Abstract
Piper longum L. (Family: Piperaceae), is a widely used herb in several Ayurvedic formulations prescribed for various diseases. Potential of the plant material as an antidiabetic and cardio protective agent has not been evaluated so far. In the study, we designed experiments to evaluate antioxidant, glucose uptake potential and lipid content regulating potential of extracts and compound from P. longum fruits. Solvent extracts from Piper longum fruits using hexane, ethyl acetate, methanol, 70 % methanol-water were taken and apigenin 7, 4'-dimethyl ether (ADE) was isolated from ethyl acetate extract. Antioxidant activity, glucose uptake potential and adipocyte differentiation assay was performed with extract and pure compound. Antioxidant activity in terms of TRP (196.03 μg/mg GAE), DPPH assay (IC50–173.09 μg/mL), hydroxyl radical scavenging assay (IC50–20.42 μg/mL), inhibiting LDL oxidation (IC50–51.99 μg/mL) and to enhance SOD activity (25.3 %) was higher in ethyl acetate extract (EAP). Phenolic and flavonoid content was measured and showed a positive correlation with antioxidant activity. Presence of apigenin 7, 4'-dimethyl ether (ADE) and piperine (Pip) in EAP was determined by HPTLC analysis and was isolated. ADE inhibited α-glucosidase and α-amylase enzymes and enhanced 2-NBDG uptake in L6 cells. Hypolipidemic effect of ADE on mouse pre-adipocyte (3T3L1) cell lines also showed a dose dependent reduction on lipid droplet content and effective concentration range was determined as 1–2.5 μg/mL. The results suggested that Piper longum fruits can provide a natural source of antioxidants with antidiabetic and anti obesity potential.
Keywords: Antioxidant; Antidiabetic; Hypolipidemic; Flow cytometry; Apigenin 7, 4'-dimethyl ether; Glucose uptake
Introduction
Diabetes and cardiovascular diseases are counted as common metabolic disorders prevailing present world community. Role of oxidative stress in the onset of diabetes as well as in development of vascular and neurological disorders is considered crucial. Free radicals are generated as by-products of normal cellular metabolism; however, several conditions are known to disturb the balance between ROS production and cellular defence mechanisms (Philips et al. 2004). Diabetes mellitus and cardiovascular disease are interlocked with basic mechanisms underlying. Diabetes is considered as one of the major risk factor for atherosclerosis (Gleissner et al. 2007). Type 2 diabetes is associated with hyperlipidemia characterized by elevated triglycerides, decreased HDL and increased LDL levels (Grundy et al. 2004). Accumulation of lipids inside muscle cells and specific increases in muscle long-chain fatty acyl-CoA content have been implicated in causing insulin resistance (Moller 2001).
Piper longum
L. (Family: Piperaceae), a plant of South Asian origin is commonly used as spice and seasoning. Medicinally, P. longum is most commonly used to treat respiratory infections such as stomach ache, bronchitis, diseases of the spleen, cough, tumors, and asthma. The fruits of this plant have been used traditionally for treating jaundice and allergy (Kirtikar and Basu 1933; Bishit 1963). Multiple biological properties have been described for the herb including anti-tubercular, anti-allergic, anti-asthmatic, antipyretic, hypotensive, anti-fertility, hypoglycemic and coronary vasodilatory effects (Uma and Seetharam 2009). The plant is a major constituent of Indian medicinal system and also in Indonesian and Malaysian cooking.
The present study was designed to evaluate efficacy of P. longum as an effective remedy from oxidative stress, diabetes and associated disorders. Extracts from the plant was investigated for antioxidant activity based on different assays. Based on the results, active extract was selected to evaluate glucose uptake effect in L6 (mouse myoblast) using fluorescent dye 2-NBDG. Hypolipidemic effect of extract was checked in vitro in 3T3L1 (Mouse pre-adipocytes) cells. Both cells were analysed by flow cytometry. Phytochemical analysis of active fraction by HPTLC and further isolation led to separation of major compounds. Potential of isolated compound on free radical scavenging, oxidative stress reduction, glucose uptake and variation in lipid content level were evaluated as part of the study. The study is supposed to enhance the importance of P. longum as natural medicine and food ingredient.
Materials and methods
Experimental section
General
Readymade HPTLC plates (kieselgel 60 F254, 20 cm × 20 cm, 0.2 mm thickness, Merck, Darmstadt, Germany) were kept at 60 °C for 5 min. The active extracts and their fractions of dried Piper longum fruit were spotted as bands of width 6 mm with Hamilton micro litre syringe using a Camag Linomat V (Camag, Switzerland) at constant application rate of 0.1 mL/s and the space between two bands was maintained as 5 mm. The standardisation of the HPTLC developing solvents for best separation of the constituents present in the fractions of the active extract of Piper longum and its fractions were carried out. It was found that toluene-acetone (70:30) as the best solvent system for the separation of components of the EAP. The developed phase were dried and scanned within the wavelength range of 250–300 nm (TLC scanner 3, Camag, Switzerland). Data processing was performed using the software WinCATS planar chromatography manager.
Isolation of apigenin 7, 4'-dimethyl ether (ADE) from the most active extract of piper longum
The ethyl acetate extract of Piper longum fruit, which contained more amount of flavonoids, was subjected to column chromatography using various mobile phases: hexane, ethyl acetate, methanol and their mixtures in different ratios. Elution with ethyl acetate: methanol (8:2) afforded yellow needle shaped crystal up on evaporation at room temperature in an undisturbed environment. The structure of this flavonoid was elucidated by spectroscopic means.
Plant material collection and extraction
Piper longum
fruits were purchased locally from Thiruvanathapuram, Kerala, India and were authenticated by the plant taxonomist, Department of Botany, University of Kerala, India. A voucher specimen was kept at National Institute for Interdisciplinary Science and Technology (NIIST), India (No.PL F-1). The fruits were collected, dried, powdered, and extracted successively with hexane (HP), ethyl acetate (EAP), methanol (MP), 70 % methanol-water (MWP) and water (WP). The extracts were filtered through a filter paper and concentrated in a rotary evaporator under reduced pressure. Each of the extracts was made up to a definite volume, keeping it as stock solution and stored below 4 °C in a refrigerator. These extracts were then used for further studies.
Chemicals
Fetal Bovine Serum (FBS) was purchased from Gibco, Auckland, NZ. Dulbecco’s modified Eagle’s media (DMEM), Streptomycin-Ampicillin-Amphotericin B mix, insulin, dexamethasone, isobutyl methylxanthine (IBMX), roziglitazone, DPPH and MTT (3–(4,5-dimethylthiazol-2-yl)–2,5-diphenyl tetrazolium bromide) were from Sigma-Aldrich Chemicals, India. 2-NBDG was purchased from Molecular probes, Invitrogen Life Tech. USA. Anti PPAR-γ and IgG-FITC were purchased from Santacruz Biotechnology, CA, USA. All other chemicals and reagents used for the study were of analytical grade.
Animal cell culture
L6 (Mouse myoblast) and 3T3L1 (Mouse pre-adipocytes) were purchased from National Centre for Cell Science (NCCS, India). Cells were maintained in DMEM with 10 % antibiotic-antimycotic mix, 10 % FBS and 10 mM NaHCO3. Cells were subcultured as per ATCC instructions and maintained at 37 °C in a humidified atmosphere with 5 % CO2 inside incubator.
Total flavonoids determination
Aluminium chloride colorimetric method was used for flavonoids determination (Chang et al. 2002). Ethyl acetate extract of Piper longum (0.5 mL of 1:10 g/mL) in methanol were separately mixed with 1.5 mL of methanol, 0.1 mL of 10 % aluminium chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water. After incubation for 30 min at room temperature, absorbance of the reaction mixture was measured at 415 nm with a double beam UV-Visible spectrophotometer (UV1601, Shimadzu, Japan). The calibration curve was prepared by preparing quercetin solutions at concentrations 12.5 to 100 mg/mL in methanol. The percentage of total flavonoid (%TF) is determined on the basis of the equation 1 (Eq.1).
Antioxidant activity
Total phenolic content (TPC)
The total phenolic content was determined using Folin-Ciocalteu reagent (McDonald et al. 2001). Appropriately diluted extracts and standard gallic acid were made up to 3.5 mL using distilled water and 0.5 mL 2 N Folin-Ciocalteu’s reagent was added to it. After 3 min, 20 % sodium carbonate (1.0 mL) was added to the mixture and incubated in darkness for 1 h. The absorbance was measured at 750 nm using a UV-Visible spectrophotometer. Amount of TPC were calculated using gallic acid calibration curve.
Determining total reducing power (TRP)
The reducing power of various extracts of Piper longum fruit was measured according to method published earlier (Oyaizu 1986). Different concentrations of various extracts ranging from 0.1–5 mg/mL and standard gallic acid (0.1–5 mg/mL) were made up to 1 mL with distilled water along with 2.5 mL phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1 % potassium ferricyanide. The reaction mixture was incubated at 50 °C for 20 min. After the incubation 2.5 mL trichloroacetic acid (TCA) (20 %) was added and mixed well. Then 2.5 mL of the solution was taken and mixed with 2.2 mL of distilled water and added 0.5 mL of 0.1 % ferric chloride. The absorbance of the reaction mixtures was read at 700 nm with a spectrophotometer. Higher the absorbance of the reaction mixture indicated greater the reducing power. Gallic acid equivalence (GAE) of sample is expressed in mg/g from the values obtained after plotting absorbance values of gallic acid.
DPPH assay
Antioxidant activity of the extracts were measured using 2,2’-diphenyl–1-picrylhydrazyl (DPPH) radical scavenging assay (Singh et al. 2002). In this assay, the radical scavenging activity was calculated by measuring the absorbance of the remaining concentrations of DPPH in presence of extract at 0–50 μg/mL concentrations. The radical scavenging activities of the tested samples, expressed as percentage of inhibition, were calculated according to the following equation (Eq.2).
where A0 is the absorbance of the control and A1 is the absorbance after adding sample.
From the calibration curve obtained with different concentrations of various extracts of Piper longum fruit, the IC50 value was calculated.
Hydroxyl radical scavenging capacity
Hydroxyl radical-scavenging activity was measured by using deoxyribose (Nagai et al. 2005). The reaction mixture contained 0.45 mL 0.2 M sodium phosphate buffer (pH 7.0), 0.15 mL 10 mM 2-deoxyribose, 0.15 mL 10 mM FeSO4−, EDTA, 0.15 mL 10 mM H2O2, 0.525 mL H2O, and 0.075 mL sample solution in a test tube. The reaction was started by the addition of H2O2. After incubation at 37 °C for 1 h, the reaction was stopped by adding 0.75 mL 2.8 % trichloroacetic acid and 0.75 mL 1.0 % TBA in 50 mM NaOH. The solution was boiled for 10 min and then cooled in water. The absorbance of the solution was measured at 520 nm. The hydroxyl radical scavenging ability was evaluated as the inhibition rate of 2-deoxy-D-ribose oxidation by hydroxyl radicals. Ascorbic acid (1 and 5 mM) and α-tocopherol (1 mM) were used as positive controls.
SOD activity
Activity of superoxide dismutase (SOD) was assayed using nitroblue tetrazolium (NBT) dye (Rook et al. 1985). NBT assay is a common and convenient indirect method that utilizes conversion of nitroblue tetrazolium to NBT diformazan via. superoxide radical. HepG2 cells were selected for the study and were treated with extracts at different concentration. Superoxide radicals were generated non-enzymatically in PMS-NADH systems by the oxidation of NADH and assayed by the reduction of NBT. The superoxide radicals were generated in 1 mL of Tris–HCl buffer (16 mM, pH 8.0) containing NBT (50 μM) solution and NADH (78 μM). PMS (10 μM) was added to the mixture and incubated at 25 °C for 5 min, and the absorbance was measured at 560 nm against blank samples.
Inhibition of human LDL oxidation in vitro
Oxidation of LDL leads to the production of malondialdehyde (MDA) which was measured by reaction with TBA. The method reported earlier (Murthy et al. 2002) was followed with slight modification. LDL (50 μg/mL) was incubated with different concentrations of extract and the oxidation of LDL was initiated by the addition of 50 μl copper sulphate (2 mM) at 37 °C for 2 h. Final volume of the reaction mixture was made up to 1.5 mL with phosphate buffer (pH 7.4). After incubation, 500 μl of reaction mixture was mixed with 250 μl of TBA (1 % in 50 mM of NaOH) and TCA (0.28 %). Samples were again incubated at 95 °C for 45 min. After cooling and centrifugation at 2,000 rpm for 10 min, fluorescence was taken at 515 nm excitation and 553 nm emission. The results were expressed as percentage of inhibition of LDL oxidation. Using the amount of MDA formed, percentage of inhibition can be calculated using the Eq. 2.
Antidiabetic effect of EAP
α-Glucosidase inhibition assay
α-glucosidase (20 μl, 1.5 U/mL) was premixed with 200 μl of extracts at varying concentrations prepared in 50 mM phosphate buffer (pH 6.8) and incubated for 5 min at 37 °C (Shinde et al. 2008). 1 mM para-nitrophenyl-α-D glucopyranoside (200 μl) in 50 mM of phosphate buffer was added to initiate the reaction, and the mixture was further incubated at 37 °C for 20 min. The reaction was terminated by the addition of 500 μl of 1 M Na2CO3, and the final volume was made up to 1,500 μl. The absorbance was recorded at 405 nm and acarbose served as positive control.
α-Amylase inhibition assay
500 μl of extract and 500 μl of 0.02 M sodium phosphate buffer (pH 6.9 with 6 mM sodium chloride) containing α-amylase solution (0.5 mg/mL) were incubated at 25 °C for 10 min (Apostolidis et al. 2007). After pre-incubation, 500 μL of a 1 % starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 6 mM sodium chloride) was added to each tube at timed intervals. The reaction mixtures were then incubated at 25 °C for 10 min and stopped with 1 mL of dinitrosalicylic acid color reagent. After incubating in boiling water bath for 5 min, the samples were cooled and diluted to 10 mL prior to measurement of absorbance at 540 nm using multimode reader (Biotek, USA). The results were expressed as percentage inhibition of enzyme activity and calculated according to Eq. 2.
MTT assay
Sub-toxic concentration of apigenin 7, 4'-dimethyl ether (ADE) was checked against 3T3L1 and L6 after 24 and 48 h incubation by MTT assay. The method was carried out as per previously described protocol (Wilson 2000). Briefly, 5 × 104 cells/mL were seeded at log phase in 24 well plates and incubated overnight. Growth media was removed and fresh media added with active compound at different concentration (0.1, 1, 5, 10, 50, 100 μg/mL) or DMSO as control and incubated. Media was removed and replaced with MTT. After incubation for 3–4 h, DMSO was added to dissolve insoluble crystals and absorbance read at 570 nm.
Glucose uptake assay by 2-NBDG
Glucose uptake assay was performed according to the method of Chen et al. (2010). L6 rat skeletal muscle cells were cultured in DMEM with 10 % fetal bovine serum. Cells were maintained at 37 ºC in a humidified 5 % CO2 environment. Cells were plated at 1 × 104 cells/well in 96-well plates and used at sub confluence after 24 h incubation. The culture medium was then removed from each well and replaced with 100 μl of culture medium containing ADE at 0.1, 1, 2.5, 5, and 10 μg/mL concentrations for 1 h pre-incubation. Media was removed and cells washed twice with pre cooled Kreb’s Ringer Buffer (KRB). The fluorescent analogue of glucose, 2-NBDG (100 μM) was given and incubated for 10 min prior to cell isolation.
The 2-NBDG uptake was stopped by removing the incubation medium and cells were washed twice with pre-cold KRB. Cells were trypsinized and re-suspended in 400 μl pre-cold fresh growth medium. For each measurement, data from 10,000 single cell events was recorded using flow cytometry (BD FACS Aria II, USA). Insulin (100 U) and rosiglitazone (100nM) served as control. Rosiglitazone is an antidiabetic drug that works as an insulin sensitizer by binding to the PPAR receptors in fat cells and making the cells more responsive to insulin.
Adipocyte differentiation assay
Effect of ADE extract to inhibit the differentiation of 3T3L1 preadipocyte to mature adipocyte was carried out based on reported protocol (Kim et al. 2007). Briefly, 3T3L1 preadipocyte cells were maintained by 10 % FBS in DMEM containing 4.5 g/L glucose, 100 U/mL penicillin, 0.1 mg/mL streptomycin and 0.25 mg/mL amphotericin B at 37 °C in 5 % CO2 incubator. Confluent cells were treated with differentiation media containing 100 U insulin, 100 nM dexamethasone, 0.5 mM IBMX and 10 % FBS with or without ADE at 0.1, 1, 2.5, 5, 10 μg/mL for 48 h. Roziglitazone (100 nM) served as control. Cells were maintained in post-differentiation DMEM containing 100 U insulin, 100 ng/mL dexamethasone in 10 % FBS, and the media was replaced in every 2 days. In control cells, normal media was replaced in every 24 h. Differentiation, as measured by the appearance of lipid droplets and change in cell size, was completed at day 10 and cells were analyzed cytometrically (FACSAria II, Becton-Dickinson, San Jose, CA) within 30 min of isolation.
Statistical analysis
The experimental results were expressed as means ± SD of three parallel measurements. The results were processed using Origin Pro 8 and the data were subjected to one-way analysis of variance (ANOVA) and the significance of differences between sample mean were calculated.
Results
Total flavanoids content
The total flavonoid content of P. longum extracts were checked by aluminium chloride colorimetric method. Out of all extracts checked, ethyl acetate extract of Piper longum fruit (EAP) was also found to be of higher flavanoids content (17.273 mg/g of quercetin) (Table 1). Generally, flavanoids possess comparatively good radical scavenging potential and total flavanoids content can be roughly correlated with antioxidant activity.
Table 1.
The total flavanoids content, phenolic content, DPPH assay, hydroxyl radical scavenging assay and total reducing power of Piper longum fruit extracts. Values are means of triplicate determinations (n = 3) ± standard deviation
| P. longum extract | Flavonoid content (mg/g of QAE) | TPC (mg/g GAE) | DPPH scavenging activity IC50 (μg/mL) | Hydroxyl radical scavenging activity IC50 (μg/mL) | TRP (mg/g GAE) |
|---|---|---|---|---|---|
| Hexane | 16.11 ± 0.21 | 0.361 ± 0.11 | – | 192.35 ± 11.65 | 3911.922 ± 25.21 |
| EtoAc | 17.273 ± 0.15 | 3.348 ± 0.24 | 173.0872 ± 9.45 | 20.42 ± 2.17 | 196.030 ± 9.34 |
| MeOH | 18.55 ± 0.11 | 1.263 ± 0.13 | 397.3903 ± 13.68 | 94.19 ± 5.43 | 481.139 ± 13.76 |
| 70 % MeOH-H2O | 14.22 ± 0.08 | 1.878 ± 0.09 | 286.5953 ± 16.76 | 52.60 ± 3.32 | 421.352 ± 11.42 |
| H2O | 9.05 ± 0.24 | 1.229 ± 0.12 | 433.1257 ± 14.32 | 146.06 ± 12.10 | 594.084 ± 10.21 |
Antioxidant activity
Total phenolic content (TPC)
The total phenolic content of the extracts was determined by Foiln-Ciocalteau method. Gallic acid was used as the standard. The total phenolic content was expressed as gallic acid equivalents (GAE) per gram of dry material. Of all extracts analyzed, ethyl acetate extract possess highest phenolic content, 3.348 g GAE. The order of total phenolic content in extracts is EAP > MWP > MP > WP > HP (Table 1).
Total reducing power (TRP)
Total reducing ability was determined by the Fe3+ - Fe2+ transformation method. The reductants (antioxidants) present in the sample can cause the reduction of ferric cyanide complex to ferrous form. Among all the extracts studied the ethyl acetate extract (196.030 mg GAE) possesses higher reducing power in terms of gallic acid equivalence. The low concentration values of the extract as gallic acid equivalents indicate the higher reducing power of the sample. The order of total reducing power of the extracts is EAP > MWP > MP > WP > HP (Table 1).
DPPH radical scavenging capacity
The DPPH radical has been widely used to evaluate the antioxidant activity of plant extracts and foods. Though all the extracts exhibited a dose dependent radical scavenging activity, ethyl acetate extract (IC50–173.09 mg/L) was found to have more antioxidant activity. The order of activity of these extracts is EAP > MWP > MP > WP (Table 1). Hexane extracts doesn’t give any significant results and might be due to insoluble components in reaction system.
Hydroxyl radical scavenging capacity
This assay shows the ability of the extract to inhibit hydroxyl radical-mediated deoxyribose degradation in Fe3+-EDTA-ascorbic acid and H2O2 reaction mixture. Similar to the reducing power and DPPH radical scavenging activity, the amount of hydroxyl radical scavenging activity appeared to depend on the phenolic concentration of the extracts. The ethyl acetate extract (IC50–20.42 mg/L) was found to have high radical scavenging ability and the order of activity for other extracts is EAP > MWP > MP > WP > HP (Table 1).
SOD activity
Ethyl acetate extract of P. longum showed a concentration dependent SOD enhancing activity in NBT assay. HepG2 cells treated with extract at 50, 100, 200 and 500 μg/mL concentrations were lysed and absorbance of lysate measured in presence of NBT. Superoxide radicals were generated using phorbol myristate acetate (PMA). At a concentration of 200 μg/mL, EAP was able to increase SOD activity by 25.3 % compared to untreated control well.
LDL oxidation inhibitory potential of EAP
The potential of extracts from Piper longum to inhibit human LDL oxidation was checked. Ethyl acetate extract of fruit inhibited the LDL oxidation very effectively (IC50–51.99 ± 2.1 μg/mL) compared to other extracts.
HPTLC analysis of ethyl acetate extract and fractions
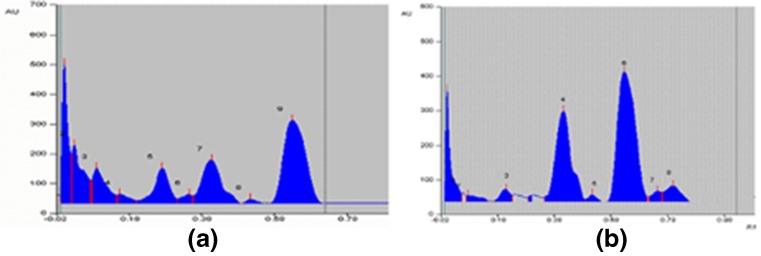
Of the extracts from Piper longum, ethyl acetate showed maximum antioxidant activity and was fractionated into three, namely hexane fraction (HFP), ethyl acetate fraction (EAFP) and methanol fraction (MFP). All the fractions were tested for antioxidant activity and it is seen that the EAFP is giving best activity amongst the three. Ethyl acetate extract and all the three fractions were analyzed for components by HPTLC. On comparison with standards, ethyl acetate extract showed peaks corresponding to apigenin 7, 4'-dimethyl ether (ADE) and piperine (Pip). Ethyl acetate fraction of EAP retained most portions of active compounds present in the extract (Fig. 1).
Fig 1.
HPTLC densitogram of EAP and separated fractions. a) ethyl acetate extract of P. longum (EAP) and b) ethyl acetae fraction of EAP . 4 – Apigenin 7, 4'-dimethyl ether; 5 – Piperine
Characterization of Apigenin 7, 4'-dimethyl ether
Yellow needle shaped crystals; Melting point: 166–168 °C
UV-Visible λmax (MeOH): 326, 276 nm; UV-Visible λmax (+AlCl3/HCl): 348, 293 nm; UV-Visible λmax (+NaOAc): 324, 271 nm; UV-Visible λmax (+NaOAc/H3BO3): 325, 271 nm.
FAB-MS (m/z) (rel.int.): 299 (M + H)+ (100), 298 (M)+ (18), 284 (M-CH3)+ (7), 269 (M-2 × CH3)+ (13), 224 (16), 166 (8), 135 (36), 132 (14).
IRνmax (cm−1): 2,922, 1,660, 1,600, 1,508, 1,440, 1,268, 1,160, 1,011, 818.
The 1H NMR spectrum of the compound exhibited a signal at δ 12.82 (1H, s), attributed to a chelated hydroxyl group. Further a signal observed at δ 6.58 (1H, s) was due to an H-3 proton. The two singlets observed at δ 3.89 (3H, s) and 3.90 (3H, s) were assigned to the two methoxy groups at positions 4’ and 7’ respectively. The 1H NMR also demonstrated two protons doublets at δ 7.86 (2H, d, J = 9Hz), 7.03 (2H, d, J = 9Hz), assignable to H2’/H6’ and H3’/H5’ protons. The two doublets observed at δ 6.38 (1H, d, J = 2Hz) and 6.50 (1H, d, J = 2Hz) were assigned to the protons H-6 and H-8 respectively. The 13C NMR of the compound showed a total of 15 signals for 17 carbons. A signal observed at δ 182.31 was assigned to C-4. Signals observed at δ 55.53 and 55.78 were assigned to two methoxy groups at C-4 and C-7. The 13C NMR further showed signals at δ 128.05 (C-2/6) and 114.51 (C-3/5). The signals at δ 98.04 and 92.63 were assigned to C-6 and C-8.
The results of 1H and 13C NMR were further confirmed by HMBC spectral studies. The HMBC experiments showed 3 J correlation of the proton at δ 6.50 with C-6 (98.04), C-7 (164.04) and C-9 (162.60) and 4 J correlation of the proton at δ 6.38 with C-5 (157.71), C-7 (164.04), C-8 (92.63) and C-10 (105.57). The position of methoxyl groups were authenticated through HMBC. The methoxyl proton at δ 3.90 correlates with C-7 (164.04) and the other at δ 3.89 correlates with C-4 (162.21). The proton at δ 6.58 correlates with C-2 (165.44), C-4 (182.31), C-10 (105.57) and C-1 (123.60) and is supposed to be with C-3. The proton at positions 2’/6’ (δ 7.86) correlates with C-2 (165.44), C-3 (114.51), C-4 (162.21) and C-5 (114.51). Further the proton at 3’/5’ (δ 7.03) correlates with C-2 (128.05), 4’ (162.21) and C-6 (128.05).
On the basis of above spectral data, the compound was confirmed to be Apigenin7, 4'-dimethyl ether.
Determining subtoxic concentration of ADE by MTT assay
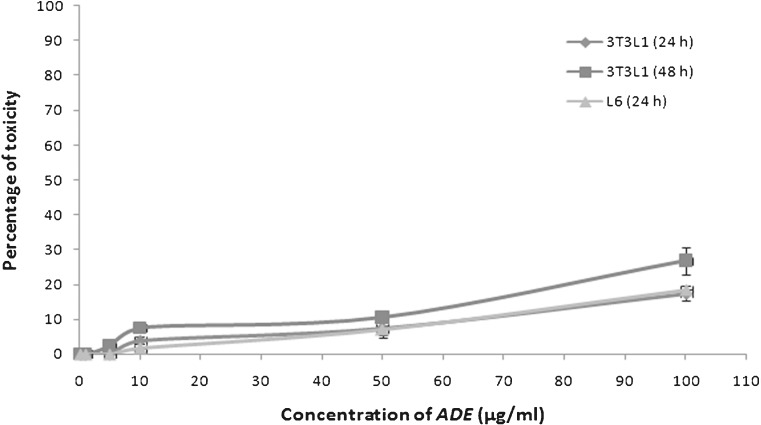
To determine the sub toxic concentrations against 3T3L1 and L6 cell lines, ADE was checked at 0.1, 1, 5, 10, 50 and 100 μg/mL concentrations and cell viability was determined by MTT assay. For further experiments, toxicity of extracts against 3T3L1 cells was checked after 24 and 48 h incubation in separate plates. Viability of L6 was assayed after 24 h incubation. Percentage of cell viability was calculated and plotted against concentration of compound (Fig. 2). Results showed that, at a concentration of 50 μg/mL, apigenin 7’4’ dimethyl ether was less toxic to both cell lines. After 48 h incubation, ADE reduced the viability of 3T3L1 cells up to 73 %.
Fig 2.
MTT assay: Cytotoxicity of 4 – Apigenin 7, 4'-dimethyl ether (ADE) against 3T3L1 and L6 cell lines in a concentration dependent manner is plotted against percentage of toxicity. Toxic effect of ADE in 3T3L1 cells after 48 h incubation was also checked
Antidiabetic potential of ADE
α-glucosidase and α-amylase inhibition assay
α-glucosidase and α-amylase are two crucial enzymes in Type-2 Diabetes Mellitus (T2DM). Table 2 indicates the enzyme inhibitory effect of ethyl acetate fraction of ethyl acetate extract showing comparatively high inhibition on α-glucosidase enzyme and moderate inhibition to α-amylase enzyme. Compared to standard compound acarbose (IC50 value 175.35 and 45.20 μg/mL for α-glucosidase and α-amylase respectively), activity of ethyl acetate extract is significantly high. Apigenin 7’4’ dimethyl ether (ADE) isolated from ethyl acetate fraction was tested for activity. Results showed that activity of ADE is 6.4 times higher than acarbose for α-glucosidase and half of activity against α-amylase inhibition.
Table 2.
α-glucosidase and α-amylase inhibitory potential of different fractions of EAP
| P. longum extract | IC50 value (μg/mL) | |
|---|---|---|
| α-glucosidase assay | α-amylase assay | |
| EtOAc extract | 202.5800 | 90.5803 |
| Hexane fraction | 18.7559 | Not significant |
| EtOAc fraction | 36.3323 | 114.2646 |
| MeOH fraction | Not significant | 92.2988 |
| Apigenin 7’4’ dimethyl ether | 27.342 | 98.143 |
Glucose uptake assay by 2-NBDG
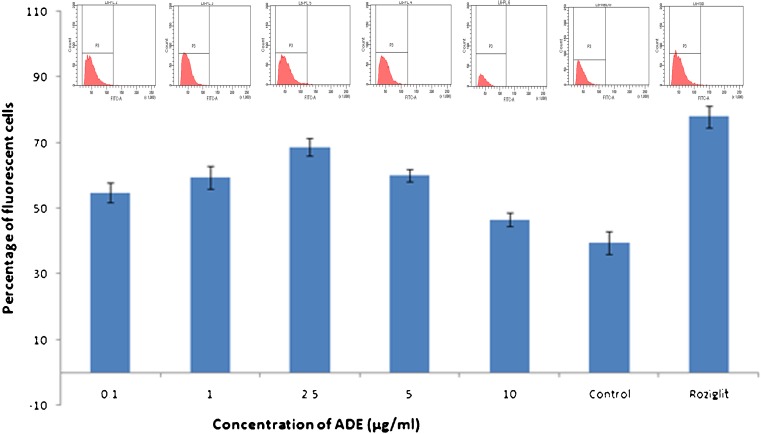
Effect of ADE on glucose uptake was checked by 2-NBDG based assay. L6 cells were pre-incubated with the compound at different concentrations from 0.1–10 μg/mL. From the flow cytometry data of 10,000 events recorded, the change in fluorescence with respect to concentration of ADE was clearly evident (Fig. 3). Cells showed an increase in 2-NBDG uptake up to 2.5 μg/mL concentration after which the effect was found to be reduced. At a concentration of 2.5 μg/mL, L6 showed 42.7 % increase in fluorescence with respect to control cells. The effect was compared with standard drug roziglitazone (100nM), which showed 66.2 % increase in dye uptake.
Fig 3.
2-NBDG uptake assay: Percentage of cells that showed fluorescence of 2-NBDG with and without ADE treatment was measured using flow cytometer. Bar diagram showing percentage of cells showing fluorescence. Corresponding histogram generated upon flow cytometric analysis is also shown above bar graph. Experiment was carried out in triplicate and values expressed as mean ± SD
Antiobesity effect of ADE by inhibiting adipocyte differentiation
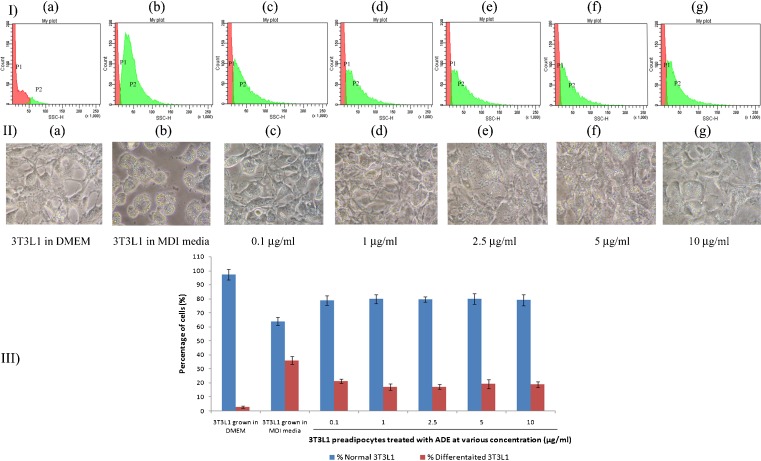
To investigate the antiobesity effect of P. longum, confluent 3T3L1 cells were treated with ADE at 0.1, 1, 2.5, 5, 10 μg/mL for 48 h in differentiation media containing 100 U insulin, 100 nM dexamethasone and 0.5 mM IBMX. After that, media was switched to post differentiation for 10 days. Cells were detached carefully from plates using trypsin and washed in cold PBS. Around 10,000 events were recorded and analyzed by flow cytometer based on difference in size and granularity which gives a separation between undifferentiated and differentiated cells (Fig. 4-I). Results showed that within a concentration 1–5 μg/mL, ADE seems to reduce adipocyte differentiation and lipid droplet content by 16 %. Fig. 4-II clearly visualizes the effect of the compound in inhibiting 3T3L1 differentiation with respect to control cells. Many of the cells remain undifferentiatied without any change in cell morphology. Change in percentage of differentiated and undifferentiated pre-adipocytes with or without ADE treatment is expressed in Fig. 4-III.
Fig 4.
Hypolipidemic effect of ADE at various concentrations were measured based on size and granularity of cells using flow cytometer. I) a-g: Histogram from FACS data showing change in differentiated 3T3L1 population (P2). II) a-g: Figures clearly visualize the change in lipid droplets content in control and treated samples. III) Graph showing change in percentage of normal and differentiated cells in the presence of ADE
Discussion
Piper longum
is a well known spice with immense medicinal potential. We, in search of natural medicines for diabetes and cardiovascular diseases, selected this plant due to its presence in food and medicine in many countries. The plant is a major ingredient in many herbal formulations and drugs available in market (Manoj et al. 2004). Although earlier studies on antioxidant and hypoglycaemic effect of crude extracts of P. longum showed antidiabetic activity, the study didn’t focus on lipid level and correlation between chemical constituents and activity (Kumar et al. 2011).
Role of oxidative stress in onset and progression of many diseases is an invariably proved fact. This led us to screen plant extracts based on antioxidant activity. From the results, the activity of EAP and EAFP against free radicals of biological importance is proved experimentally. Total flavanoids and phenolics in the selected extract are also high compared to others. A number of mechanisms are likely to contribute to the inhibition of LDL oxidation by flavonoids (Vaya et al. 2003). Polyphenolic compounds are known to have antioxidant activity and it is likely that the activity of the extracts may also be due to these compounds (Djeridane et al. 2006; Luximon-Ramma et al. 2005). The results indicate that a good correlation exist between total phenolic content, reducing power and DPPH radical scavenging activity of the extracts.
The bathochromic shift with AlCl3/HCl is due to the presence of hydroxyl group at fifth position of the flavonoid ring. There were no shifts associated with NaOAc and NaOAc/H3BO3, which suggests the absence of hydroxyl group at positions 3’, 4’ and 7’ (Fathiazad et al. 2006). The compound shows the characteristic fragmentation of flavones resulting from the retro-Diels-Alder cleavage of the chromonyl moieties (peaks corresponding to an m/z of 166 and 132) as in Fig. 3. The IR spectra of the compound showed absorption bands for chelated hydroxyl group (2,922 cm−1), chelated α, β-unsaturated carbonyl attached with aromatic nucleus (1,660, 1,508, 1,440 cm−1), aromatic C = C (1,600 cm−1), methoxy group (1,011 cm−1), and p-substituted benzene ring (818 cm−1) functionalities (Mabry et al. 1970). On the basis of UV, IR, 1H and 13C NMR, HMBC and FAB MS the compound obtained was identified as apigenin 7, 4'-dimethyl ether (ADE). All the spectral data were in good concurrence with those reported in the literature (Dhar et al. 1970). This compound has been reported previously from Baccharis rhomboidalis (Silva et al. 1971), but not yet from Piper longum.
Significant effect of ADE against α-glucosidase and α-amylase enzymes showed the antidiabetic effect of P. longum. Previous studies on flavanoids and flavanoid fraction from plants has reported its effect on enzymes related to glucose metabolism (Jung et al. 2006; Mennen et al. 2004). Food additives that can lower blood glucose level are of much importance in present scenario. On comparison with a well known antidiabetic medicinal plant Aloe ferox, the phenolic content of P. longum was 4-fold higher and flavonoids content was 32-fold higher (Loots et al. 2007). The plant also showed same phenolic content to that of Allium sativum (garlic), a common ingredient of food (Beato et al. 2011). Garlic was able to double the glucose uptake at a concentration of 3,000 μg/mL and 24 h incubation time (Noipha et al. 2010). We selected 2-NBDG assay and adipocyte differentiation assay based on the observations that overweight and obesity are closely associated with both adipocyte differentiation and type II diabetes and are a primary risk factor for the latter (O’Rahilly 2007). P. longum was able to enhance 42.7 % glucose uptake from basal level at 2.5 μg/mL within an incubation time of 1 h. The activity was higher than control cells at all subtoxic concentrations and slightly lower than thiazolidinedione (66 %) treated cells. Reduction in 2-NBDG uptake after 2.5 μg/mL concentration might be due to the increase in concentration of any other compounds in extract that can interact with active compound (s). Chance of other compounds in extract that prevents expression of glucose transporters to cell surface can also be a reason for the phenomenon.
Within 0.1–10 μg/ml concentration, ADE was able to show anti lipid peroxidative effect and hypo lipidemic effect in pre adipocyte cells. Bright field images of 3T3L1 showed in Fig. 4-II demonstrates the potential of ADE to regulate adipocyte differentiation. PPAR-γ plays a major role in adipocyte differentiation. This ligand activated nuclear receptor, along with RXR, directly binds to target genes and enhance expression of genes related to adipocyte differentiation. Drugs that modulate obesity through PPAR will act either by enhancing the expression of PPAR or by agonistic action.
Chemical fingerprinting of EAFP by HPTLC revealed the presence of apigenin 7, 4'-dimethyl ether and piperine as major components. A recent study on insulinotropic effect of Teucrium polium extract showed that the major component present is apigenin 7, 4'-dimethyl ether flavone (Mirghazanfari et al. 2010). Effect of piperine on galactose metabolism has been studied previously (Reen et al. 1993). No reports are available on glucose uptake or lipid lowering potential of apigenin 7, 4'-dimethyl ether or piperine.
Thus, the results presented here suggest Piper longum could be more suitable candidate for the search of new antidiabetic drug molecule. The differentiation preventing effect of extract in 3T3L1 can be considered as a positive factor compared to the side effect of oral hypoglycemic drugs presently in use. The advantage of this plant over existing types is its presence in general food items and herbal formulations. In summary, the work reveals the presence of water soluble antidiabetic and antiobesity compounds in P. longum and about the molecular mechanism underlying. More detailed studies on the effect of these compounds on gene level regulation are under investigation.
Acknowledgement
We thank the Director of National Institute for Interdisciplinary Science and technology, CSIR, India for providing facilities to carry out the work. We also thank CSIR for providing financial support for the progress of work.
Declaration of interest
The authors hereby report no declaration of interest
References
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Inn Food Sci Emer Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Beato VM, Orgaz F, Mansilla F, Montano A. Changes in phenolic compounds in garlic (Allium sativum L.) owing to the cultivar and location of growth. Plant Foods Hum Nutr. 2011;66:218–223. doi: 10.1007/s11130-011-0236-2. [DOI] [PubMed] [Google Scholar]
- Bishit BS. Pharmcognosy of ‘piplamul’-the root and stem of Piper longum linn. Planta Med. 1963;11:410–416. doi: 10.1055/s-0028-1100257. [DOI] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chen QC, Zhang WY, Jin W, Lee IS, Min BS, Jung HJ, Na M, Lee S, Bae K. Flavonoids and isoflavonoids from sophorae flos improve glucose uptake in vitro. Planta Med. 2010;76:79–81. doi: 10.1055/s-0029-1185944. [DOI] [PubMed] [Google Scholar]
- Dhar KL, Atal CK, Pelter A. Flavonoid compounds from Piper peepuloides. Planta Med. 1970;18:332–335. doi: 10.1055/s-0028-1099787. [DOI] [PubMed] [Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- Fathiazad F, Delazar A, Amiri R, Sarker SD. Extraction of flavonoids and quantification of rutin from waste tobacco leaves. Iranian J Pharm Res. 2006;3:222–227. [Google Scholar]
- Gleissner CA, Galkina E, Nadler JL, Ley K. Mechanisms by which diabetes increases cardiovascular disease. Drug Disc Today: Disease Mech. 2007;4:131–140. doi: 10.1016/j.ddmec.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the national heart, lung, and blood institute/american heart association conference on scientific issues related to definition. Circul. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 2006;38:1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Kim J, So H, Youn M, Kim H, Kim Y, Park C, Kim S, Ha Y, Chai K, Kim S, Kim K, Park R. Hibiscus sabdariffa L. water extract inhibits the adipocyte differentiation through the PI3-K and MAPK pathway. J Ethnopharm. 2007;114:260–267. doi: 10.1016/j.jep.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. Indian Medicinal Plants. 2. Allahabad, India: LalitMohan Basu Publication; 1933. pp. 2131–2133. [Google Scholar]
- Kumar S, Sunil S, Suman, Jitpal (2011) In vivo anti-hyperglycemic and antioxidant potential of Piper longum fruit. J Pharm Res 4:471–474
- Loots DT, van der Westhuizen FH, Botes L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J Agric Food Chem. 2007;55:6891–6896. doi: 10.1021/jf071110t. [DOI] [PubMed] [Google Scholar]
- Luximon-Ramma A, Bahorun T, Soobrattee AM, Aruoma OI. Antioxidant activities of phenolic, proanthocyanidin and flavonoid components in extracts of Acacia fistula. J Agric Food Chem. 2005;50:5042–5047. doi: 10.1021/jf0201172. [DOI] [PubMed] [Google Scholar]
- Mabry TJ, Markham KR, Thomas MB. The systematic identification of flavonoids. The systematic identification of flavonoids. NY: Springer; 1970. [Google Scholar]
- Manoj P, Sonia EV, Banarjee NS, Ravichandran P (2004) Recent studies on well known plant, Piper longum Linn. Nat Prod Rad 3:222–227
- McDonald S, Prenzler PD, Autolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. doi: 10.1016/S0308-8146(00)00288-0. [DOI] [Google Scholar]
- Mennen LI, Sapinho D, Bree A, Arnault N, Bertrais S, Galan P, Hercberg S. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J Nutr. 2004;134:923–926. doi: 10.1093/jn/134.4.923. [DOI] [PubMed] [Google Scholar]
- Mirghazanfari SM, Keshavarz M, Nabavizadeh F, Soltani N, Kamalinejad M. The effect of Teucrium polium L. extracts on insulin release from in situ isolated perfused rat pancreas in a newly modified isolation method: the role of Ca2+ and K+ channels. Iran Biomed J. 2010;14:178–185. [PMC free article] [PubMed] [Google Scholar]
- Moller ED. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–827. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- Murthy KNC, Singh RP, Jayaprakasha GK. Antioxidant activities of grape (Vitis vinifera) pomace extracts. J Agric Food Chem. 2002;50:5909–5914. doi: 10.1021/jf0257042. [DOI] [PubMed] [Google Scholar]
- Nagai T, Myoda T, Nagashima T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005;91:389–394. doi: 10.1016/j.foodchem.2004.04.016. [DOI] [Google Scholar]
- Noipha K, Ratanachaiyavong S, Ninla-aesong P. Enhancement of glucose transport by selected plant foods in muscle cell line L6. Diabetes Res Clin Pract. 2010;89:e22–e26. doi: 10.1016/j.diabres.2010.04.021. [DOI] [PubMed] [Google Scholar]
- O’Rahilly S. Human obesity and insulin resistance: lessons from experiments of nature. Biochem Soc Trans. 2007;35:33–36. doi: 10.1042/BST0350033. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Japan J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Philips M, Cataneoa RN, Cheema T, Greenberg J. Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin Chim Acta. 2004;344:189–194. doi: 10.1016/j.cccn.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Reen RK, Jamwal DS, Taneja SC, Koul JL, Dubey RK, Wiebel FJ, Singh J. Impairment of UDP-glucose dehydrogenase and glucuronidation activities in liver and small intestine of rat and guinea pig in vitro by piperine. Biochem Pharmacol. 1993;46:229–238. doi: 10.1016/0006-2952(93)90408-O. [DOI] [PubMed] [Google Scholar]
- Rook GA, Steele J, Umar S, Dockrell HM. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by I3-interferon. J Immunol Meth. 1985;82:161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Shinde J, Taldone T, Barletta M, Kunaparaju N, Hu B, Kumar S, Placido J, Zito SW. α-Glucosidase inhibitory activity of Syzygium cumini (Linn.) Skeels seed kernel in in-vitro and in Goto–Kakizaki (GK) rats. Carbohydr Res. 2008;343:1278–1281. doi: 10.1016/j.carres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Silva M, Mundaca JM, Sammes PG. Flavonoid and triterpene constituents of Baccharis rhomboidalis. Phytochem. 1971;10:1942–1943. doi: 10.1016/S0031-9422(00)86469-9. [DOI] [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Uma BR, Seetharam YN. Anthelmintic activity of trikatu churna and its ingredients. Ethnobot Leaflets. 2009;13:532–539. [Google Scholar]
- Vaya J, Mahmood S, Goldblum A, Aviram M, Volkova N, Shaalan A, Musa R, Tamir S. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry. 2003;62:89–99. doi: 10.1016/S0031-9422(02)00445-4. [DOI] [PubMed] [Google Scholar]
- Wilson AP. Cytotoxicity and viability assays. In: Masters JRW, editor. Animal cell culture: A practical approach. Oxford: Oxford Univ Press; 2000. [Google Scholar]