Abstract
Physical, functional and physicochemical properties of flours of five cocoyam (Colocasia spp and Xanthosoma spp) cultivars were evaluated. Colour (L*a*b*) parameters of corms and flours, pasting and functional properties of the flours were determined. Xanthosoma spp showed significantly higher length (95.16–151.46), width (75.29–78.03) and weight (179.20–605.94) than the Colocasia spp., but the parameters did not vary significantly within either Xanthosoma and Colocasia spp. Generally, colour of peeled corms [L* (72.08–78.93); a* (+1.06 − +3.5); b* (+17.65 − +35.80)] was lighter than the flours [L* (69.35–84.97); a* (+0.30 − + 4.76); b* (+4.44 − +23.48)]. The NXs001 showed significantly higher peak (201.71RVU), trough (186.75 RVU), final (289.75 RVU) and setback (103 RVU) viscosities that the other cultivars. Pasting profiles of the cocoyam flours showed similar trend with the NXs001 showing a steeper curve. Pasting temperature and peak time ranged from 87.33 to 92.53 °C and 5.17–6.34 min, respectively. Water absorption capacity, gelling point, pH, foam capacity, bulk density and swelling power varied from 32–69 %, 6.56–7.59, 58.5–72.5 °C, 7.19–14.72 %, 0.94–1.01 g/mL and 3.18–7.36, respectively.
Keywords: Cocoyam cultivars; Flours; Pasting properties, Colour; Functional properties
Introduction
Cocoyams (Colocasia esculenta (L.) Schott and Xanthosoma sagittifolium (L.) Schott) are the important species of edible aroids, grown in tropical and sub-tropical countries. Cocoyams are used as subsistence staples in many parts of the tropics and sub-tropics. Cocoyams are grown primarily for their edible starch laden corms and cormels, and secondarily their leaves are used as vegetable (Aregheore and Perera 2003). According to Odebunmi et al. (2007), Xanthosoma corm contains moisture (80.99 %), ash (1.03 %), crude protein (5.47 %), crude fat (0.20 %), crude fibre (1.28 %) and total carbohydrate of 11.03 %. Colocaisa corm contains (dry matter) protein (10.88 %), fat (0.18 %), ash (3.42 %), crude fiber (3.38 %) and carbohydrate (82.14 %) (Chinnasarn and Manyasi 2010). Nigeria is the largest producer of cocoyam in the world with an annual production of 3.450 million metric tonnes in 2012, representing 72.2 %, 57.7 % and 45.9 % of total production in West Africa, Africa and the World, respectively (FAOSTAT 2012).
Cocoyam is said to be a giant crop, because its corms, cormels, leaves, stalks and inflorescence are all utilized for human consumption (Chukwu et al. 2008). According to Falade and Okafor (2013), the granule size of cocoyam starch (length = 6.47–13.63 μm; width = 5.36–8.45 μm) was within the range reported for native cocoyam starch (4–18.7 μm) from Malawian (Mweta 2009). Colocasia spp is reported more suitable for several food products, especially as food for potentially allergic infants, and persons with gastro-intestinal disorders (Opara 2002). The cocoyam corms could be processed into many products including poi (fresh or fermented paste, canned, and canned-acidified), flour, cereal base, beverage powders, chips, sun-dried slices, grits, and drum-dried flakes (Owuamanam et al. 2010). However, cocoyam consumption has been affected by the presence of oxalates which imparts acrid taste, and causes sharp irritation and burning sensation in the throat and mouth on ingestion (Akpan and Umoh 2004). This high moisture content (>70 %) associated with high rate of deterioration after harvest, results in its limited availability. Therefore, processing of fresh cocoyam corms and cormels into flours will reduce food losses and transportation cost and increased versatility and utilization in food formulations.
Application of flours in the food industry is primarily governed by their functional and physicochemical properties. Physicochemical properties are those properties that affect the physical and chemical attributes of food during processing. Investigation of these properties is necessary to know the requisite characteristics of cocoyams if their utilization would be enhanced. Many articles have been published on the functional and physicochemical, and pasting characteristics of cocoyam starches (Mepba et al. 2009; Falade and Okafor 2013). However, little information is available of these properties as it pertains to flours of cocoyam cultivars. Therefore the objective of this research was to evaluate the physical, functional and physicochemical properties of five cocoyam (Colocasia spp. and Xanthosoma spp.) cultivars.
Materials and methods
Cocoyam (Colocasia esculenta (taro) and Xanthosoma sagittifolium (tannia)) cultivars were harvested after seven to eight months of planting, were obtained from the National Root Crops Research Institute, Umudike, Abia State, Nigeria. The Colocosia spp. were NCe001 (Coco India), NCe002 (Ede ofe green), NCe003 (Ede ofe purple), while the Xanthosoma spp. were NXs001 (Ede ocha), and NXs003 (Okorokoro). The cocoyam samples were harvested in January 2011 (harmattan/dry season), at Umudike. Umudike is situated in Ikwuano, Abia, Nigeria, and its geographical coordinates are 5° 28′ 0″ North, 7° 33′ 0″ East.
Measurement of the physical parameters
Length and width dimensions of the tubers were determined using a digital veneer caliper (Model 500−196, Mitutoyo Products, America). The weight of the corms was determined using a weighing balance (Model GF-2000, A&D company Ltd., Japan) of an accuracy of ± 0.01.
Preparation of cocoyam flour
Corm samples of each cultivar (2 kg) were sorted, washed with potable water to remove adhering soil, and peeled manually with stainless steel knife. The peeled roots were washed with portable water and sliced into 2 mm thickness with a manual stainless steel slicer, and the thin slices were air dried in a cross flow Precision Gravity Convection Oven (Model No. STG40, Precision group, Chicago) at 50 °C for 36 h, and pulverised using an electric hand mill (Romer serial II mill, Romer labs, USA), and sieved using 425 μm Tyler screen. The flour obtained from each cultivar was packaged separately in transparent polypropylene (100 μm) bags, sealed and stored at 4–7 °C.
CIE L* a* b* colour determination
Surface colour of the peeled cocoyam corms and flours were evaluated objectively using a Colourimeter (Colour Tec PCMTM Colour Tec Associates, Inc, Clinton, NJ, USA). Each cocoyam cultivar was sliced into halves vertically and longitudinally, and L* a* b* parameters of the interior were evaluated. The L* a* b* readings were taken at ten different points on the cut. Similarly, the colour parameters of the cocoyam flours were determined at ten different points. L* values range from 100 (white) to 0 (black), a* values range from + a* (green) to -a* (red), and b* value range from + b* (yellow) to -b* (blue). Averages of the readings were computed and reported. Hue angle (H) was calculated using as H = Tan−1(b/a).
Determination of pasting properties
Pasting properties of flours were determined using a Rapid Visco Analyzer (Model RVA-4; Newport Scientific Pty. Ltd, Warriewood, Australia). Three grammes of the flour samples were weighed into a dried empty canister; 25 mL of distilled water was dispensed into the canister containing the sample. The mixture was thoroughly stirred and the canister was fitted into the RVA as per manufacturer’s instructions. The slurry was heated from 50 to 95 °C with a holding time of 2 min followed by cooling to 50 °C with 2 min holding time. The rate of heating and cooling were at a constant rate of 11.25 °C min−1. The pasting profile were determined with the aid of Thermocline for Windows Software connected to a computer.
Swelling power and solubility determination
Swelling power and solubility of cocoyam flours were determined by heating a flour-water slurry (0.35 g flour in 12.5 mL of distilled water) in a water bath at 60 °C for 30 min, with constant stirring (Crosbie 1991). The slurries were centrifuged using a Super-speed centrifuge (Model No. L-708-2, Phillips Drucker, Oregon, USA) at 168 × g for 15 min, the supernatant was decanted into a weighed evaporating dish and dried at 100 °C for 20 min. The difference in weight of the evaporating dish was used to calculate flour solubility. Swelling power was obtained by weighing the residue after centrifugation and dividing by original weight of flour on dry weight basis.
Gelling and boiling points determination
The method of Narayana and Narasinga-Rao (1982) was adopted. The flour sample (10 g) was dispersed in distilled water, in a 250 mL beaker and made up to 100 mL. A thermometer was clamped on a retort stand such that its bulb submerged in the suspension. With a magnetic stirrer the suspension was continuously stirred and heated. This continued until the suspension began to gel and the corresponding temperature recorded. The temperature as soon as boiling commenced was also noted and recorded. This analysis was done in duplicate.
Foam capacity and foam stability
The method described by Narayana and Narasinga-Rao (1982) was used for the determination of foam capacity and foam stability. About 2 g of flour sample was added to 50 mL distilled water at 30 ± 2 °C in a 100 mL measuring cylinder. The mixture was stirred and the volume was noted. The suspension was blended using a warring blender (Model HGBTWT; Warring Commercial, Torrington, USA) at 160 × g for 5 min to create foam, then returned to the measuring cylinder and the volume of the foam after 30 s was recorded. The foam capacity was expressed as a percentage increase in volume using the formula of Abbey and Ibeh (1988). Foam volume was recorded in 1 h after whipping to determine the foam stability as a percentage of the initial foam volume.
Determination of pH
The pH of a 10 % (w/v) flour-water suspension of each sample prepared into a clean beaker (200 mL) and allowed to settle at 30 ± 2 °C for 15 min was determined.
Determination of water absorption capacity
Water absorption capacity was determined using the method described by Beuchat et al. (1975)). About 1 g of sample was weighed into graduated 25 mL conical centrifuge tubes, and 10 mL of distilled water was added. The suspensions were allowed to stand at 30 ± 2 °C for 1 h. The suspension was centrifuged (Model No. L-708-2, Phillips Drucker, Oregon, USA) at 200 × g for 30 min. The supernatant was decanted and then the sample was reweighed. Change in weight was expressed as percent water absorption based on the original sample weight.
Determination of oil absorption capacity
The method of Sosulski (1962)) as described by Abbey and Ibeh (1988) was adopted. One gramme of each sample was weighed into a dry, clean centrifuge tube and both weight noted. Grand soya oil (10 mL) with density of 0.98 gcm−3, was poured into the tube and properly mixed with the samples using a stainless steel spatula. The suspension was centrifuged (Model No. L-708-2, Phillips Drucker, Oregon, USA) at 350 × g speed for 15 min. The supernatant was discarded, the tube and its content were then re-weighed. The gain in mass expressed as a percentage of oil bound is the oil absorption capacity of the sample.
Bulk density determination
Bulk density was determined by the method of Narayana and Narasinga-Rao (1984). Each sample (50 g) was filled into graduated cylinder and their weight noted. The cylinder was tapped continuously until there was no further change in volume. The weight and final volume of flour in the cylinder was noted, and the difference in weight and volume were determined. Bulk density was computed as grams per millilitre (g/mL) of the sample.
Statistical data analysis
All analyses were determined in triplicates. Data were analyzed using the Analysis of Variance (ANOVA) statistical method (Statistical Analysis System version 9.2 program, SAS Inc., (2008), USA.). Means were separated using Duncan’s (1955) multiple range test. Significant differences were established at p ≤ 0.05.
Results and discussion
For the purposes of this study the cultivars: Coco India, Ede ofe green, Ede ofe purple, Ede ocha, and Okorokoro will be discussed as NCe001, NCe002, NCe003 (Colocosia spp.), NXs001 and NXs003 (Xanthosoma spp.), respectively. The corms varied in geometry (length, width), and weight. The shapes were round, and some could be described as long, semi-circle and gourd (Table 1). However, the shapes of some cocoyam corms of the same cultivars were not homogeneous. Width and length varied significantly (p < 0.05) from 25.65 mm (NCe002) to 78.03 mm (NXs001) and 41.58 mm (NCe001) to 151.46 mm (NXs003) respectively. The Xanthosoma spp (NXs003) showed significantly higher length, width and weight than other samples. The Colocasia spp showed significantly smaller length, width and weight than the Xanthosoma spp (Table 1). Within each species, cocoyam samples showed similar width, length and weight. Knowledge of the physical characteristics is important for proper planning of storage and transportation spaces as well design of processing equipment.
Table 1.
Physical properties of unpeeled cocoyam corms
| Cultivars | Weight (g) | Length (mm) | Width (mm) | Visual Surface colour | Shape |
|---|---|---|---|---|---|
| NCe001 | 36.21b ± 18.36 | 41.58b ± 2.85 | 41.33b ± 11.84 | Brown | Semi-circle |
| NCe002 | 14.26b ± 9.13 | 41.98b ± 1.77 | 25.65b ± 6.27 | Brown | Gourd |
| NCe003 | 39.64b ± 33.75 | 46.12b ± 5.75 | 36.24b ± 10.20 | Brown | Gourd |
| NXs001 | 179.20ab ± 120.74 | 95.16ab ± 15.40 | 78.03a ± 20.08 | Brown | Round |
| NXs003 | 605.94a ± 547.01 | 151.46a ± 91.44 | 75.29a ± 26.34 | Brown | Long |
Means in a column with the same letter are not significantly different (p > 0.05). Means of ten replicates
Colour parameters of peeled cocoyam flesh and flours of cocoyam
Visually, the colour of the flesh of the cocoyam tubers could be described as cream to yellow to red. The colour of the flesh was generally lighter than the peel colour, which was brown in colour. The L* (lightness) value of the flesh varied significantly from 72.08 (NCe001) to 78.93 (NCe002), higher value indicated lighter (whiter) colour. The NCe002 and NCe003 cultivars showed similar L* value. However, NCe001 with the significant lower L* value showed darker flesh colour. The a* value of the flesh varied significantly from +1.06 (NCe001) to +3.45 (NXs003), while the b* varied from +17.65 (NCe002) to +35.8 (NXs003). The b* of the Xanthosoma spp. flesh was significantly higher than the Colocasia spp. The + a* and + b* values appear as red, and orange to yellow colour, respectively. Difference in the colour characteristics of the flours could be due to inherent pigments, which depends on the botanical origin of the plant and composition of the flour (Aboubakar et al. 2008). The intensity of yellow is dependent on the concentration of the beta carotene pigment. Higher + b* showed brighter yellow colour: NXs003 cultivar showed higher + b* value, and is locally called “egg yolk cocoyam” because of its yellowish appearance. The higher + a* and + b* values of the NXs003 and NCe001 cultivars, may adversely affect starch quality. According to Moorthy (2002), the extraction and leaching of the colour pigments would result in discolouration of the starch granules. Also, calculated hue angle of the flesh of the cocoyam corms ranged between 80.42 (NXs003) and 86.19 (NCe002) (Table 2). Hue angle of the cocoyam corms were statistically similar.
Table 2.
Colour parameters of the flesh and flours of cocoyam corms
| Cultivars | Flesh (peeled) cocoyam corms | Cocoyam flours | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Hue angle | L* | a* | b* | Hue angle | |
| NCe001 | 72.08c ± 2.33 | 3.25ab ± 1.03 | 22.06bc ± 1.03 | 81.70a ± 2.03 | 72.99d ± 0.52 | 3.67b ± 0.00 | 21.12b ± 0.05 | 80.18d ± 0.02 |
| NCe002 | 78.93a ± 1.13 | 1.06b ± 0.68 | 17.65c ± 2.59 | 86.19a ± 3.08 | 84.97a ± 0.00 | 0.35e ± 0.00 | 4.44e ± 0.00 | 85.49a ± 0.00 |
| NCe003 | 78.69a ± 1.05 | 2.15ab ± 3.80 | 19.9bc ± 3.81 | 81.86a ± 15.70 | 80.62b ± 0.01 | 2.30c ± 0.08 | 20.90c ± 0.10 | 83.72c ± 0.26 |
| NXs001 | 76.85ab ± 2.59 | 3.50a ± 1.52 | 27.58ab ± 7.32 | 81.85a ± 4.53 | 76.84c ± 0.08 | 2.02d ± 0.04 | 19.78d ± 0.13 | 84.17b ± 0.16 |
| NXs003 | 76.47b ± 1.42 | 3.45a ± 0.81 | 35.80a ± 15.53 | 80.42a ± 11.59 | 69.35e ± 0.24 | 4.76a ± 0.06 | 23.48a ± 0.18 | 78.54e ± 0.08 |
Means in a column with the same letter are not significantly different (p > 0.05). Means of ten replicates
The L* (lightness) value of the flour varied significantly (p < 0.05) ranging from 69.35 (NXs003) to 84.97 (NCe002). The NXs003 flour which showed significantly lower L* value appeared darker than other cultivars, while NCe002 and NCe003 were visually lighter, and further confirmed by their higher L* value. The a* of cocoyam flours varied significantly from +0.35 (NCe002) to +4.76 (NXs003), while b* varied significantly from +4.44 (NCe002) to +23.48 (NXs003). The NCe002 appeared lighter than the other cocoyam flour samples while, NXs003 appeared darkest. Hence, the NCe002 cultivar is expected to have a better starch quality, because the possibility of discolouration due to leaching of colour pigments would be least. Calculated hue angle of the cocoyam flours varied significantly from 78.54 (NXs003) to 85.49 (NCe002).
Functional properties of cocoyam cultivars
Colocasia spp. showed lower pH values when compared to their Xanthosoma spp. pH values of all the samples fell within the neutral pH range but varied significantly from 6.56 (NCe001) to 7.59 (NXs003). The pH values of the cocoyam flours are within the low acid to neutral range (4–8) of which might support the growth and proliferation of microorganisms, particularly in the slurry form. Similar pH values (4.2–5.78) were observed some other cocoyam cultivars (Owuamanam et al. 2010). Therefore the extension of storage life may necessitate preservation including reduction of water activity, addition of chemical anti-microbial agents and anti-oxidants (Owuamanam et al. 2010). pH correlated negatively with gelling point (r = −0.85) and foam capacity (r = −0.63).
Water absorption capacity refers to the water retained by a food product following filtration and application of mild pressure of centrifugation. Table 3 shows that the water absorption capacity of the flour samples varied significantly (p < 0.05) between NXs003 (69 %) and NXs001 (32 %). Water absorption capacity of flour enables the processor to add more water during food preparation thereby improving handling characteristics. Higher water absorption of flour helps to maintain the freshness of bread, cakes and sausage. High water absorption capacities of the flours favour their use as a soup thickener. Water absorption capacity had a negative correlation with oil absorption capacity (r = −0.84), foam capacity (r = −0.72) and foam stability (r = −0.75). Oil absorption capacity showed a strong positive correlation with solubility in lipids (r = 0.94). Oil absorption capacity of the flour samples varied significantly between 22.0 % (NCe001) and 27.5 % (NCe003 and NXs001) (Table 3). Oil absorption capacity reflects the emulsifying capacity and the amount of oil that can be picked up by a sample during frying, a highly desirable characteristic in products such as mayonnaise (Table 3).
Table 3.
Functional properties of cocoyam flours
| Sample | pH | Water absorption capacity (%) | Oil absorption capacity (%) | Gelling point (°C) | Boiling point (°C) | Foam capacity (%) | Foam stability (%) | Bulk density (g/mL) | Swelling power (%) | Solubility (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| NCe001 | 6.56e ± 0.01 | 61.0ab ± 1.41 | 22.0c ± 1.41 | 72.5a ± 3.54 | 87.0a ± 1.41 | 13.43b ± 0.18 | 4.91c ± 0.04 | 0.95bc ± 0.02 | 4.81b ± 0.06 | 23.18a ± 0.02 |
| NCe002 | 6.92c ± 0.01 | 43.5ab ± 30.41 | 27.0ab ± 1.41 | 66.0b ± 2.83 | 81.5bc ± 0.71 | 18.11a ± 0.17 | 9.92b ± 0.10 | 0.94c ± 0.01 | 3.95c ± 0.03 | 14.63e ± 0.15 |
| NCe003 | 6.75d ± 0.01 | 44.0ab ± 0.00 | 27.5a ± 0.71 | 68.0ab ± 2.83 | 80.0c ± 1.41 | 18.23a ± 0.01 | 1.95d ± 0.04 | 0.99ab ± 0.01 | 3.18d ± 0.03 | 14.95d ± 0.08 |
| NXs001 | 7.45b ± 0.01 | 32.0b ± 1.41 | 27.5a ± 0.71 | 58.5c ± 0.71 | 88.5a ± 0.71 | 14.30b ± 0.02 | 14.72a ± 0.08 | 0.97abc ± 0.02 | 7.36a ± 0.01 | 16.68c ± 0.13 |
| NXs003 | 7.59a ± 0.01 | 69.0a ± 1.41 | 24.0bc ± 1.41 | 64.0bc ± 1.41 | 83.5b ± 0.71 | 7.19c ± 1.11 | 1.93d ± 0.06 | 1.01a ± 0.03 | 3.97c ± 0.03 | 18.50b ± 0.11 |
Means in a column with the same letter are not significantly different (p > 0.05); Means of three replicates
Gelling temperature of the flour samples varied significantly from 58.5 °C (NXs001) to 72.5 °C (NCe001) (Table 3). These gelling temperatures obtained from our work are in agreement with values reported by Owuamanam et al. (2010). Colocasia spp showed higher gelling point than the Xanthosoma spp. This indicated higher stability of Colocasia spp flours crystals upon heating. Gelling temperature is the temperature at which a food solution forms an observable thicker consistency when heat is applied (Sai-Ut et al. 2009). The relatively high gelling temperature of the flour samples could be due to the presence of other components in cocoyam flours such as proteins and lipids that would obstruct the swelling of granules and thus increase the amount of heat required to reach the final swelling. Similar observations have been reported previously (Jane et al. 1992). Boiling point of cocoyam flours varied significantly from 80 °C (NCe003) to 88.5 °C (NXs001). The NCe001 and NXs001 flours showed similar high boiling points, while NCe002 and NCe003 showed significantly lower values. Boiling point correlated strongly with swelling power (r = 0.89). Foam stability correlated positively swelling power (r = 0.82). When heat is applied to a liquid or solution, its temperature rises until the vapour pressure of the liquid equals the pressure of the surrounding gases. The temperature at which this occurs is known as the boiling point temperature.
Foam capacity varied significantly from 7.19 % (NXs003) to 18.23 % (NCe003), while foam stability ranged from 1.93 % (NXs003) to 14.72 % (NXs001). The NXs003 flour showed significantly lower foam capacity and stability (Table 3). However, foam capacity of cocoyam cultivars showed lower values than that earlier reported for wheat flour (60 %) and African breadfruit kernel flour (80 %) (Akubor and Badifu 2004). Foam capacity and stability show the level of adsorbed air on the air-liquid interface during whipping or bubbling, and by its ability to form a cohesive viscoelastic film by way of intermolecular interactions (Zhou et al. 2011). Also, foam stability is related to the amount of solubilized protein, and the amount of polar and non-polar lipids in a sample (Zhou et al. 2011).
Bulk density of cocoyam flours varied significantly from 0.94 g/mL (NCe002) to 1.01 g/mL (NXs003). The NXs003 showed significantly higher bulk density than other cultivars. Xanthosoma spp. showed similar bulk density values, however, only NXs001 showed similarity with the Colocasia spp. Bulk density of the flour samples, is higher than the 0.689 g/mL and 0.57–0.71 g/mL reported for taro flours by (Kaur et al. 2013) and Njintang et al. (2007). Bulk density is a measure of heaviness of solid samples, which is important for determining packaging requirements, material handling and application in the food industry. Flours with high bulk densities (>0.7 g/mL) are used as thickeners in food products (Akubor and Badifu 2004), therefore the cocoyam flours in this study could also be suitable as thickeners.
Solubility of the flour samples varied significantly between 14.63 % (NCe002) and 23.18 % (NCe001) (Table 3). Solubility is the ability of solids to dissolve or disperse in an aqueous solution (mostly water). Swelling power significantly varied from 3.18 (NCe003) to 7.36 (NXs001), however, NCe002 and NXs003 flours were similar. The NXs001 flour showed higher swelling power, because of its shorter cooking time when compared to other cultivars, showing that its starch granules have better tendency to swell and soften. Swelling power is a measure of hydration capacity, because the determination is a weight measure of swollen starch granules and their occluded water. Food eating quality is often connected with retention of water in the swollen starch granules.
Pasting properties of cocoyam flours
Peak viscosity of cocoyam cultivars varied significantly from 97.25 RVU (NXs003) to 134.33 RVU (NCe002) (Table 4). Peak viscosity showed a strong significant correlation with trough viscosity (r = 0.98), final viscosity (r = 0.91) and water absorption capacity (r = 0.90). However, negative correlation was observed between the peak viscosity and pasting temperature (r = −0.76) and gelling point (r = −0.78). Peak viscosity, which shows the maximum swelling of the starch granule prior to disintegration, has also been described as the equilibrium point between swelling and breakdown of the granules (Liu et al. 2006). Hoover (2001) stated that, granules with high peak viscosity have weaker cohesive forces within the granules than those with lower values and would disintegrate more easily. Falade and Okafor (2013) showed that the peak viscosity and other physicochemical parameters including amylose, amylopectin and granule sizes of the starches of the cocoyam cultivars varied significantly. Pasting properties are greatly influenced by plant source, starch content, interaction among the components, and testing conditions (Liu et al. 2006).
Table 5.
Correlation matrix between the pasting and functional parameters of cocoyam flours
| Name | Peak visc. | Trough visc. | Breakdown visc. | Final visc | Setback visc. | Peak time | Pasting temp | pH | WAC | OAC | Gelling point | Boiligpoint | Foam cap. | Foam stab. | Bulk density | Swelling pow. | Solubility |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak viscosity | 1.00 | ||||||||||||||||
| Trough viscosity | 0.98* | 1.00 | |||||||||||||||
| Breakdown | 0.43 | 0.23 | 1.00 | ||||||||||||||
| Final viscosity | 0.91* | 0.98* | 0.04 | 1.00 | |||||||||||||
| Setback | 0.83 | 0.93* | −0.13 | 0.99* | 1.00 | ||||||||||||
| Peak time | −0.43 | −0.23 | −0.97* | −0.06 | 0.10 | 1.00 | |||||||||||
| Pasting temp | −0.76 | −0.79 | −0.12 | −0.82 | −0.82 | 0.14 | 1.00 | ||||||||||
| pH | 0.34 | 0.42 | −0.24 | 0.54 | 0.55 | 0.03 | −0.35 | 1.00 | |||||||||
| WAC | −0.90* | −0.79 | −0.75 | −0.65 | −0.52 | 0.69 | 0.58 | 0.01 | 1.00 | ||||||||
| OAC | 0.72 | 0.55 | 0.88 | 0.42 | 0.26 | −0.93* | −0.42 | 0.21 | −0.84 | 1.00 | |||||||
| Gelling point | −0.78 | −0.79 | −0.16 | −0.82 | −0.77 | 0.30 | 0.61 | −0.85 | 0.51 | −0.60 | 1.00 | ||||||
| Boiling point | 0.40 | 0.58 | −0.60 | 0.69 | 0.78 | 0.65 | −0.49 | 0.24 | −0.08 | −0.35 | −0.29 | 1.00 | |||||
| Foam capaciy | 0.35 | 0.17 | 0.86 | −0.04 | −0.17 | −0.72 | −0.02 | −0.63 | −0.72 | 0.61 | 0.18 | −0.38 | 1.00 | ||||
| Foam stability | 0.86 | 0.88 | 0.23 | 0.82 | 0.76 | −0.18 | −0.42 | 0.25 | −0.75 | 0.45 | −0.63 | 0.55 | 0.29 | 1.00 | |||
| Bulk density | −0.21 | −0.18 | −0.23 | −0.05 | 0.00 | 0.04 | −0.26 | 0.59 | 0.39 | −0.01 | −0.28 | −0.20 | −0.62 | −0.55 | 1.00 | ||
| Swelling power | 0.77 | 0.89* | −0.21 | 0.94 | 0.97 | 0.24 | −0.70 | 0.40 | −0.48 | 0.12 | −0.62 | 0.89* | −0.13 | 0.82 | −0.22 | 1.00 | |
| Solubility | −0.46 | −0.28 | −0.87 | −0.15 | 0.01 | 0.96* | 0.13 | −0.23 | 0.63 | −0.94* | 0.50 | 0.60 | −0.53 | −0.24 | −0.08 | 0.17 | 1.00 |
Breakdown viscosity, a measure of the resistance to heat and shear, of the cocoyam cultivars varied significantly between 3.67 RVU (NCe001) and 24.71 RVU (NCe003). Since breakdown viscosity is an estimation of paste resistance to disintegration in response to heat and shear, lower breakdown viscosity showed greater resistance which would be expected of flours with lower peak viscosities. Breakdown viscosity had a strong positive correlation with oil absorption capacity (r = 0.89) and foam capacity (r = 0.86), however it showed negative correlation with peak time (r = −0.97) (Table 5). Setback, defined as the difference between the breakdown viscosity and the viscosity at 50 °C, determines the tendency of starch to retrogradation. The setback values differed significantly (p < 0.05) between 31.34 RVU (NCe003) and 103.00 RVU (NXs001). The higher the setback value, the lower the retrogradation during cooling and the lower the staling rate of the products made from the flour. Setback viscosity correlated positively with boiling point (r = 0.79), foam stability (r = 0.76) and swelling power (r = 0.94), while negative correlation was shown between the setback viscosity and the pasting temperature (r = −0.82) and gelling point (r = −0.77). Trough viscosity correlated positively with final viscosity (r = 0.98), setback viscosity (r = 0.93), swelling power (r = 0.89) and foam stability (r = 0.88), but correlated negatively with pasting temperature (r = −0.79), water absorption capacity (r = −0.79) and gelling point (r = −0.79).
Table 4.
Pasting properties of flours of cocoyam cultivars
| Sample | Peak viscosity (RVU) | Trough viscosity (RVU) | Breakdown viscosity (RVU) | Final viscosity (RVU) | Setback viscosity (RVU) | Peak time (min) | Pasting temperature (°C) |
|---|---|---|---|---|---|---|---|
| NCe001 | 98.96c ± 1.36 | 95.30b ± 0.88 | 3.67c ± 0.47 | 137.80b ± 3.36 | 42.50bc ± 2.47 | 6.34a ± 0.07 | 91.15ab ± 0.35 |
| NCe002 | 134.33b ± 22.63 | 112.13b ± 20.68 | 22.21a ± 1.94 | 145.42b ± 30.41 | 33.30bc ± 9.72 | 5.31d ± 0.14 | 92.53a ± 1.73 |
| NCe003 | 127.38bc ± 9.26 | 102.67b ± 5.89 | 24.71a ± 3.36 | 134.00b ± 5.42 | 31.34c ± 0.47 | 5.17d ± 0.12 | 89.58c ± 0.25 |
| NXs001 | 201.71a ± 10.78 | 186.75a ± 11.43 | 14.96b ± 0.65 | 289.75a ± 11.43 | 103.00a ± 0.00 | 5.63c ± 0.16 | 87.33c ± 0.60 |
| NXs003 | 97.25c ± 1.65 | 91.67b ± 2.71 | 3.92c ± 0.28 | 146.83b ± 6.01 | 44.28b ± 2.34 | 6.01b ± 0.08 | 91.10ab ± 1.03 |
Means in a column with the same letter are not significantly different (p > 0.05). Means of three replicates; Viscosity; unit: 1 RVU = 0.01 Pa.s
Final viscosity of the flours ranged from 134.00 RVU (NCe003) to 289.75 RVU (NXs001). This marked increase could be due to the alignment of the chains of amylose in the starch (Flores-Farias et al. 2000). Positive correlation occurred between the final viscosity and setback (r = 0.99), foam capacity (r = 0.82) and swelling power (r = 0.94). However, negative correlation was observed between the final viscosity and pasting temperature (r = −0.82) and gelling point (r = −0.82). Pasting temperatures of the flours varied significantly (p < 0.05) from 87.33 °C (NXs001) to 92.53 °C (NCe002). Pasting temperature depends on the size of the starch granules in the flour; small starch granules are more resistant to rupture and loss of molecular order (Zeng et al. 2013). Therefore, Colocasia spp., with higher pasting temperature was found to have smaller granular size.
The ability of starch to imbibe water and swell is primarily dependent on the pasting temperature. Pasting properties indicate the tendency to form paste, the higher the pasting temperature, the faster the tendency for paste to be formed. Starch granules swell and form paste by imbibing water in the presence of water and heat (Rincon and Padilla 2004). Peak time of the of cocoyam flour samples ranged from 5.17 min (NCe003) to 6.34 min (NCe001). However, the cultivar with higher peak time showed low peak viscosity (Table 4). This is to be expected as high peak times characterize low swelling starch granules in the flour. Peak time showed a positive correlation with water absorption capacity (r = 0.69) and boiling point (r = 0.65) but, a negative correlation with oil absorption capacity (r = −0.93) and foam capacity (r = −0.72).
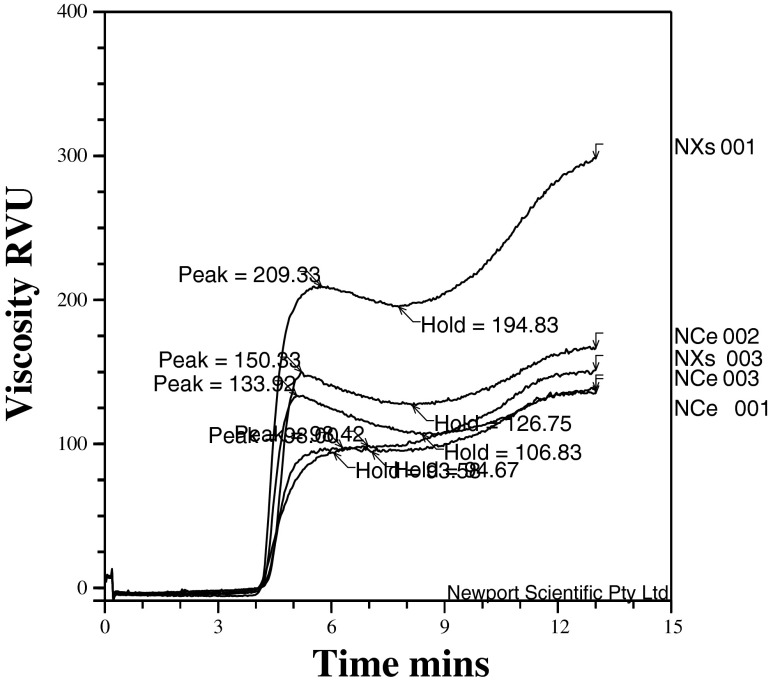
After 4 min of heating, the cocoyam flours showed patterns characteristics of rise in viscosity until peak, with subsequent decrease in viscosity at holding and thereafter a rise up to final viscosity (Fig. 1). Peak paste viscosity of the flours did not show a showed a sharp peak curve (Fig. 1). Instead, cocoyam flours showed patterns characteristics of steady rise in viscosity up to the peak and viscosity at holding, and thereafter a rise up to final viscosity. The Xanthosoma spp., particularly the NXs 001 showed greater steep curve than other cultivars. Pasting behaviours in starch-based systems have been classified as types A, B, C and D (Chen et al. 2003). After peak paste viscosity, the samples showed differences in their patterns of pasting properties (Fig. 1), which can be grouped to predict the cooking and other food utilization properties of the cultivars (Falade and Okafor, 2013). The Colocosia NCe 002- Ede ofe green and Xanthosoma (NXs 001- Ede ocha, NXs 003- okorokoro) showed slightly shear thinning (Type C) while the Colocosia (NCe 001- Coco India, , NCe 003-Ede ofe purple) cultivars showed shear thickening (Type D) behaviours. Type-C did not produce peaks because the viscosity remains constant or increased during cooking while type-D pastes did not show significant peaks. These researchers proposed that types C and D pasting classes were changeable by increasing the starch concentrations. However, pasting behaviours that result from interactions between starch and non-starch components (e.g. as in sorghum) or high-amylose starches may not change by increasing starch concentrations (Waramboi et al. 2011). Also, surface lipids and proteins, rehydration time, method of sample preparation (e.g. milling and particle size), presence of impurities, pH, type of cultivar, and presence of endogenous enzymes can affect starch swelling, pasting and gelatinization properties (Chen et al. 2003; Mahasukhonthachat et al. 2010). The broad grouping consists of samples with low pasting homogeneous starch granules with no apparent retrogradation, e.g. NCe001 and NXs003 (Table 4). Such products which would show relative low viscosity can be used as fillings because of their low retrogradation and paste clarity.
Fig. 1.
Rapid Visco-Analyser patterns of flours of Colocosia (NCe 001- Coco India, NCe 002- Ede ofe green, NCe 003-Ede ofe purple) and Xanthosoma (NXs 001- Ede ocha, NXs 003- okorokoro) cultivars of cocoyam
Conclusion
The Xanthosoma spp (NXs001 and Nxs003) with the higher physical, OAC, swelling power, peak viscosity and less steep pasting patterns showed better potential as a thickener than other cultivars. Generally, Xanthosoma and Colocasia spp flours showed slightly shear thinning and shear thickening properties respectively. However, the functional and physicochemical parameters of the cocoyam cultivars compares effectively well with other roots and tuber crops.
Acknowledgments
Authors are grateful to the University of Ibadan, Ibadan for the award of the Senate Research grant SRG/FT/2010/7A. Sincere gratitude goes to Dr Chukwu of the National Root Crop Research Institute (NRCRI), Umudike, Abia State for making available the cocoyam cultivars used for this research.
References
- Abbey BW, Ibeh CO. Functional properties of raw and heat processed cowpea (Vigna unguiculata walp) flour. Food Sci. 1988;53:1775–1777. doi: 10.1111/j.1365-2621.1988.tb07840.x. [DOI] [Google Scholar]
- Aboubakar NYN, Scher J, Mbofung CMF. Physicochemical, thermal properties and micro structure of six varieties of taro (Colocasia esculenta L. Schott) flours and starches. J Food Eng. 2008;86:294–305. doi: 10.1016/j.jfoodeng.2007.10.006. [DOI] [Google Scholar]
- Akpan EJ, Umoh IB. Effect of heat and tetracycline treatments on the food quality and acridity factors in cocoyam (Xanthosoma sagittifolium (L.) Schott) Pak J Nutr Biochem. 2004;3:240–243. doi: 10.3923/pjn.2004.240.243. [DOI] [Google Scholar]
- Akubor PI, Badifu GIO (2004) Chemical composition, functional properties and baking potential of African breadfruit kernel and wheat flour blends. Int J Food Sci Tech 39:223–229
- Aregheore E, Perera D. Dry matter, nutrient composition and palatability/acridity of eight exotic cultivars of cocoyams-taro (Colocasia esculenta) in Samoa. Plant Foods Hum Nutr. 2003;58:1–8. doi: 10.1023/B:QUAL.0000041164.22363.e1. [DOI] [Google Scholar]
- Beuchat LR, Cherry YP, Quinn MR (1975). Physiochemical. Latinomericanos de Nutricion, Caracas,\Venezuela. 140–149
- Chen Z, Schols HA, Voragen AGJ (2003) Starch granule size strongly determines starch noodle processing and noodle quality. J Food Sci 68:1584–1589
- Chinnasarn M, Manyasi R. Chemical and physical properties of taro flour and the application of restructured taro strip product. World Appl Sci J. 2010;9(6):600–604. [Google Scholar]
- Chukwu GO, Nwosu KI, Madu TU, Chinaka C, Okoye BC (2008). Development of Gocing storage method for Cocoyam. Retrieved September 17, 2010, from http://mpra.ub.uni-muenchen.de/17444/
- Crosbie GB. The relationship between swelling properties, 510 paste viscosity and boiled noodle quality in wheat flours. J Cereal Sci. 1991;13:145–150. doi: 10.1016/S0733-5210(09)80031-3. [DOI] [Google Scholar]
- Duncan DB (1955). Multiple range amd multiple F-tests. Biometrics 11:1–5
- Falade KO and Okafor CA (2013) Physicochemical properties of five cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) starches. Food Hydrocoll 30:173–181
- FAOSTAT 2012. Retrieved March 27, 2014, from http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor
- Flores-Farias F, Martinez-Bustos Y, Salinas-Moreno YK, Chang JS, Hernandez R, Rios E. Physicochemical and rheological characteristics of commercial nixtamalised Mexican corn flours for tortillas. J Sci Food Agric. 2000;80:657–664. doi: 10.1002/(SICI)1097-0010(20000501)80:6<657::AID-JSFA576>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Hoover R. Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym. 2001;45:253–267. doi: 10.1016/S0144-8617(00)00260-5. [DOI] [Google Scholar]
- Jane JL, Shen L, Lim S, Kasemsuwan T, Nip WK (1992) Physical and chemical studies of taro starches and flours. Cereal Chem 69:528–535
- Kaur M, Kaushal P, Sandhu KS. Studies on physicochemical and pasting properties of Taro (Colocasia esculenta L.) flour in comparison with a cereal, tuber and legume flour. J Food Sci Technol (January–February 2013) 2013;50(1):94–100. doi: 10.1007/s13197-010-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Donner E, Yin Y, Huang RL, Fan MZ. The physicochemical properties and in vitro digestibility of selected cereals, tubers and legumes grown in China. Food Chem. 2006;99:470–477. doi: 10.1016/j.foodchem.2005.08.008. [DOI] [Google Scholar]
- Mahasukhonthachat K, Sopade PA, Gidley MJ (2010) Kinetics of starch digestion in sorghum as affected by particle size. J Food Eng 96:18–28
- Mepba HD, Eboh L, Eko CB, Ukpabi UJ. Composition and pasting properties of starch from two cocoyam cultivars. J Food Qual. 2009;32:522–537. doi: 10.1111/j.1745-4557.2009.00257.x. [DOI] [Google Scholar]
- Moorthy SN. Physicochemical and functional properties of tropical tuber starches: a review. Starch-Starke. 2002;54:559–592. doi: 10.1002/1521-379X(200212)54:12<559::AID-STAR2222559>3.0.CO;2-F. [DOI] [Google Scholar]
- Mweta DE (2009) Physicochemical, functional and structural properties of native malawian Cocoyam and Sweetpotato starches. PhD. Thesis. Dept. of Chemistry and Plant Sciences, University of the Free State, Bloemfontein, South Africa. 5–32
- Narayana K, Narasinga-Rao MS. Functional properties of raw and heat processed winged bean flour. J Food Sci. 1982;47:1534–1538. doi: 10.1111/j.1365-2621.1982.tb04976.x. [DOI] [Google Scholar]
- Narayana K, Narasinga-Rao MS. Effect of partial proteolysis on the functional properties of winged pea (Psophocarpus tetragonolobus) flour. J Food Sci. 1984;49:944–947. doi: 10.1111/j.1365-2621.1984.tb13247.x. [DOI] [Google Scholar]
- Njintang YN, Mbofung CMF, Moates KG, Parker L, Fauld CB, Smith AC. Functional properties of five varieties of taro flour and relationship to creep recovery and sensory characteristics of achu (taro based paste) J Food Eng. 2007;82:114–120. doi: 10.1016/j.jfoodeng.2006.12.023. [DOI] [Google Scholar]
- Odebunmi EO, Oluwaniyi OO, Sanda AM, Kolade BO. Nutritional compositions of selected tubers and root crops used in Nigerian food preparations. Int J Chem. 2007;17(1):37–43. [Google Scholar]
- Opara LU (2002). Edible Aroids: Postharvest Operation Watts, B.M., G.L. Ylimaki, L.E. Jeffrey and L.G. in: AGST/FAO: Danilo, M. (Ed.). Massey University, Ekics,1989. Basic sensory methods for food New Zealand. 14–15.
- Owuamanam CI, Ihediohanma NC, Nwanekezi EC. Sorption isotherm, particle size, chemical and physical properties of cocoyam corm flours. Res. 2010;2(8):11–19. [Google Scholar]
- Rincon AM, Padilla FC. Physicochemical properties of breadfruit (Artocarpus altilis) starch from Margarita island, Venezuela. Arch Latinoam Nutr. 2004;8:95–97. [PubMed] [Google Scholar]
- Sai-Ut S, Ketnawa S, Chaiwut P, Rawdkuen S. Biochemical and functional properties of proteins from red kidney, navy and adzuki beans. Asian J Food Agro-Ind. 2009;2(4):493–504. [Google Scholar]
- Sosulski FW. The centrifuge methods for determining flour absorption in hard red spring wheat. Cereal Chem. 1962;39:344. [Google Scholar]
- Waramboi JG, Dennien S, Gidley MJ, Sopade PA. Characterisation of sweetpotato from Papua New Guinea and Australia: physicochemical, pasting and gelatinisation properties. Food Chem. 2011;126:1759–1770. doi: 10.1016/j.foodchem.2010.12.077. [DOI] [PubMed] [Google Scholar]
- Zeng F, Liu H, Liu G (2013) Physicochemical properties of starch extracted from Colocasia esculenta (L.) Schott (Bun-long taro) grown in Hunan, China. Starch/Stärke 65:1–7
- Zhou T., Zhang, T. Liu W. & Zhao, G. (2011). Physicochemical characteristics and functional properties of grape (Vitis vinifera L.) seeds protein. International Journal of Food Science and Technology 2011, 46, 635–641