Abstract
In present study, response surface methodology was used to optimize extraction condition of phenolic compounds from licorice root by microwave application. Investigated factors were solvent (ethanol 80 %, methanol 80 % and water), liquid/solid ratio (10:1–25:1) and time (2–6 min). Experiments were designed according to the central composite rotatable design. The results showed that extraction conditions had significant effect on the extraction yield of phenolic compounds and antioxidant capacities. Optimal condition in microwave assisted method were ethanol 80 % as solvent, extraction time of 5–6 min and liquid/solid ratio of 12.7/1. Results were compared with those obtained by soxhlet extraction. In soxhlet extraction, Optimum conditions were extraction time of 6 h for ethanol 80 % as solvent. Value of phenolic compounds and extraction yield of licorice root in microwave assisted (MAE), and soxhlet were 47.47 mg/g and 16.38 %, 41.709 mg/g and 14.49 %, respectively. These results implied that MAE was more efficient extracting method than soxhlet.
Keywords: Optimization, Microwave assisted extraction, Soxhlet extraction, RSM
Introduction
Licorice (Glycyrrhiza glabra L.) is a well-known medicinal herb that grows in various parts of the world. It is native to Eurasia and cultivated in Europe (Spain, Italy, France, etc.), Middle East (Syria, Iran, Turkey, Iraq. etc.), and Asia (e.g., China). Its dried roots, which are collected in autumn, are widely used (Eng et al. 2007) in traditional medicine. The chemical constituents of the roots include several bioactive compounds, such as glycyrrhizin (~16 %), different sugars (up to 18 %), flavonoids, saponoids, sterols, starches, amino acids, gums and essential oils (Mukhopadhyay and Panja 2008). The main constituent found in the root is glycyrrhizin (Chen et al. 1991). This compound is 50 times sweeter than sugar, which makes it a widely used ingredient in food industry (Acharya et al. 1993) and it is usually o formed the as calcium and potassium salts within its root (Baran and Fenercio 1991). Licorice is mostly used for its antimicrobial, antioxidant, antiinflammatory, anticoagulative, antiulcer, expectorant, antiallergic and anxiolytic activities and a number of other pharmacological actions (Khanzadi and Thomas 2010). Limitations of existing extraction methods, such as high energy consumption (more than 10 % of total process required energy) and usage of harmful chemicals, have forced food and chemical industries to find new “green” separation techniques which typically use less solvent and energy, such as microwave extraction (MAE), supercritical fluid extraction, ultrasound extraction (Ping et al. 2010; Vellingiri et al. 2011). Microwaves have been used for the extraction of some biological compounds, such as extraction of essential oils from the leaves of rosemary and peppermint (Chen and Spiro 1994), extraction of glycyrrhizic acid from licorice root (Pan et al. 2000), extraction of taxanes from Taxus biomass (Mattina et al. 1997) and extraction of ergosterol and total fatty acids from fungal hyphae and spores, mushrooms, filtered air, artificially contaminated corn, naturally contaminated grain dust, and soil (Young 1995). Also, Pan et al. (2003) have found that MAE extraction improves the efficiency of the extraction of polyphenols and caffeine from green tea leaves.
For optimization of complex processes where many factors and interactions affect the responses, response surface methodology (RSM) becomes a useful technique (Loh et al. 2005). RSM allows the user to identify optimal conditions for a selected response while minimizes the number of required experiments. RSM comprises a collection of mathematical and statistical procedures that can be used to study the relationship between a number of factors (independent variables) and one or more responses (dependent variables) (Rai et al. 2009).
In present study the effect of different methods (soxhlet and microwave assisted extraction) and conditions on polyphenolic compounds content and extraction yield of licorice root were studied and optimized using RSM. Free radical-scavenging and antioxidant properties of licorice extract which extracted by optimized conditions were also evaluated.
Materials and methods
Solvents and reagents
Methanol, ethanol and Sodium carbonate were purchased from Merck Company (Darmstat, Germany). gallic acid, Folin-Ciocalteu and 2,2-diphenyl-1picryl-hydrazyl (DPPH) were purchased from fluca chemical co, Buchs/Switzerland. All chemical used were of analytical grade.
Plant material
The roots of Glycyrrhiza glabra were collected from research farm of Gorgan agricultural research institute, Iran on October 2010. After removing dust, plant materials were dried at 40 °C in an oven and then ground to fine powders and passed through a sieve with a mesh No = 20.
Microwave-assisted extraction
Samples were soaked in different solvents (ethanol 80 %, methanol 80 % or water) for 90 min as recommended by Pan et al. (2003). A domestic microwave oven (CE3260F CE3260FS) was used in this study. The suspensions were placed in microwave oven and heated according to the method proposed by Mirzapour et al. (2010). Time (X1) and liquid/solid ratio (X2) were independent variables while phenolic compounds contents and extraction yield were dependent variables. The extract was then filtered through Whatmann No 1 filter paper. The resulting extract was lyophilized and the extraction yield was calculated.
Soxhlet extraction
Soxhlet extraction was performed in a Soxhlet apparatus. Exhaustive extraction with different solvent (ethanol 80 %, methanol 80 % or water) was performed using 25 g Glycyhhriza glabra powder, wrapped in filter paper and impregnated with solvent. Extraction was performed with 250 mL of solvent. The extract was then filtered through Whatmann No 1 filter paper. The resulting extract was lyophilized and then the extraction yield was calculated.
Determination of total phenolic content
After isolation of the phenolic compounds by the extraction methods described in previous sections, the concentration of total phenolic content was estimated by the Folin-Ciocalteu assay, according to the method presented by Lin and Tang (2007). gallic acid was used as standard and results were expressed as mg of gallic acid per g of dried sample.
DPPH radical scavenging activity
The ability of extracts to scavenge DPPH radicals was determined according to the method of Florence Suma and Urooj (2011). 1 mL of a 1 mM methanolic solution of DPPH was mixed with 3 mL of extract solution in methanol (containing 50–500 μg of dried extract). The mixture was then vortexed vigorously and left for 30 min at room temperature in the dark. The absorbance was measured at 517 nm by Spectrophotometry (Cecil CE 2502, cecil Ins., England) and activity was expressed as percentage DPPH scavenging relative to control using the following equation:
The DPPH free radical scavenging activity was also expressed as IC50 value which is the concentration of extract required to decrease the absorbance at 517 nm by 50 %. Measurements were performed in triplicates.
Yield of licorice root extract
In this study, extraction efficiency was calculated as following (Pan et al. 2000):
Experimental Design and Statistical Analysis
RSM with a central composite rotatable design (CCRD) was used to determine the optimum condition for microwave-assisted extraction. The experimental design and statistical analysis were performed using Minitab 16 software, English version (Minitab Inc., State College, PA, USA). Time (X1) and liquid/solid ratio (X2) in microwave assisted extraction (MAE) were selected as independent variables based on a literature survey and preliminary experiments. The maximum and minimum values in microwave assisted extraction for liquid/solid ratio were set at 10:1 and 25:1, time 2 min and 6 min, respectively. Also in microwave, solvents were water, ethanol 80 % or methanol 80 % measured responses were total phenolic compounds and yield of the licorice root extract. The complete design consisted of 13 combinations in microwave assisted extraction (Tables 1, 2 and 3). All trials were carried out in triplicate and all the data were reported as means ± SD (standard deviation). The statistics significance was evaluated using P < 0.05 or 0.01 was taken as significant.
Table 1.
Central composite rotatable design with the experimental values of the response variables (Methanol solvent)
| Run order | Time (min) | Liquid/Solid ratios X2 | Total phenol (mg/g) Y1 | Yield (%) |
|---|---|---|---|---|
| X1 | Y2 | |||
| 1 | 6 | 17.5 | 43.55 | 14.50 |
| 2 | 4 | 10 | 37.6 | 12.52 |
| 3 | 2 | 10 | 32.8 | 12.81 |
| 4 | 4 | 17.5 | 42.99 | 14.31 |
| 5 | 2 | 25 | 33.19 | 12.96 |
| 6 | 4 | 17.5 | 42 | 13.98 |
| 7 | 4 | 17.5 | 42.43 | 14.10 |
| 8 | 4 | 17.5 | 43.15 | 14.30 |
| 9 | 4 | 17.5 | 43 | 14.35 |
| 10 | 6 | 25 | 37.41 | 14.61 |
| 11 | 4 | 25 | 36.88 | 13.85 |
| 12 | 2 | 17.5 | 38.64 | 12.86 |
| 13 | 6 | 10 | 41.89 | 16.36 |
Table 2.
Central composite rotatable design with the experimental values of the response variables (Ethanol solvent)
| Run order | Time (min) | Liquid/Solid ratios X2 | Total phenol (mg/g) Y1 | Yield (%) |
|---|---|---|---|---|
| X1 | Y2 | |||
| 1 | 6 | 17.5 | 47.43 | 16.5 |
| 2 | 4 | 10 | 41.73 | 14.35 |
| 3 | 2 | 10 | 36.48 | 11.83 |
| 4 | 4 | 17.5 | 46.15 | 15.3 |
| 5 | 2 | 25 | 35.95 | 13.7 |
| 6 | 4 | 17.5 | 46.17 | 15.78 |
| 7 | 4 | 17.5 | 46 | 15.5 |
| 8 | 4 | 17.5 | 45.95 | 15.81 |
| 9 | 4 | 17.5 | 46.18 | 15.58 |
| 10 | 6 | 25 | 40.83 | 15 |
| 11 | 4 | 25 | 39.88 | 14.98 |
| 12 | 2 | 17.5 | 41.07 | 13.87 |
| 13 | 6 | 10 | 43.53 | 15.50 |
Table 3.
Central composite rotatable design with the experimental values of the response variables (Water solvent)
| Run order | Time (min) | Liquid/Solid ratios X2 | Total phenol (mg/g) Y1 | Yield (%) |
|---|---|---|---|---|
| X1 | Y2 | |||
| 1 | 6 | 17.5 | 19.68 | 8.3 |
| 2 | 4 | 10 | 17.88 | 7.52 |
| 3 | 2 | 10 | 16.5 | 6.83 |
| 4 | 4 | 17.5 | 18.5 | 7.96 |
| 5 | 2 | 25 | 16.61 | 6.5 |
| 6 | 4 | 17.5 | 18.52 | 7.9 |
| 7 | 4 | 17.5 | 18.54 | 7.88 |
| 8 | 4 | 17.5 | 18.55 | 7.87 |
| 9 | 4 | 17.5 | 18.3 | 7.93 |
| 10 | 6 | 25 | 19 | 8 |
| 11 | 4 | 25 | 18.2 | 7.12 |
| 12 | 2 | 17.5 | 16.84 | 7.09 |
| 13 | 6 | 10 | 18.85 | 7.85 |
Result and discussion
Total phenolic compounds (Y1) and yield of licorice root extract (Y2) in licorice root obtained from all experiments are listed in Tables 1, 2 and 3. These experimental data were used to calculate the coefficients of the second-order polynomial equations which are presented in Tables 4, 5 and 6. For any terms in model, a large regression coefficient and a small p-value would indicate a more significant effect on the respective response variables. ANOVA showed that the resultant second-order polynomial model adequately represented the experimental data with the coefficient of multiple determinations for the response of extraction yield and total phenolic compounds (R2) of 0.98 and 0.98 using methanol, 0.98 and 0.99 using ethanol, 0.98 and 0.96 using water, respectively.
Table 4.
Analysis of variance results representing linear, quadratic and interaction terms of each variable and coefficient for the prediction model (solvent = methanol)
| Source | df | Total phenol | Yield | ||
|---|---|---|---|---|---|
| Coef | P value | Coef | P value | ||
| Model | 5 | 42.61 | 0.000 | 14.19 | 0.000 |
| X1 | 1 | 3.036 | 0.000 | 1.011 | 0.000 |
| X2 | 1 | −0.8017 | 0.018 | −0.2667 | 0.02 |
| X1 2 | 1 | −1.2803 | 0.013 | −0.4312 | 0.013 |
| X2 2 | 1 | −5.1353 | 0.000 | −1.71 | 0.000 |
| X1X2 | 1 | −1.2175 | 0.007 | −0.405 | 0.008 |
| Lack of fitness | 5 | 0.176 | 0.186 | ||
| R2 | 0.984 | 0.983 | |||
| Adj-R2 | 0.972 | 0.971 | |||
| CV | 0.641 | 0.2184 |
Xi, linear; Xi2, quadratic and XiXj: interaction of variables (1: time, 2: liquid/solid ratio)
Table 5.
Analysis of variance results representing linear, quadratic and interaction terms of each variable and coefficient for the prediction model (solvent = ethanol)
| Source | df | Total phenol | Yield | ||
|---|---|---|---|---|---|
| Coef | P value | Coef | P value | ||
| Model | 5 | 46.057 | 0.000 | 15.62 | 0.000 |
| X1 | 1 | 3.0483 | 0.000 | 1.2667 | 0.000 |
| X2 | 1 | −0.8067 | 0.000 | 0.333 | 0.003 |
| X1 2 | 1 | −1.7278 | 0.000 | −0.529 | 0.002 |
| X2 2 | 1 | −5.1728 | 0.000 | −1.049 | 0.000 |
| X1X2 | 1 | −0.5425 | 0.000 | −0.5925 | 0.008 |
| Lack of fitness | 5 | 0.149 | 0.772 | ||
| R2 | 0.999 | 0.987 | |||
| Adj-R2 | 0.999 | 0.978 | |||
| CV | 0.1481 | 0. 184 |
Xi, linear; Xi2, quadratic and XiXj: interaction of variables (1: time, 2: liquid/solid ratio)
Table 6.
Analysis of variance results representing linear, quadratic and interaction terms of each variable and coefficient for the prediction model (solvent = water)
| Source | df | Total phenol | Yield | ||
|---|---|---|---|---|---|
| Coef | P value | Coef | P value | ||
| Model | 5 | 12.79 | 0.000 | 4.22 | 0.000 |
| X1 | 1 | 1.184 | 0.006 | 0.415 | 0.014 |
| X2 | 1 | 0.222 | 0.041 | 0.264 | 0.000 |
| X1 2 | 1 | −0.0659 | 0.089 | −0.03 | 0.069 |
| X2 2 | 1 | −0.0061 | 0.037 | −0.008 | 0.000 |
| X1X2 | 1 | −0.001 | 0.896 | 0.008 | 0.039 |
| Lack of fitness | 5 | 0.598 | 0.014 | ||
| R2 | 0.969 | 0.98 | |||
| Adj-R2 | 0.947 | 0.94 | |||
| CV | 0.2215 | 0. 014 |
Xi, linear; Xi2, quadratic and XiXj: interaction of variables (1: time, 2: liquid/solid ratio)
Microwave-assisted extraction
Total phenolic compounds and yield of extraction using methanol as solvent
Response surface analysis (RSA) of the data in Table 4 demonstrates that the relationship between the total phenolic compounds, yield of extraction and extraction parameters is quadratic with good regression coefficient (R2 = 0.98). Equations (1, 2) show the relationship between phenolic compounds (Y1), yield of extraction (Y2) and extraction parameters (X1 and X2).
| 1 |
| 2 |
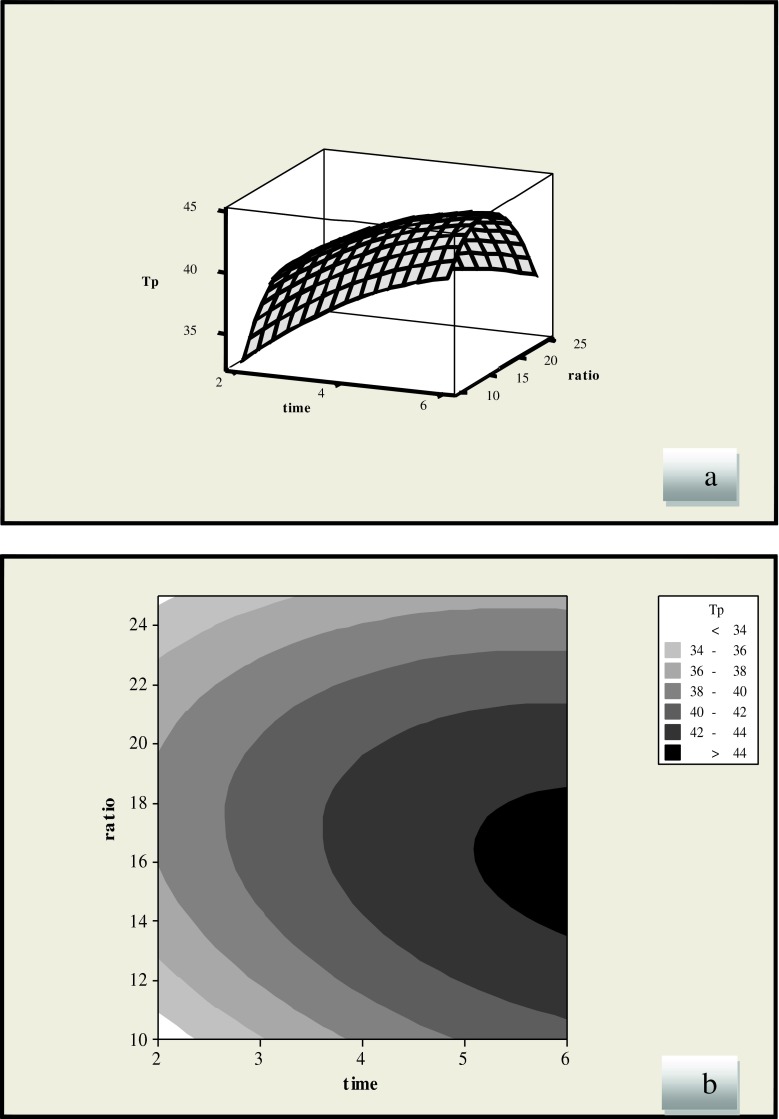
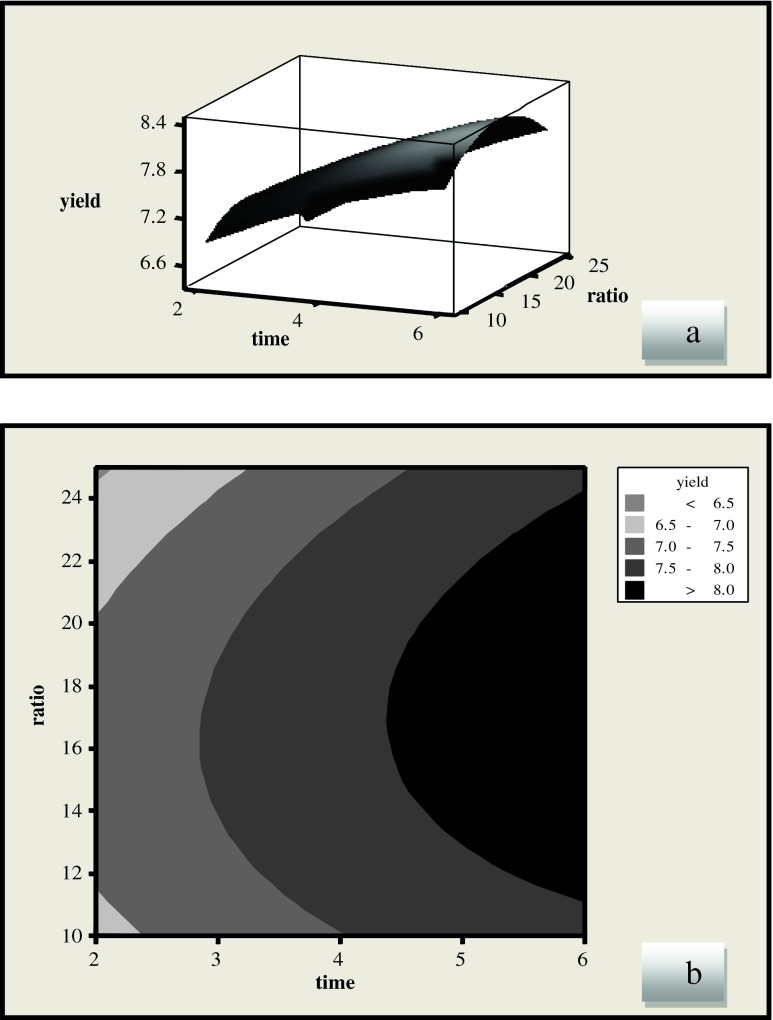
Figure 1 shows the effect of extraction time and liquid/solid ratio on the extraction of phenolic compounds. The liquid/solid ratio has showed as a negative linear effect (p < 0.05), while the extraction time had positive linear effects (p < 0.001) on the yield of phenolic compounds. The yield of phenolic compounds increased and then decreased with increase in liquid/solid ratio, which revealed that medium liquid/solid ratio is favorable for extraction of licorice root. At low levels of the liquid/solid ratio, the solubility of phenolic compounds improves by increasing the liquid/solid ratio. It has been reported that the breakage degree of the raw materials cell membrane enhances by increasing solvent (Vatai et al. 2009; Zhang et al. 2006). Generally in conventional extraction techniques a higher volume of solvent will increase the recovery, but in MAE a higher solvent volume may give lower recoveries (Guo et al. 2001; Chen et al. 2007; Ping et al. 2010).
Fig. 1.
Response surface (a) and contour plots (b) of the total phenolic compounds (TP) (Y1) as a function of liquid/solid ratio and extraction time (methanol solvent)
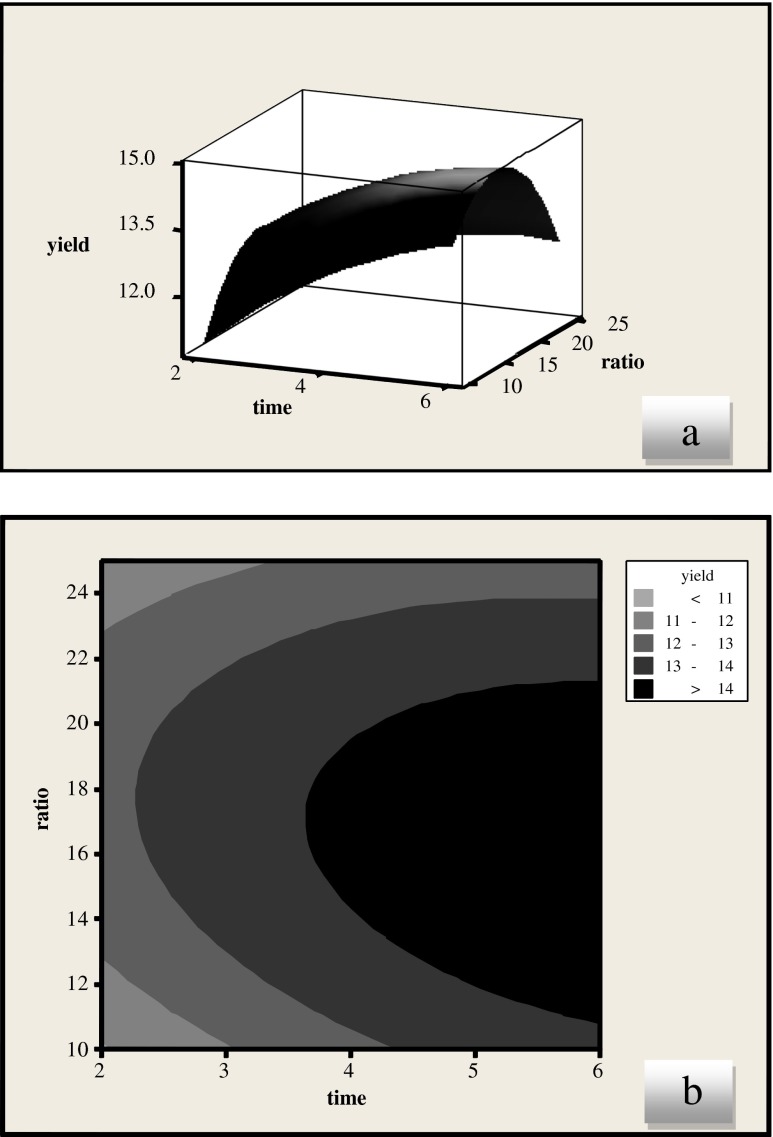
The effect of extraction time and liquid–solid ratio on yield of licorice root extract is presented in the Fig. 2. Our results showed that liquid/solid ratio had a linear effect (p < 0.05) while time showed a positive linear effect on the yield (p < 0.001). At the lowest level of ratio, the yield of licorice root extract showed rapid increase at the beginning but decreased towards the end. There was direct relation between the extraction time and the licorice root extract yield. Same results were reported by Pan et al. (2000) using microwave-assisted extraction (MAE) technique for the extraction of glycyrrhizic acid (GA) from licorice root.
Fig. 2.
Response surface (a) and contour plots (b) for the effects of extraction time and ratio on the yield of licorice root extract (methanol solvent)
Total phenolic compounds and yield of extraction using ethanol as solvent
The statistical analysis of obtained results (Table 5) shows a high regression value (R2) and Eqs. (3, 4) represent the relationship between the extraction of phenolic compound (Y1) or the yield of extraction (Y2) from licorice root and extraction parameters such as liquid/solid ratio (X1), and extraction time (X2):
| 3 |
| 4 |
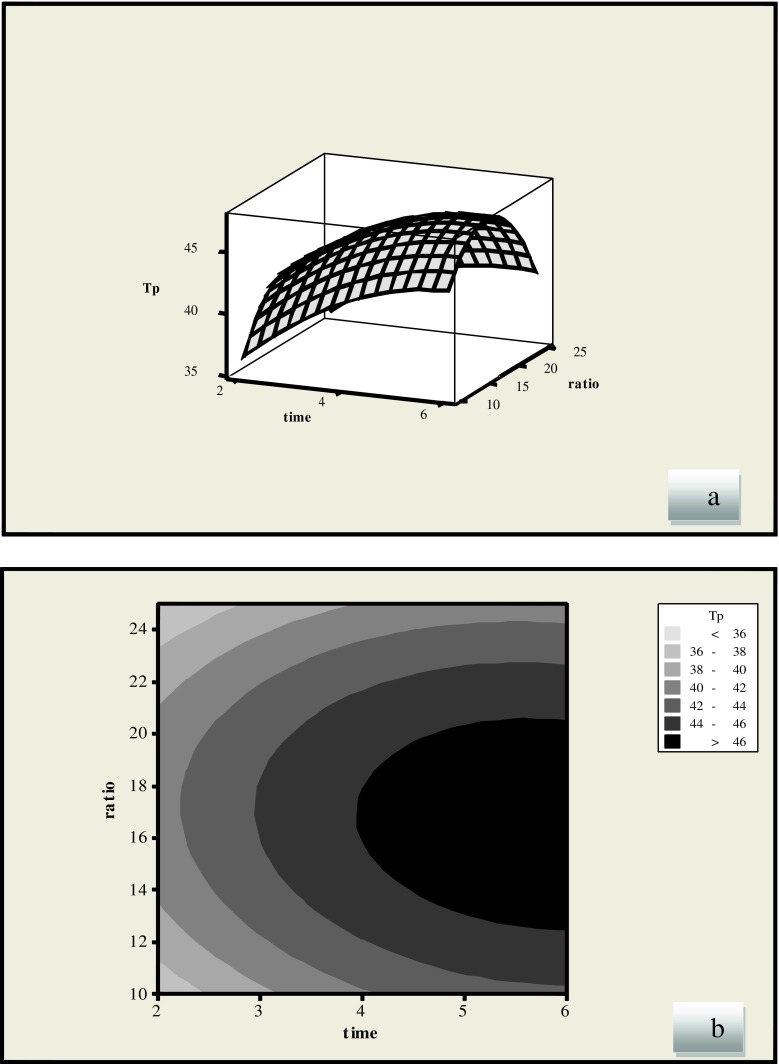
Figure 3 shows the effect of extraction time and liquid/solid ratio on the extraction of phenolic compounds using ethanol. The liquid/solid ratio showed as a negative linear effect (p < 0.001), while the extraction time had positive linear effects (p < 0.001) on the yield of phenolic compounds. It can be seen in Fig. 3 that the yield of phenolic compounds increased by increasing the amount of solvent before the ratio of solvent to material reach 15 at which the phenolic compounds reached its highest value, and then it fell down rapidly. This was probably due to an inadequate stirring of the solvent when the microwaves are applied at larger volumes. Generally in conventional extraction techniques a higher volume of solvent will increase the recovery, but in MAE a higher solvent volume leads to lower recoveries (Chen et al. 2007). Results indicated that the phenolic compounds increased with the increase of MAE time in the beginning of extraction and then it fell down slowly. The extraction percentage of phenolic compounds decreased with the increase of MAE time because phenolic compounds easily decomposed if they were kept at high temperature for a long period of time (Chen et al. 2007). Previous study reported that extended extraction time would lead to a decrease in the phenolic content of crude extract as oxidation of phenolic compounds was possible to be occurred by prolonging the exposure to environment factors such as light and oxygen (Juntachote et al. 2006; Chan et al. 2009). This was also observed in the extraction of aromatic amines from leather, where the recovery of some amines decreases with increasing extraction time (Eskilsson and Bjorklund 2000). Figure 4 indicates that the extraction yield increases by increasing the time.
Fig. 3.
Response surface (a) and contour plots (b) of the total phenolic compounds (TP) ((Y1) as a function of liquid/solid ratio and extraction time (Ethanol solvent)
Fig. 4.
Response surface (a) and Contour plots (b) for the effects of extraction time and ratio on the yield of licorice root extract (Ethanol solvent)
Total phenolic compounds and yield of extraction using water as solvent
Statistical analysis presented in Table 6 shows a high regression value (R2) and Eqs. (5, 6) represent the relationship between the extraction of phenolic compound (Y1) or yield of extraction (Y2) and extraction parameters of liquid/solid ratio (X1), extraction time (X2):
| 5 |
| 6 |
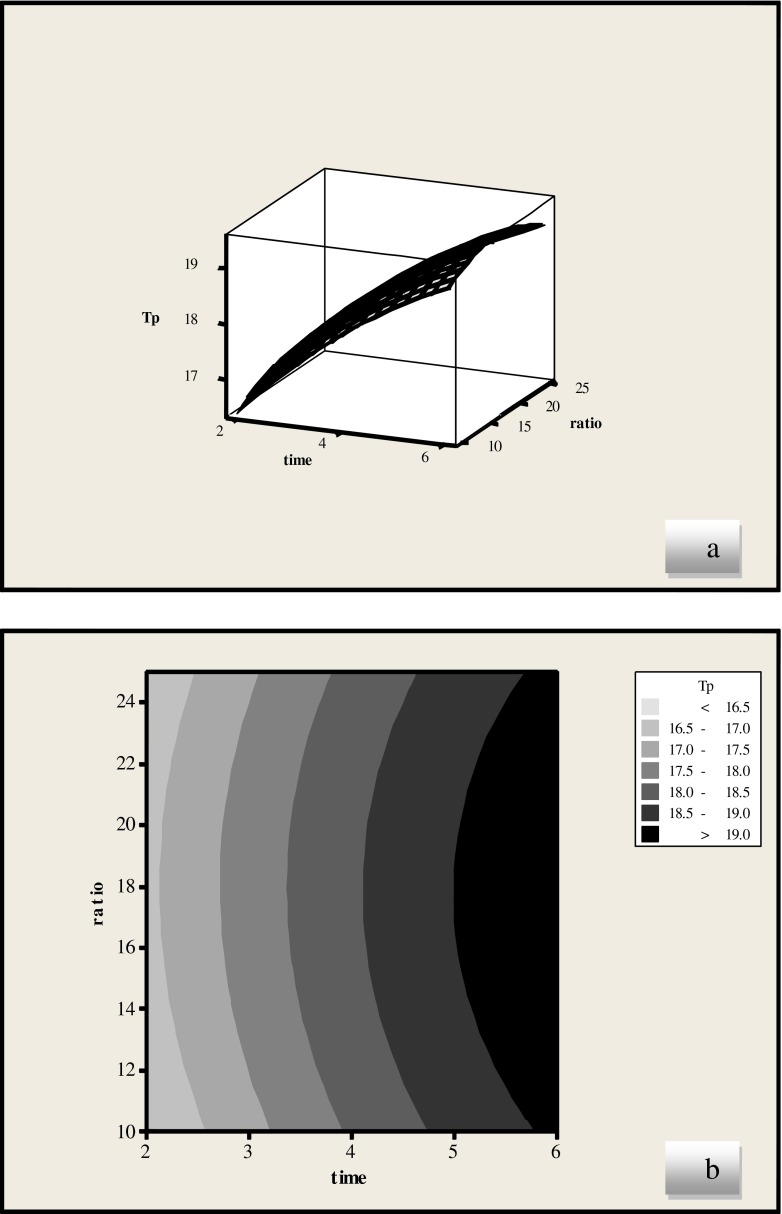
Figures 5 and 6 present the effect of ratio and extraction time on the yield of phenolic compounds. Extraction time showed a positive linear effect (p < 0.01) while the interaction between ratio and extraction time had no significant effect on the yield of phenolic compounds (Table 6).
Fig. 5.
Response surface (a) and contour plots (b) of the total phenolic compounds (TP) ((Y1) as a function of liquid/solid ratio and extraction time (water solvent)
Fig. 6.
Response surface (a) and Contour plots (b) for the effects of extraction time and ratio on the yield of licorice root extract (water solvent)
Optimization
Optimum conditions for extraction of phenolic compounds and yield of extract from licorice root were determined to obtain maximum phenolic compounds and yield of extraction. The second-order polynomial models obtained in this study were utilized for each response in order to determine optimum conditions. The operating parameter ranges were determined by considering economical, industrial and product quality constraints.
In this study, in microwave-assisted extraction, liquid/solid ratio and extraction time were selected in the range of 10:1–25:1 and 2–6 min. By applying the desirability function method, the optimum values were found 12.7:1 for liquid/solid ratio and 6 min for time in methanol solvent extraction. The desirability value of the optimum extraction was 100 %. At this point, total phenolic compounds and yield content were calculated as 43.5 mg/g and 14.5 %. In ethanol solvent extraction, By applying the desirability function method, the optimum value was found 16.5:1 for liquid/solid ratio and 6 min for time. At this point, total phenol content yield were calculated as 47.43 mg/g and 16.38 %. Optimum values for water as solvent were found 18.15:1 for liquid/solid ratio and time of 2 min in water solvent extraction. At this point, total phenolic compounds and yield content were calculated as 19.4 mg/g and 8.36 %.
After optimal extraction conditions for extraction of phenolic compounds and yield of extraction were derived from RSM, experiments based on the optimum process parameters were performed and repeated three times. The result of experiments and predicted value (mean of three measurements), presented in Table 7 that show insignificant differences (p > 0.05) in the extraction of phenolic compound and yield of extraction, then corresponding models are suitable.
Table 7.
Experimental and predicted result for Tp and yield
| Response | Tp (mg/g) S.E | Yield (%) S.E | Tp (mg/g) S.M | Yield (%) S.M | Tp (mg/g) S.W | Yield (%) S.W |
|---|---|---|---|---|---|---|
| Predicted value | 47.43 | 16.38 | 43.5 | 14.5 | 19.4 | 8.3 |
| Actual value | 47.1 ± 0.15 | 16.24 ± 0.06 | 43.063 ± 0.15 | 14.23 ± 0.225 | 19.3 ± 0.264 | 8.27 ± 0.11 |
Tp total phenol, SE solvent ethanol, SM Solvent methanol, SW solvent water
Comparison between different solvents in microwave assisted extraction
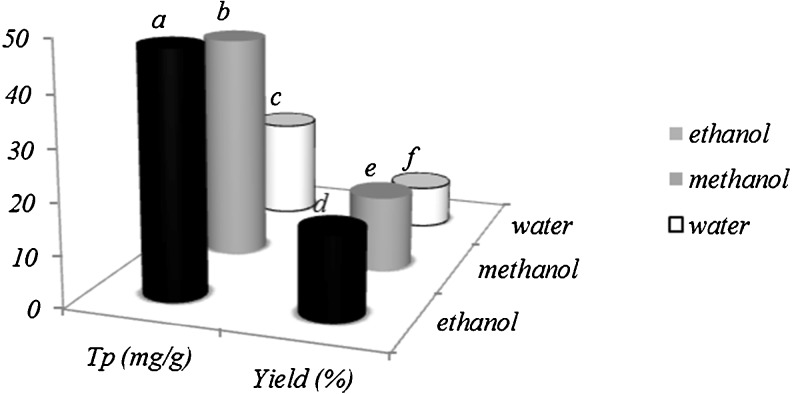
Our results showed that water gives lower amounts of extractable phenolic compounds than methanol and ethanol (Fig. 7). Water has the highest dielectric constant (ε = 80) among common solvents. However, its dissipation factor is significantly lower than other solvents (δ = 1,500 × 104) Hence, the rate at which water absorbs microwave energy is higher than the rate at which the system can dissipate heat. These phenomena which called “superheating” effect occurs when water is used. So, intense heating may have caused degradation of the analyte. This is why it is best to choose a solvent that has a high dielectric constant as well as a high dissipation factor, to facilitate heat distribution through the matrix (Proestos and Komaitis 2008). Also, results show that maximum extraction observed with ethanol solvent. The principle of heating using microwave energy is based on the direct effect of microwaves on molecules by ionic conduction and dipole rotation. Polar molecules (such as polyphenols) and ionic solutions absorb microwave energy strongly because they have a permanent dipole moment. This results in rapid rise of the temperature and fast completion of the reaction (Eskilsson and Bjorklund 2000; Gfrerer and Lankmayr 2005; Venkatesh and Raghavan 2004). Results in Fig. 7 show that the higher yield were obtained when 80 % ethanol and 80 % methanol were used as solvents. Knowing that however ethanol is a safe solvent providing both good yield and high concentration for phenolic compounds but methanol is a toxic solvent. So it should be considered as the best solvent in this study.
Fig. 7.
Comparison between the solvent in microwave assisted extraction. Values with different letters are significantly different (p < 0.05) from each other. (Tp total phenol). Values are means of triplicate determinations (n = 3) ± standard deviation
Soxhlet extraction
Effect of solvent type and time on the total phenolic compounds
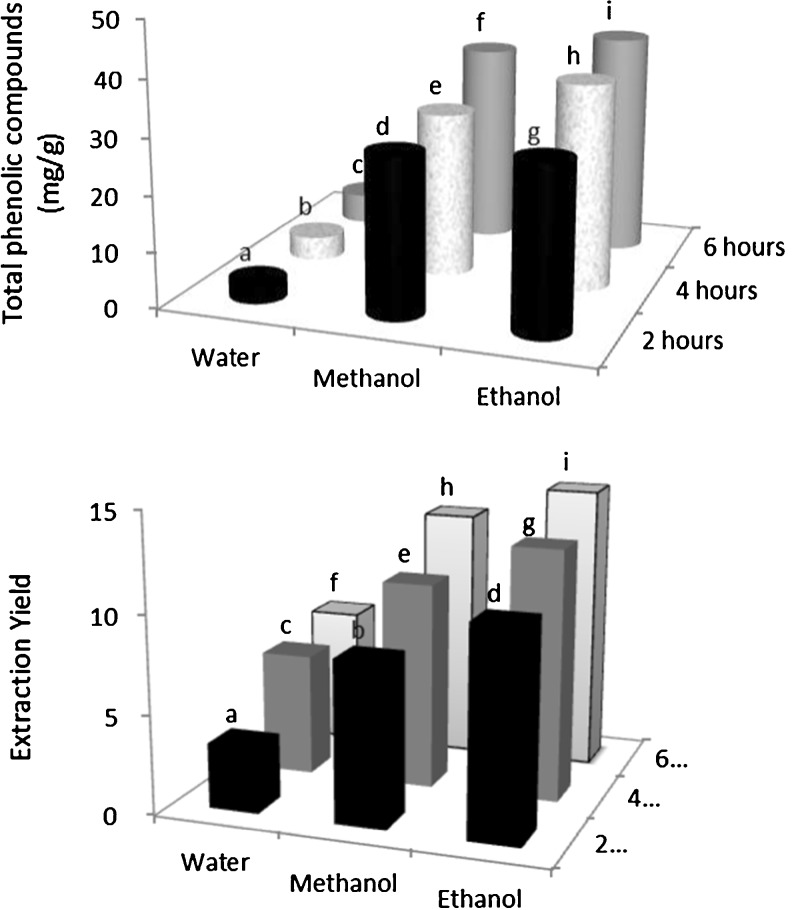
The water, methanol 80 % and ethanol 80 % were used as solvent for the extraction of phenolic compounds from licorice root were. Figure 8 shows the effect of solvent and extraction time on the total phenolic compounds. It is clear that an increase in time led to an increase in the total phenolic compounds. In this method, water as solvent played a less important role in extracting of phenolic compounds compared with ethanol and methanol as solvent. Higher extracted amount was obtained 41.7 mg/g by ethanol usage (Fig. 8). Also, variations in antioxidant activity and the yield of phenolic compounds have been reported to be influenced by extracting solvents (Annegowda et al. 2011).
Fig. 8.
Effect of time and solvent on total phenolic compounds and yield of extraction. Values with different letters are significantly different (p < 0.05) from each other. Values are means of triplicate determinations (n = 3) ± standard deviation
Effect of different extraction solvent and time on the yield of extraction
It was observed that the yield of extraction increased with increasing time, and ethanol was more efficient on the yield of extraction (14.49 %). Also, water extraction than other solvents was found to give lower amounts of extraction yield while ethanol and methanol showed higher amounts of extraction yield (Fig. 8).
Determination of free radical scavenging activity with DPPH
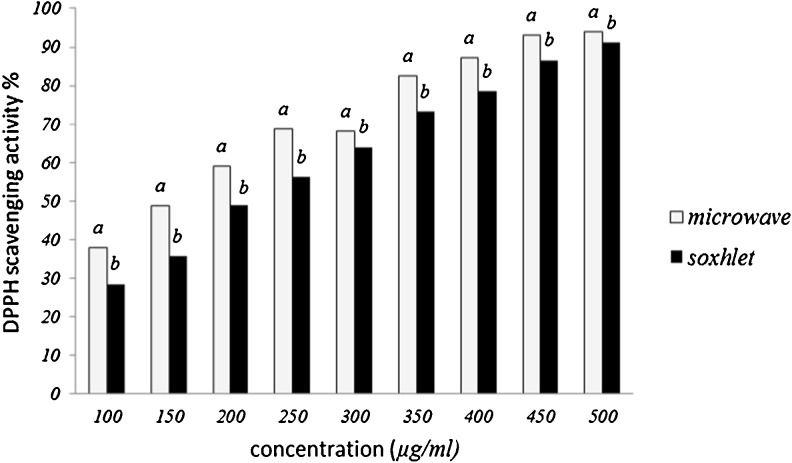
The antioxidant activity of the extract obtained using optimal condition in different methods (microwave and soxhlet) were determined using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical (Blois 1958). DPPH is a stable free radical with a purple colour and has a maximum absorption at 517 nm. The free radial scavenging assay is based on the decolouration of the compound when reduced by a free radical scavenger (Loganayaki et al. 2011). The scavenging activity of two extracts against DPPH˚ was concentration-dependent (Fig. 9). Microwave extract, was found to be the more active radical scavenger than soxhlet extract. Obtained results from the DPPH radical experiments were also expressed as IC50 values. A low IC50 value is indicative of strong DPPH radical scavenging activity. The IC50 were found to be 0.155 ± 0.0005, 0.206 ± 0.002 for microwave extract and soxhlet extract, respectively. Maximum antioxidant activity was observed at microwave assisted extraction. It is obvious that extraction with the MAE was of better quality and richer in antioxidant compounds than other methods. The higher total phenol content values and antioxidant activity found with MAE may be explained by a phenomenon that has been observed in plant cells after exposure to microwave heating. The dried plant material used for extraction contains minute traces of moisture and, as microwave energy is absorbed and subsequently converted into heat, moisture begins evaporating. The vaporization of water generates pressure within the cell wall that eventually leads to cell rupture, thereby facilitates the leaching out of active constituents into the surrounding solvent and improves extraction yield (Mandal et al. 2007). Zhou and Liu (2006) observed this phenomenon upon investigating the surface of tobacco leaves after MAE treatment using scanning electron microscopy (SEM). They found that the surface of the tobacco leaves was severely damaged, owing cellular disruption, which enhanced the extraction of solanesol from the leaves. Similarly, Kratchanova et al. (2004) used SEM to observe the effects of microwave heating on fresh orange peels. It was determined that MAE treatment resulted in the destruction of parenchymal cells of fresh orange peels, which increased the extraction yield of pectin. Treatment of the peanut skins with MAE likely initiated cell rupture, which allowed more of the polyphenolic compounds from the skins to be extracted by the solvent (Ballard et al. 2010).
Fig. 9.
DPPH radical scavenging activities of optimal extracts of microwave and soxhlet from licorice root. Values with different letters are significantly different (p < 0.05) from each other. Values are means of triplicate determinations (n = 3) ± standard deviation
Conclusion
Soxhlet which has been used for many decades is very time consuming and requires relatively large quantities of solvents. Our results proved that microwave–assisted extraction is a more effective technique compared to the conventional method (soxhlet). Using microwave assisted extraction could be completed in minutes instead of hours with high reproducibility, reducing the consumption of solvent and energy due to its shorter time. Moreover, the final product usually showed less thermal degradation than traditional methods. As shown by our results, among different solvents ethanol was more effective for the extraction of phenolic compounds from licorice root. It can be concluded that increasing solvent/sample ratio can increase the extraction yield however it has less effect on the phenoloic compounds. Regarding total phenolic compounds, the time of extraction has more distinct effect and it can be increased by increasing the extraction time.
Acknowledgments
This research was founded by the Vice Chancellors for Research, Universities of Tehran and Gorgan, Iran.
References
- Acharya SK, Dasarathy S, Tandon A, Joshi YK, Tandon BN. A preliminary open trial on interferon stimulator (SNMC) derived from Glycyrrhiza glabra in the treatment of subacute hepatic failure. Indian J Med Res. 1993;98:69–74. [PubMed] [Google Scholar]
- Annegowda HV, Bhat R, Tze LM, Karim AA, Mansor SM. The free radical scavenging and antioxidant activities of pod and seed extract of Clitoria fairchildiana (Howard)- an underutilized legume. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard TS, Mallikarjunan P, Zhou K, O’Keefe S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010;120:1185–1192. doi: 10.1016/j.foodchem.2009.11.063. [DOI] [Google Scholar]
- Baran A, Fenercio H. A research study on the determination of the properties and preservation of licorice extract. Gida. 1991;16:391–396. [Google Scholar]
- Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
- Chan SW, Lee CY, Yap CF, Wan Aida WM, Ho CW. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int Food Res J. 2009;16:203–219. [Google Scholar]
- Chen SS, Spiro M. Study of microwave extraction of essential oil constituents from plant materials. J Microw Power Elect Energy. 1994;29:231–241. [Google Scholar]
- Chen MF, Shimada F, Kato H, Yano S, Kanaoka M. Effect of oral administration of glycyrrhizin on the pharmacokinetics of prednisolone. Endocrinol Jpn. 1991;38:167–174. doi: 10.1507/endocrj1954.38.167. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xie MY, Gong XF. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J Food Eng. 2007;81:162–170. doi: 10.1016/j.jfoodeng.2006.10.018. [DOI] [Google Scholar]
- Eng ATW, Heng MY, Ong ES. Evaluation of surfactant assisted pressurized liquid extraction for the determination of glycyrrhizin and ephedrine in medicinal plants. Anal Chim Acta. 2007;583:289–295. doi: 10.1016/j.aca.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Eskilsson SC, Bjorklund E. Analytical-scale microwave assisted extraction. J Chromatogr A. 2000;902:227–250. doi: 10.1016/S0021-9673(00)00921-3. [DOI] [PubMed] [Google Scholar]
- Florence Suma P, Urooj A. Antioxidant activity of extracts from foxtail millet (Setaria italica) J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gfrerer M, Lankmayr E. Screening, optimization and validation of microwave-assisted extraction for the determination of persistent organochlorine pesticides. Anal Chim Acta. 2005;533:203–211. doi: 10.1016/j.aca.2004.11.016. [DOI] [Google Scholar]
- Guo Z, Jin Q, Fan G, Duan Y, Qin C, Wen M. Microwave-assisted extraction of effective constituents from a Chinese herbal medicine Radix puerariae. Anal Chim Acta. 2001;436:41–47. doi: 10.1016/S0003-2670(01)00900-X. [DOI] [Google Scholar]
- Juntachote T, Berghofer E, Bauer F, Siebenhandl S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int J Food Sci Technol. 2006;41(2):121–133. doi: 10.1111/j.1365-2621.2005.00987.x. [DOI] [Google Scholar]
- Khanzadi FK, Thomas JS. Effect of gamma irradiation on the antimicrobial and free radical scavenging activities of Glycyrrhiza glabra root. Radiat Phys Chem. 2010;79:507–512. doi: 10.1016/j.radphyschem.2009.10.005. [DOI] [Google Scholar]
- Kratchanova M, Pavlova E, Panchev I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr Polym. 2004;56(2):181–185. doi: 10.1016/j.carbpol.2004.01.009. [DOI] [Google Scholar]
- Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh SK, Man YBC, Tan CP, Osman A, Hamid NSA. Process optimisation of encapsulated pandan (Pandanus amaryllifolius) Powder Using Spray-drying Method. J Sci Food Agri. 2005;85:1999–2004. doi: 10.1002/jsfa.2169. [DOI] [Google Scholar]
- Mandal V, Mohan Y, Hemalatha S. Microwave-assisted extraction – an innovative and promising extraction tool for medicinal plant research. Pharm Rev. 2007;1(1):7–18. [Google Scholar]
- Mattina MJI, Berger WAI, Denson CL. Microwave assisted extraction of taxanes from Taxus biomass. J Sci Food Agri. 1997;45:4691–4696. doi: 10.1021/jf970454o. [DOI] [Google Scholar]
- Mukhopadhyay M, Panja P. A novel process for extraction of natural sweetener from licorice (Glycyrrhiza glabra) roots. Separ Purif Technol. 2008;63:539–545. doi: 10.1016/j.seppur.2008.06.013. [DOI] [Google Scholar]
- Mirzapour M, Hamedi M, Rahimipanah M. Sunflower oil stabilization by Persian walnut leaves extract during oven storage test. Food Sci Technol Res. 2010;16(5):443–446. doi: 10.3136/fstr.16.443. [DOI] [Google Scholar]
- Pan X, Liu H, Jia G, Shu Y. Microwave-assisted extraction of glycyrrhizic acid from licorice root. J Biochem Eng. 2000;5:173–177. doi: 10.1016/S1369-703X(00)00057-7. [DOI] [PubMed] [Google Scholar]
- Pan X, Niu G, Liu H. Microwave-assisted extraction of tea polyphenols, tea caffeine from green tea leaves. Chem Eng Process. 2003;42:129–133. doi: 10.1016/S0255-2701(02)00037-5. [DOI] [Google Scholar]
- Ping S, Jinzhe H, Peilong S, Peicheng Z. Analysis of conditions for microwave-assisted extraction of total water-soluble flavonoids from Perilla Frutescens leaves. J Food Sci Technol. 2010 doi: 10.1007/s13197-011-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proestos C, Komaitis M. Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT. 2008;41:652–659. doi: 10.1016/j.lwt.2007.04.013. [DOI] [Google Scholar]
- Rai AK, Nived C, Sakhare PZ, Suresh PV, Bhaskar N, Mahendrakar NS. Optimization of acid hydrolysis conditions of delimed tannery fleshings by response surface method. J Sci Ind Res. 2009;68:967–974. [Google Scholar]
- Vatai T, Škerget M, Knez Ž. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J Food Eng. 2009;90:246–254. doi: 10.1016/j.jfoodeng.2008.06.028. [DOI] [Google Scholar]
- Vellingiri M, Deivamarudhachalam TP, Jagathala MS. Effects of processing conditions on the stability of polyphenolic contents and antioxidant capacity of Dolichos lablab L. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh MS, Raghavan GSV. An overview of microwave processing and dielectric properties of agri-food materials. Biosys Eng. 2004;88:1–18. doi: 10.1016/j.biosystemseng.2004.01.007. [DOI] [Google Scholar]
- Young JC. Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J Agric Food Chem. 1995;43:2904–2910. doi: 10.1021/jf00059a025. [DOI] [Google Scholar]
- Zhang S, Chen R, Wu H, Wang C. Ginsenoside extraction from Panax quinquefolium L. (American ginseng) root by using ultrahigh pressure. J Pharm Biomed Anal. 2006;41:57–63. doi: 10.1016/j.jpba.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Liu CZ. Microwave-assisted extraction of solanesol from tobacco leaves. J Chromatogr. 2006;1129(1):135–139. doi: 10.1016/j.chroma.2006.07.083. [DOI] [PubMed] [Google Scholar]