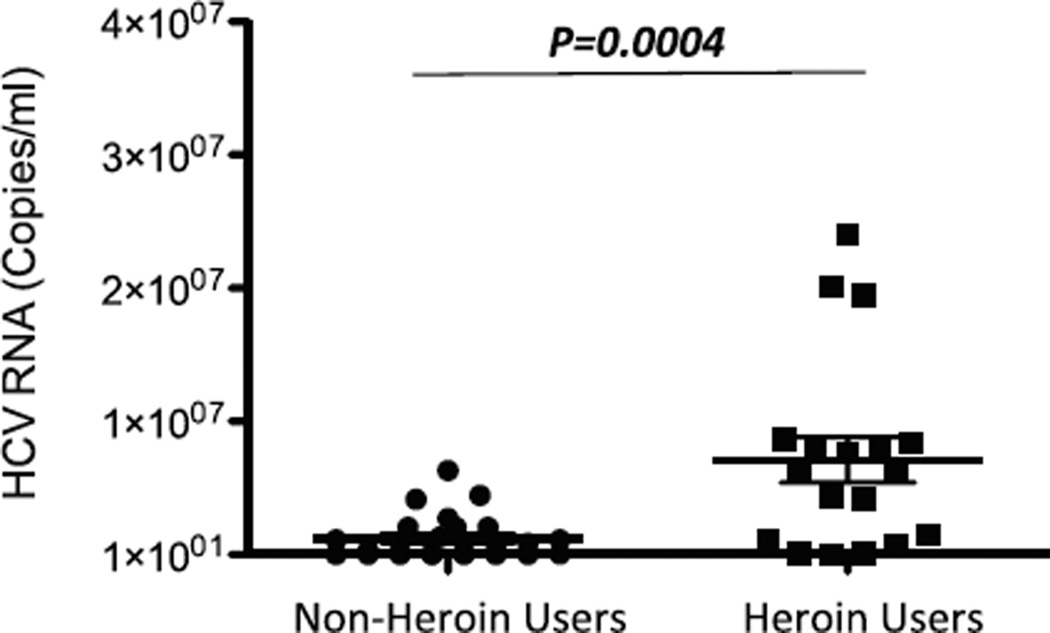
We previously showed that morphine could enhance HCV replicon infection in human hepatocytes (Li et al. 2003). To determine the in vivo impact of heroin use on HCV, we measured plasma HCV RNA levels in HCV-infected subjects with or without heroin use. As shown in Fig. 2, the heroin users had significantly higher plasma levels of HCV RNA than the non-heroin users (p = 0.004).
Fig. 2.
Comparison of HCV loads between heroin users and non-heroin users. Total RNA was extracted from plasma specimens of HCV infected subjects with (n = 20) or without heroin use (n = 20) and then subjected to real-time RT PCR for quantification of HCV RNA. Quantity of HCV RNA is expressed as copies/ml of plasma. The data were shown as mean for RNA copy numbers with standard deviation. Statistical significance was calculated by the Students t test
Heroin Use and/or HCV Infection Dysregulate the Circulating miRNAs Related to HCV
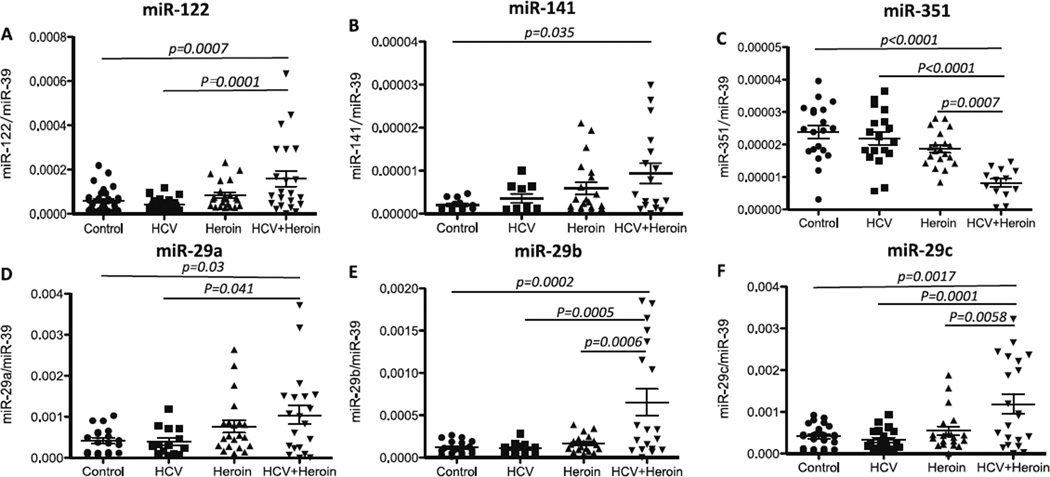
Using the specimens from the 4 groups of study subjects (Table 1), we determined the in vivo impact of heroin use and/or HCV on the expression of circulating miRNAs related to HCV infection (miRNAs-29a, 29b, 29c, 122, 141, and 351) (Pedersen et al. 2007; Henke et al. 2008; Kumar 2011; Zhang et al. 2012; Mortimer and Doudna 2013). As shown in Fig. 3, heroin use and/or HCV infection dysregulated the expression of these miRNAs. MiRNA-122 and miRNA-141, both of which can promote HCV replication (Pedersen et al. 2007; Jopling 2012), were found to be increased in heroin users with HCV infection (Fig. 3a and b). In contrast, HCV-suppressive miRNA-351 was down-regulated in heroin users with HCV infection synergistically (Fig. 3c). In addition, although HCV infection alone had little effect on miRNA29 family members (Fig. 3d, e and f), HCV infection and heroin use synergistically enhanced the expression of these miRNAs (Fig. 3d, e and f). Heroin use and/or HCV infection had little effect on the expression of other circulating miRNAs (miRNAs-196a, 196b, 199, 296, and 448) that are associated with HCV (data not shown).
Fig. 3.
Impact of heroin use and/or HCV infection on HCV-related circulating MicroRNAs. Plasma specimens were collected from control subjects (n = 20), heroin users with (n = 20) or without (n = 20) HCV infection, and subjects with HCV infection but without heroin use (n= 20), respectively. Total RNA was extracted from plasma and subjected to real-time RT-PCR to quantify circulating miRNA-122 (a), -141 (b), -351 (c), -29a (d), -29b (e), and -29c (f). Synthetic C. elegans miRNA-39 (celmiR-39) was used as a spiked-in miRNA. Levels of target circulating miRNAs were expressed by normalization to the spiked-in cel-miR-39 and compared between the 4 groups. Data are shown as scatter plots. Mean values are indicated by horizontal bars. Statistical significance was calculated by the one-way ANOVA with Tukey-Kramer adjustment for multiple comparisons
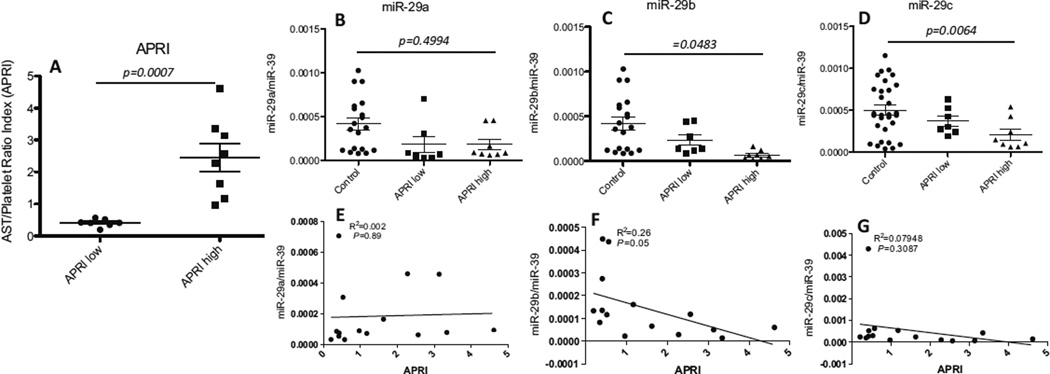
Circulating miR-29 is Correlated with AST/Platelet Ratio Index in HCV Infection
The aspartate aminotransferase (AST) to platelet ratio index (APRI) is one of the non-invasive markers for liver fibrosis and positively indicates the severity of HCV disease progression (Antonello et al. 2010; Macias et al. 2010). We divided the HCV-infected subjects into 2 sub-groups based on their APRI (APRI-low: APRI<0.68; APRI-high: APRI>0.68 (Fig. 4a). To determine whether circulating miRNA-29 family members are associated with HCV infection severity, we examined the levels of these circulating miRNAs in HCV-infected subjects with low or high APRI as compared to those in the control subjects, and found a significant decrease in miR-29 family members (Fig. 4b, c and d) in APRI high group when comparing with control. As shown in Fig. 4, the level of miRNA-29b was negatively correlated with HCV infection (Fig. 4f).
Fig. 4.
Correlation analysis of circulating miR-29 family with HCV severity (APRI). HCV-infected patients were stratified into APRI-low group (APRI<0.68, n = 7) and APRI-high group (APRI>0.68, n = 8). The levels of circulating miRNAs-29a, 29b, and 29c were compared between the APRI-low and APRI-high groups as well as with the healthy controls. a APRI values between APRI-low and APRI-high groups of HCV-infected patients. APRI was calculated using Wai’s formula (AST/upper limit of normal considered as 40 IU/L)/platelet count (expressed as platelets × 109 L−1) × 100. b–d Comparison of the levels of circulating miRNAs-29a (b), 29b (c), and 29c (d) among the APRI-low, APRI-high and control groups. e–g Correlation analysis for circulating miRNA 29a (e), 29b (f), and 29c (g) with APRI level in HCV-infected subjects
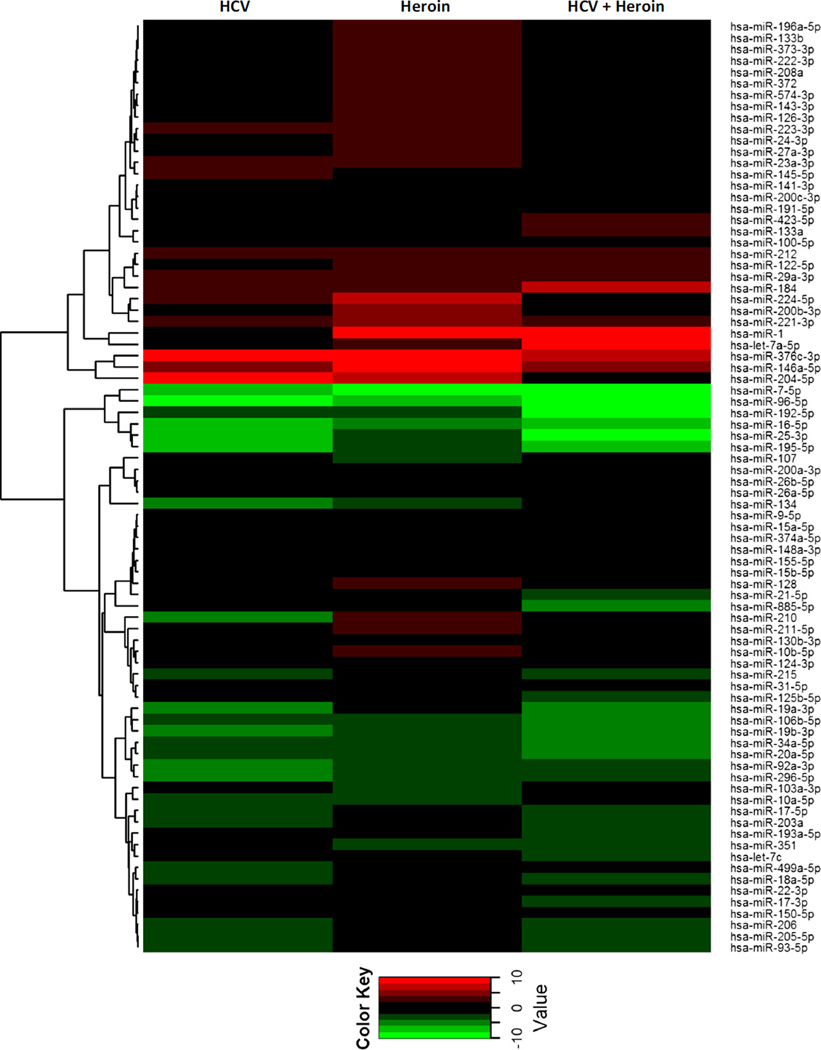
Heroin Use and/or HCV Infection Dysregulate Circulating miRNA Profile
We also examined the impact of heroin use and/or HCV infection on a panel of plasma miRNAs. As shown in Fig. 5 and Table 2, a number of circulating miRNAs were dysregulated by heroin use and/or HCV infection (Fig. 5, Table 2). In heroin users with HCV infection, 6 miRNAs were up-regulated, and 14 miRNAs were down-regulated; 11 miRNAs were up-regulated and 9 miRNAs were down-regulated in heroin users without HCV infection; in HCV infected subjects, 3 miRNAs were up-regulated and 17 miRNAs were down-regulated (Table 2).
Fig. 5.
Dysregulation of circulating miRNA profile by heroin abuse and/or HCV infection. Plasma specimens were collected from control subjects, HCV-infected patients, and heroin users with or without HCV infection, respectively. Total RNA was extracted and subjected to circulating miRNA profiling by real-time PCR. Data were first normalized to the spiked-in cel-miR-39, expressed as fold change relative to control subject, and then shown as relative up-regulation (red) and down-regulation (green) as indicated in the heat-map graph with dendrogram
Table 2.
Dys-regulated miRNAs in heorin users (with or without HCV infection) and HCV infected subjects
| miRNAs ↑ | Heroin | HCV | Heroin + HCV+ |
| miR-376c-3p | 22.72 | 7.89 | 13.08 |
| miR-146a-5p | 11.41 | 4.29 | 5.82 |
| miR-1 | 40.41 | 72.86 | |
| miR-204-5p | 6.26 | 10.88 | |
| miR-211-5p | 3.31 | ||
| miR-221-3p | 4.65 | ||
| miR-24-3p | 3.31 | ||
| miR-214-3p | 3.14 | ||
| miR-223-3p | 3.56 | ||
| miR-224-5p | 6.50 | ||
| miR-27a-3p | 3.44 | ||
| miR-184 | 6.05 | 3.26 | |
| let-7a-5p | 14.47 | ||
| miRNAs ↓ | Heroin | HCV | Heroin + HCV+ |
| miR-25-3p | −3.58 | −8.04 | −6.13 |
| miR-296-5p | −3.81 | −3.84 | −8.97 |
| miR-20a-5p | −3.35 | −4.05 | −3.34 |
| miR-7-5p | −16.69 | −13.70 | −6.16 |
| miR-96-5p | −7.81 | −27.57 | −9.95 |
| miR-16-5p | −4.94 | −6.93 | −6.34 |
| miR-192-5p | −3.20 | −12.36 | −3.59 |
| miR-195-5p | −3.81 | −7.28 | −7.32 |
| miR-107 | −3.25 | ||
| miR-19b-3p | −5.89 | −4.16 | |
| miR-92a-3p | −3.46 | −4.16 | |
| miR-19a-3p | −4.80 | −4.63 | |
| miR-106b-5p | −4.67 | −3.47 | |
| miR-203a | −3.20 | −3.16 | |
| miR-134 | −4.22 | ||
| miR-34a-5p | −4.30 | ||
| miR-885-5p | −5.79 | ||
| miR-17-5p | −3.22 | ||
| miR-122-5p | −4.52 |
Discussion
MiRNAs are important in regulating host-virus interactions and antiviral immunity (Iwama et al. 2011; Bhanja Chowdhury et al. 2012; Aalaei-Andabili and Rezaei 2013; Li and Shi 2013). Among the viruses, HCV appears in particular to be influenced by the host miRNAs. Roles of miRNAs in regulating HCV infection and anti-HCV immunity have been intensively studied, which has led to identification of a number of HCV-related miRNAs that can either promote or inhibit HCV infection/replication (Pedersen et al. 2007; Gupta et al. 2012; Wilson and Huys 2013). For examples, miR-122, a liver-specific miRNA involved in fatty-acid metabolism, can enhance HCV infection by binding to the 5 prime untranslated region (5’UTR) of HCV genome, thus stabilizing viral transcripts and preventing the viral RNA from being targeted by host cytoplasmic viral sensors (Henke et al. 2008; Goergen and Niepmann 2012; Mortimer and Doudna 2013). Similarly, miRNA-141 could enhance HCV infection of human hepatocytes by inhibiting the tumor-suppressor DLC-1 (Deleted in Liver Cancer 1) (Banaudha et al. 2011). A similar result was observed in a non-human primate model where miRNA-122 in liver was increased during acute HCV infection (Choi et al. 2013). In contrast, miR-448, miR-196, miR-431, miR-351, and miR-296 inhibit HCV through different mechanisms (Pedersen et al. 2007; Gupta et al. 2012). For instance, miR-448 and miR-196 were found to suppress HCV infection by targeting the regions of HCV genome that encode core protein and NS5A, respectively (Pedersen et al. 2007). Therefore, it is of importance to determine whether these miRNAs are affected by heroin use and/or HCV infection. Using unique clinical specimens from heroin users with or without HCV infection, we found that heroin users with HCV infection had significantly higher level ofmiRNA-122 and lower level of miRNA-351 than control subjects. These effects of heroin use and/or HCV appear to be specific, as other HCV-related miRNAs (miRNAs-448, 196a, 196b, 296, and 441) are not affected.
These in vivo findings provide molecular mechanism(s) not only for our earlier in vitro studies, showing that morphine-enhanced HCV replicon expression in human hepatocytes, but also for the in vivo observation that heroin use was associated with increased HCV load (Fig. 2). Unlike the majority of heroin users who use multiple drugs in the United States, 70–80 % of heroin users in China use heroin only. Therefore, to access these subjects in China provides a unique opportunity to examine the impact of heroin use on host immune system and HCV infection. However, because of the limited number of the subjects and study design in this investigation, future studies are necessary in order to determine the role of heroin use in facilitating HCV disease and decipher the precise mechanisms through which heroin alters HCV virology and the host immunity.
APRI is an important predictor of hepatic fibrosis in patients with HCV infection (Snyder et al. 2006, 2007). miRNA-29 family members were also found to inhibit liver fibrosis. Van Rooji et al. (van Rooij et al. 2008) showed that inhibition of miR-29 in mice could increase collagen expression in liver. It was also hypothesized that in Hepatic stellate cells (HSCs), miR-29 down regulation is activated by liver injury-induced TGF-β along with the increased extracellular matrix production, which ultimately leads to fibrosis (Hoffmann et al. 2012). In addition, in livers from chronic hepatitis C patients with sustained virological response (SVR), it presents higher levels of miR-29a, -29b and -29c when compared to livers of patients without SVR (Hoffmann et al. 2012). Furthermore, miR-29 was also associated with IFN-γ production (Ma et al. 2011), and appeared to have tumor suppressor activity in leukemia and HCC (Pekarsky and Croce 2010). Altogether, there is a growing line of evidence indicating the importance of the miR-29 family in host defense against HCV infection. Another study by Bandyopadhyay et al. also demonstrated that in HCV infected patients, lower levels of miR-29 was shown in liver and overexpression of miR-29 suppresses viral RNA in HCV infected hepatocytes (Bandyopadhyay et al. 2011). Experimental evidence indicates that putative targets of miR-29 family include an anti-apoptotic protein, the myeloid leukemia cell differentiation protein (MCL1), an anti-inflammatory and anti-cancer gene (Mott et al. 2007), Zinc finger protein 36 homolog (ZFP36) (Gebeshuber et al. 2009), and another cancer related gene DNA methyltransferases DNMT3A and DNMT3B (Fabbri et al. 2007). Moreover, Voltage-dependent anion channel genes VDAC1 and VDAC2 were also identified as targets of miR-29a in HEK293T cells (Bargaje et al. 2012). Due to the crucial function of miRNA-29 family in both basic cellular processes and HCV related liver pathogenesis, we also observed the correlation of miRNA-29 levels and HCV infection. When comparing the levels of miRNA-29 between HCV-infected patients and the control subjects, no significant differences were found (Fig. 3). However, when we stratified the HCV patients based on their APRI, significant decreases in the levels of circulating miRNA-29 family members were detected in the APRI-high group as compared to the control group (Fig. 4). These data indicted that miR-29 is negatively correlated with severity of HCV infection, which is consistent with a previous study showing that HCV infection and stellate cell activation can down-regulate miR-29 in liver biopsies (Bandyopadhyay et al. 2011).
In addition to cellular miRNAs, circulating miRNAs in plasma are found to be stable and readily detectable by PCR, which have made them attractive candidates for diagnosis, prognosis and targets for therapy in a variety of disease settings (Huang et al. 2010; Xu et al. 2011; Ding et al. 2012; Kawano et al. 2013). It has been shown that changes in circulating miRNA profile are strongly associated with disease progression and development and can be useful biomarkers for cancer and infections (Bartels and Tsongalis 2009; Ding et al. 2012; Farazi et al. 2013). Alterations in cellular miRNA profiles in response to HCV infection and/or drug exposure have been previously reported (Pedersen et al. 2007; Ye et al. 2010; Banaudha et al. 2011; Bandyopadhyay et al. 2011; Kumar 2011; Bhanja Chowdhury et al. 2012). In our study, we performed array analysis to characterize the circulating miRNA profiles in plasma of subjects and we demonstrated that a broad range of miRNAs, in addition to those that have been identified and well studied, were dysregulated in HCV infection and/or heroin abuse patients as compared to the control subjects (Fig. 5). These miRNA array results may provide new insights to identify novel candidate miRNAs that are involved in regulating host-HCV interactions. In terms of the origin, circulating miRNAs were thought to be from passive leakage of broken cells. While in the past couple of years, our understanding of how the extracellular miRNAs get released from cells has considerably broadened (Rayner and Hennessy 2013). It is now believed the secretion of miRNAs is an active, and under-controlled process. Recent studies demonstrated besides bounding to proteins such as Argonaute-2 (Ago2), and high- and low-density lipoprotein (HDL and LDL), extracellular miRNAs can be also packaged into cell-derived lipid-based exosomes, called exosomal miRNAs (Rayner and Hennessy 2013). Exosomal miRNAs have been indicated to be involved in many cellular biological functions (Rayner and Hennessy 2013). After exosomes being released from donor cells to recipient cells, the exosome-carried miRNAs enter the cytosol of the target cell where it may silence the related genes. For instance, it was reported that human (HMC-1) and mouse (MC/9) mast cell lines derived exosomes are able to transport miRNA to adjacent mast cells, and affect the function of target cells (Valadi et al. 2007; Schorey and Bhatnagar 2008). Additionally, a study by Bukong et al., reported that exosomes from sera of HCV infected patients contain HCV RNA, miR-122, Ago2, and HSP90, and are able to transfer HCV infection to primary human hepatocytes. Moreover, they found HCV delivery by exosomes can be blocked by miR-122 or HSP90 inhibitor within exosomes, indicating the crucial function of exosomal miR-122 in exosome-mediated HCV transmission (Bukong et al. 2014).
In summary, we here provide in vivo evidence that heroin use can affect HCV infection and affect host-HCV interactions by altering circulating miRNAs. Our study highlights the importance of investigating circulating miRNAs in HCV pathogenesis and host anti-HCV responses in clinical settings.
Acknowledgments
Supported Grant DA12815 and DA22177 to Dr. Wen-Zhe Ho
Financial Support The study was supported by grants NIDA012815, NIDA027550 and NIDA022177 (to WZH) from the National Institutes of Health.
Footnotes
Conflict of Interest The authors have no conflict of interest.
Author Contributions Conceived and designed the experiments: YZ XW WZH. Performed the experiments: YZ LS XW. Analyzed the data: YZ HQZ XW WZH. Contributed reagents/materials/analysis tools/others: LZ MQL FW JSP YZW JLL MS NR XDL XEG DJZ. Wrote the paper: YZ WZH.
Contributor Information
Yu Zhou, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, 3500 N. Broad St., Philadelphia, PA 19140, USA.
Li Sun, Center for Animal Experiment/ABSL-III Laboratory, Wuhan University School of Medicine, Wuhan 430071, China.
Xu Wang, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, 3500 N. Broad St., Philadelphia, PA 19140, USA.
Li Zhou, Center for Animal Experiment/ABSL-III Laboratory, Wuhan University School of Medicine, Wuhan 430071, China.
Jieliang Li, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, 3500 N. Broad St., Philadelphia, PA 19140, USA.
Manqing Liu, Wuhan Centers for Disease Prevention & Control, Wuhan 430015, China.
Fang Wang, Wuhan Centers for Disease Prevention & Control, Wuhan 430015, China.
Jinsong Peng, Wuhan Centers for Disease Prevention & Control, Wuhan 430015, China.
Xi’en Gui, Zhongnan Hospital, Wuhan University, Wuhan 430071, China.
Huaqing Zhao, Temple Clinical Research Institute, Temple University School of Medicine, Philadelphia, PA 19140, USA.
Nancy Reichenbach, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, 3500 N. Broad St., Philadelphia, PA 19140, USA.
Dunjin Zhou, Wuhan Centers for Disease Prevention & Control, Wuhan 430015, China.
Wen-Zhe Ho, Email: wenzheho@temple.edu, Department of Pathology and Laboratory Medicine, Temple University School of Medicine, 3500 N. Broad St., Philadelphia, PA 19140, USA; Center for Animal Experiment/ABSL-III Laboratory, Wuhan University School of Medicine, Wuhan 430071, China.
References
- Aalaei-Andabili SH, Rezaei N. Toll like receptor (TLR)-induced differential expression of microRNAs (MiRs) promotes proper immune response against infections: a systematic review. J Infect. 2013 doi: 10.1016/j.jinf.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Antonello VS, Tovo CV, Kliemann DA, Santos BR, Zaltron VF. Evaluation of APRI score in liver disease following the introduction of antiretroviral therapy in HIV and HCV coinfected versus HIV monoinfected patients. Rev Soc Bras Med Trop. 2010;43:678–681. doi: 10.1590/s0037-86822010000600015. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Wachtler M, Eichenlaub D. Hepatitis C virus infection in injection drug users in Bavaria: risk factors for seropositivity. Eur J Epidemiol. 2003;18:563–568. doi: 10.1023/a:1024603517136. [DOI] [PubMed] [Google Scholar]
- Banaudha K, Kaliszewski M, Korolnek T, Florea L, Yeung ML, Jeang KT, Kumar A. MicroRNA silencing of tumor suppressor DLC-1 promotes efficient hepatitis C virus replication in primary human hepatocytes. Hepatology. 2011;53:53–61. doi: 10.1002/hep.24016. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Friedman RC, Marquez RT, Keck K, Kong B, Icardi MS, Brown KE, Burge CB, Schmidt WN, Wang Y, McCaffrey AP. Hepatitis C virus infection and hepatic stellate cell activation downregulate miR-29: miR-29 overexpression reduces hepatitis C viral abundance in culture. J Infect Dis. 2011;203:1753–1762. doi: 10.1093/infdis/jir186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargaje R, Gupta S, Sarkeshik A, Park R, Xu T, Sarkar M, Halimani M, Roy SS, Yates J, Pillai B. Identification of novel targets for miR-29a using miRNA proteomics. PLoS One. 2012;7:e43243. doi: 10.1371/journal.pone.0043243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels CL, Tsongalis GJ. MicroRNAs: novel biomarkers for human cancer. Clin Chem. 2009;55:623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- Bassani S, Toro C, de la Fuente L, Brugal MT, Jimenez V, Soriano V. Rate of infection by blood-borne viruses in active heroin users in 3 Spanish cities. Med Clin (Barc) 2004;122:570–572. doi: 10.1016/s0025-7753(04)74311-0. [DOI] [PubMed] [Google Scholar]
- Bhanja Chowdhury J, Shrivastava S, Steele R, Di Bisceglie AM, Ray R, Ray RB. Hepatitis C virus infection modulates expression of interferon stimulatory gene IFITM1 by upregulating miR-130A. J Virol. 2012;86:10221–10225. doi: 10.1128/JVI.00882-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Lin CH, Lee CT, Chang SJ, Ko YC, Liu HW. Hepatitis C virus infection among short-term intravenous drug users in southern Taiwan. Eur J Epidemiol. 1999;15:597–601. doi: 10.1023/a:1007662315835. [DOI] [PubMed] [Google Scholar]
- Choi Y, Dienes HP, Krawczynski K. Kinetics of miR-122 expression in the liver during acute HCV infection. PLoS One. 2013;8:e76501. doi: 10.1371/journal.pone.0076501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C, Ross J, Dolan K. Hepatitis C-related discrimination among heroin users in Sydney: drug user or hepatitis C discrimination? Drug Alcohol Rev. 2003;22:317–321. doi: 10.1080/0959523031000154463. [DOI] [PubMed] [Google Scholar]
- Ding X, Ding J, Ning J, Yi F, Chen J, Zhao D, Zheng J, Liang Z, Hu Z, Du Q. Circulating microRNA-122 as a potential biomarker for liver injury. Mol Med Rep. 2012;5:1428–1432. doi: 10.3892/mmr.2012.838. [DOI] [PubMed] [Google Scholar]
- Edlin BR, Seal KH, Lorvick J, Kral AH, Ciccarone DH, Moore LD, Lo B. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N Engl J Med. 2001;345:211–215. doi: 10.1056/NEJM200107193450311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi TA, Hoell JI, Morozov P, Tuschl T. MicroRNAs in human cancer. Adv Exp Med Biol. 2013;774:1–20. doi: 10.1007/978-94-007-5590-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Conrad KD, Niepmann M. Differential stimulation of hepatitis C virus RNA translation by microRNA-122 in different cell cycle phases. Cell Cycle. 2012;11:277–285. doi: 10.4161/cc.11.2.18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, Yu XF. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33:182–188. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goergen D, Niepmann M. Stimulation of hepatitis C virus RNA translation by microRNA-122 occurs under different conditions in vivo and in vitro. Virus Res. 2012;167:343–352. doi: 10.1016/j.virusres.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Gupta A, Swaminathan G, Martin-Garcia J, Navas-Martin S. MicroRNAs, hepatitis C virus, and HCV/HIV-1 co-infection: new insights in pathogenesis and therapy. Viruses. 2012;4:2485–2513. doi: 10.3390/v4112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JI, Goergen D, Zheng J, Song Y, Schuttler CG, Fehr C, Junemann C, Niepmann M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27:3300–3310. doi: 10.1038/emboj.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TW, Duverlie G, Bengrine A. MicroRNAs and hepatitis C virus: toward the end of miR-122 supremacy. Virol J. 2012;9:109. doi: 10.1186/1743-422X-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yang S, Zhang J, Tan L, Jiang F, Li N, Cheng J, Lu Y, Dai Y. MicroRNAs as promising biomarkers for diagnosing human cancer. Cancer Investig. 2010;28:670–671. doi: 10.3109/07357901003631064. [DOI] [PubMed] [Google Scholar]
- Iwama H, Murao K, Imachi H, Ishida T. MicroRNA networks alter to conform to transcription factor networks adding redundancy and reducing the repertoire of target genes for coordinated regulation. Mol Biol Evol. 2011;28:639–646. doi: 10.1093/molbev/msq231. [DOI] [PubMed] [Google Scholar]
- Jopling C. Liver-specific microRNA-122: biogenesis and function. RNA Biol. 2012;9:137–142. doi: 10.4161/rna.18827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Iwata S, Kawada J, Gotoh K, Suzuki M, Torii Y, Kojima S, Kimura H, Ito Y. Plasma viral microRNA profiles reveal potential biomarkers for chronic active Epstein-Barr virus infection. J Infect Dis. 2013;208:771–779. doi: 10.1093/infdis/jit222. [DOI] [PubMed] [Google Scholar]
- Kumar A. MicroRNA in HCV infection and liver cancer. Biochim Biophys Acta. 2011;1809:694–699. doi: 10.1016/j.bbagrm.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Li Y, Shi X. MicroRNAs in the regulation of TLR and RIG-I pathways. Cell Mol Immunol. 2013;10:65–71. doi: 10.1038/cmi.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, Ho WZ. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–1175. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- Macias J, Gonzalez J, Ortega E, Tural C, Cabrero E, Burgos A, Pineda JA, Team GS. Use of simple noninvasive biomarkers to predict liver fibrosis in HIV/HCV coinfection in routine clinical practice. HIV Med. 2010;11:439–447. doi: 10.1111/j.1468-1293.2009.00812.x. [DOI] [PubMed] [Google Scholar]
- Mortimer SA, Doudna JA. Unconventional miR-122 binding stabilizes the HCV genome by forming a trimolecular RNA structure. Nucleic Acids Res. 2013;41:4230–4240. doi: 10.1093/nar/gkt075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan R, Putta S, Kato M. MicroRNAs and diabetic complications. J Cardiovasc Transl Res. 2012;5:413–422. doi: 10.1007/s12265-012-9368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov PV, Reinsbach SE, Muller A, Nicot N, Philippidou D, Vallar L, Kreis S. Interplay of microRNAs, transcription factors and target genes: linking dynamic expression changes to function. Nucleic Acids Res. 2013;41:2817–2831. doi: 10.1093/nar/gks1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina EG, Urazova LN, Stegny VN. MicroRNAs and human cancer. Exp Oncol. 2012;34:2–8. [PubMed] [Google Scholar]
- Palanisamy V, Jakymiw A, Van Tubergen EA, D’Silva NJ, Kirkwood KL. Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. J Dent Res. 2012;91:651–658. doi: 10.1177/0022034512437372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget. 2010;1:224–227. doi: 10.18632/oncotarget.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglio GL, Lugoboni F, Pajusco B, Sarti M, Talamini G, Mezzelani P, Des Jarlais DC, Gics Hepatitis C virus infection: prevalence, predictor variables and prevention opportunities among drug users in Italy. J Viral Hepat. 2003;10:394–400. doi: 10.1046/j.1365-2893.2003.00448.x. [DOI] [PubMed] [Google Scholar]
- Rayner KJ, Hennessy EJ. Extracellular communication via microRNA: lipid particles have a new message. J Lipid Res. 2013;54:1174–1181. doi: 10.1194/jlr.R034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth BP, O’Connor JJ, Barry J, Keenan E. Retrospective cohort study examining incidence of HIV and hepatitis C infection among injecting drug users in Dublin. J Epidemiol Community Health. 2003;57:310–311. doi: 10.1136/jech.57.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder N, Gajula L, Xiao SY, Grady J, Luxon B, Lau DT, Soloway R, Petersen J. APRI: an easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J Clin Gastroenterol. 2006;40:535–542. doi: 10.1097/00004836-200607000-00013. [DOI] [PubMed] [Google Scholar]
- Snyder N, Nguyen A, Gajula L, Soloway R, Xiao SY, Lau DT, Petersen J. The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin Chim Acta. 2007;381:119–123. doi: 10.1016/j.cca.2007.02.046. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Roles for microRNAs in the regulation of cell adhesion molecules. J Cell Sci. 2011;124:999–1006. doi: 10.1242/jcs.081513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Li Y, Douglas SD, Wang X, Metzger DS, Zhang T, Ho WZ. Morphine withdrawal enhances hepatitis C virus replicon expression. Am J Pathol. 2005;167:1333–1340. doi: 10.1016/S0002-9440(10)61220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I. Epidemiology of hepatitis C in the United States. Am J Med. 1999;107:2S–9S. doi: 10.1016/s0002-9343(99)00373-3. [DOI] [PubMed] [Google Scholar]
- Wilson JA, Huys A. miR-122 promotion of the hepatitis C virus life cycle: sound in the silence. Wiley Interdiscip Rev RNA. 2013 doi: 10.1002/wrna.1186. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu C, Che X, Wang L, Yu D, Zhang T, Huang L, Li H, Tan W, Wang C, Lin D. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog. 2011;50:136–142. doi: 10.1002/mc.20712. [DOI] [PubMed] [Google Scholar]
- Ye L, Peng JS, Wang X, Wang YJ, Luo GX, Ho WZ. Methamphetamine enhances hepatitis C virus replication in human hepatocytes. J Viral Hepat. 2008;15:261–270. doi: 10.1111/j.1365-2893.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wang X, Metzger DS, Riedel E, Montaner LJ, Ho W. Upregulation of SOCS-3 and PIAS-3 impairs IL-12-mediated interferon-gamma response in CD56 T cells in HCV-infected heroin users. PLoS One. 2010;5:e9602. doi: 10.1371/journal.pone.0009602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pu R, Du Y, Han Y, Su T, Wang H, Cao G. Non-coding RNAs in hepatitis B or C-associated hepatocellular carcinoma: potential diagnostic and prognostic markers and therapeutic targets. Cancer Lett. 2012;321:1–12. doi: 10.1016/j.canlet.2012.03.011. [DOI] [PubMed] [Google Scholar]