Abstract
The aim of the present study was to evaluate the antioxidant activity of flavoured milk enriched with antioxidative whey protein hydrolysates (WPHs) by radical scavenging method. Whey protein concentrate (WPC) was hydrolyzed by using three commercial proteases; flavouzyme, alcalase and corolase PP and these WPHs were analyzed for degree of hydrolysis and antioxidant activity. The antioxidant activities of these WPHs were evaluated using ABTS method. Trolox equivalent antioxidant activity of all the hydrolysates i.e. flavourzyme (0.81 ± 0.04), alcalase (1.16 ± 0.05) and corolase (1.42 ± 0.12) was higher than the WPC (0.19 ± 0.01). Among these, whey protein hydrolysates prepared using corolase showed maximum antioxidant activity. Total 15 β-lactoglobulin, 1 α-lactoalbumin, and 6 β-casein derived peptide fragments were identified in the WPHs by LC-MS/MS. Due to their size and characteristic amino acid composition, all the identified peptides may contribute for the antioxidant activity. The strawberry and chocolate flavoured milk was supplemented with WPC and WPHs and 2 % addition has shown increase in antioxidant activity upto 42 %. The result suggests that WPH could be used as natural biofunctional ingredients in enhancing antioxidant properties of food products.
Keywords: Whey protein hydrolysates, Antioxidant activity, Flavourzyme, Alcalase, Corolase
Introduction
Oxidation metabolism is essential for the survival of cells but it generates free radicals and other reactive oxygen species (ROS) as a side effect, which can cause oxidative damage. The body has its own defense system against ROS, oxidative stress occurs when ROS overload the body’s antioxidant defense mechanism which may be a significant causative factor of several lifestyle mediated diseases (Hernandez-Ledesma et al. 2005a). Enhancement of the body’s antioxidant defense mechanism through dietary supplementation would seem to be a practical approach to reduce the level of oxidative stress. Dairy products and their fractions have been found to be antioxidative, e.g. milk, skim milk, whey, casein and lactoferrin (Steijins and Van 2000; Tong et al.2000; Cervato et al. 1999; Colbert and Decker 1991; Taylor and Richardson 1980). Whey protein, a by-product recognized as valuable food ingredient with important nutritional and functional properties is gaining acceptance as functional food ingredient. Hydrolysis of whey protein is known to release bioactive peptides that can exihibit a number of physiological properties and enable them to exhibit antioxidative, antihypertensive, antimicrobial activity, opioid activity and antithrombotic activity (Meisel and Schlimme 1996; Korhonen and Pihlanto-Leppala 1998; Clare and Swaisgood 2000; Pihlanto Leppala 2001). WPHs had shown a broad range of antioxidant activity in an iron-catalysed liposome oxidation system (Pena-Ramos and Xiong 2001) or a copper-catalysed liposome emulsion (Colbert and Decker 1991), depending on the proteases used. Adriena et al. (2010) reported that on hydrolysis with microbial proteases (alcalase, flavourzyme, protamex and neutrase) the antioxidant activity of whey protein increased from 7–19.8 to 40–54.2 %. There are concerns about the potential health effects of synthetic antioxidants and therefore, the demand for natural antioxidants has recently increased (Park et al.2001). For this reason, there is a growing amount of research focused on the development of natural antioxidants derived from food ingredients that could retard lipid peroxidation.
Hernandez-Ledesma et al. (2005b) identified several peptides in the β-lactoglobulin A hydrolysate by corolase PP. One of the peptides, Trp–Tyr–Ser–Leu–Ala–Met–Ala–Ala–Ser–Asp–Ile, possessed higher radical scavenging activity than BHA. Whey protein ingredients have high solubility over a wide pH range. With low level of fat and lactose in these products make them ideal ingredient for formulating sugar free, low fat or no fat dairy products. Whey proteins contribute to a smooth mouth feel and a mild dairy flavour that also blend well with the popular flavours like vanilla, chocolate and strawberry. Milk beverages are considered as convenient, highly nutritional and digestible health foods associated with high protein content as well as a high content of calcium and phosphorus. Health benefits of flavoured milk can be enhanced by addition of WPHs having substantial antioxidant activity, hence the present study was designed to evaluate the effect of enrichment of WPHs on antioxidant activity of flavoured milk.
Materials and methods
Whey Protein Concentrate (WPC-70) was procured from Modern Dairies Pvt. Ltd. (Karnal, India). The commercial enzymes alcalase and flavourzyme were obtained from Novozyme (Bangalore, India) and corolase PP was collected from AB Enzymes (an ABF ingredients company, Germany). Milk was collected from Experimental Dairy, NDRI (Karnal, India). Artificial strawberry flavour and chocolate syrup was from Harshey, USA. 2,2′-Azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS) and Trolox (6- hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of whey protein hydrolysate
5 % WPC solution was preheated to 68 ºC for 10 min and then hydrolysed at the optimal conditions (Table 1). The hydrolysis process was terminated by heating to 90 ºC for 10 min. The resultant hydrolysate was subjected to centrifugation at 10,000 rpm for 30 min and the supernatant was collected and stored at -20 ºC in airtight container. Each hydrolysate was analysed for protein content by Kjeldahl method (AOAC 2005) and degree of hydrolysis by the method of Adler-Nissen (1986).
Table 1.
Conditions for hydrolysis
| Enzyme | Enzyme/substrate ratio | pH | Time (h) | Temp.(˚C) |
|---|---|---|---|---|
| Alcalase | 1/100 | 8.5 | 5 | 65 |
| Corolase PP | 1/50 | 7.5 | 7 | 45 |
| Flavourzyme | 1/100 | 6.5 | 8 | 50 |
Antioxidant activity of whey protein hydrolysate
Antioxidant activity of samples was measured using 2, 2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) spectrophotometric decolourization method described by Re et al. (1999). A stock solution of ABTS was prepared by dissolving it in deionized water and potassium persulphate added to a concentration of 140 mM. The mixture was stored at ambient temperature for 12–16 h. The solution of ABTS.+ was diluted with phosphate buffer saline, pH 7.4 to the final absorbance of 0.70 ± 0.02 at 734 nm (Specord 200 spectrophotometer, Analytik Jena AG, Germany). 30 μl of sample was added to 3 ml of diluted ABTS.+ solution. This mixture was shaken for 10s at 30 °C, and the decrease in the absorbance was recorded at 734 nm for 10 min. Appropriate solvent blanks were run in each case assay. Trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acid) was used as a reference standard. The percentage inhibition was calculated and plotted as a function of concentration of antioxidants and Trolox for the standard reference data. The antioxidant activity of sample was expressed in terms μM Trolox/mg of protein.
Identification of peptides from whey protein hydrolysates
Enrichment of the peptides with low molecular weight were done by ultrafiltrating the WPH using centrifugal ultrafiltration device (PALL Life sciences, USA) in which the hydrolysate was filtered through 3,000 Dalton molecular weight cut-off membranes. Permeate was collected and dried in lyophilizer (Labtech Istruments, Germany). The freeze dried samples were subjected to LC-MS/MS for peptide identification at Proteomics International Pvt. Ltd, Western Australia through Technoconcept Pvt Ltd., New Delhi, India. Sample were analyzed by electronspray ionization mass spectrometry using the Ultimate 3,000 nano HPLC system (Dionex) coupled to a 4,000 Q TRAP mass spectrometer (Applied Biosystems). WPH peptides were loaded onto a C18 PepMap 100, 3 mm column (LC Packings) and separated with a linear gradient of water/acetonitrile/0.1 % formic acid (v/v). Spectra were analyzed to identify proteins of interest using Mascot sequence matching software (Matrix Science) with taxonomy set at all entries.
Preparation of flavoured milk
Strawberry and chocolate flavoured milk was prepared by the method of De (2008) with addition of 1 to 2 % WPC/WPHs before pasteurization. The flavoured milk prepared with alcalase WPH (WPHA) and corolase WPH (WPHC) was bitter than that of flavourzyme WPH (WPHF). To mask the bitterness of alcalase and corolase hydrolysates the flavour concentration was optimized.
Antioxidant activity of flavoured milk
Antioxidant activity of samples was measured using ABTS by method of Re et al. (1999) as described for WPH with slight modifications, using acetate buffer, pH 5.5. The antioxidant activity of sample was expressed in terms of mM Trolox/l of sample.
Sensory evaluation
The sensory evaluation of flavoured milk using 9 point hedonic scale was carried out by a panel of trained judges (n = 9). The samples were evaluated for colour and appearance, mouth feel, sweetness, flavour and overall acceptability.
Statistical analysis
All the experiments were carried out in triplicates. The results were expressed as mean ± S.E. of mean. Significance was tested by employing analysis of variance and comparison between means made by critical difference (C.D.) value at 5 % level of significance.
Results and discussion
Preparation of whey protein hydrolysate
The protein contents in these hydrolysates are related to the degree of hydrolysis because during preparation of WPHs the unhydrolysed whey proteins were precipitated by heating as shown in Table 2.
Table 2.
Degree of hydrolysis with protein content
| WPH | DH (%) | Protein % |
|---|---|---|
| WPHF | 8.93 ± 1.2 | 53.52 ± 2.41 |
| WPHA | 19.21 ± 1.0 | 56.03 ± 2.41 |
| WPHc | 23.69 ± 1.0 | 59.8 ± 1.42 |
The value expressed as means ± SEM, n = 3
Antioxidant activity of WPHs
The antioxidant activity of WPHs was much higher than the WPC which could be attributed to the exposure of reactive groups on hydrolysis (Table 3). Amongst all WPHs, WPHC showed the maximum antioxidant activity. This result could be well correlated with result obtained by Hernandez-Ledesma et al. (2005a) who found that antioxidant activity of corolase WPH was highest amongst different commercial enzymes pepsin, trypsin, hymotrypsin, thermolysin and corolase PP to obtain antioxidant hydrolysates from α-la to β-lg. Naik et al. (2013) also found increased antioxidant activity of whey protein after hydrolysis. Yadav et al. (2011) found that WPH prepared using flavourzyme and heat denatured whey proteins possess good antioxidant activity both in vivo and in vitro, the elevation of the antioxidant enzyme activity in mice fed with WPHs was more as compared to the denatured whey protein. The hydrolysis of whey protein isolate by alcalase enhanced the antioxidant activity in liposome model systems (Xinyan et al.2010). Athira et al. (2013) reported the effect of WPH against paracetamol induced oxidative stress by reducing the level of oxidative biomarkers and increasing the activity of antioxidative enzymes in mice. Similar results were obtained by Castro and Sato (2014) who found an increase in antioxidant activity up to 205.3 % in bovine whey protein hydrolysates compared to the intact proteins.
Table 3.
Antioxidant activities of whey protein hydrolysates
| Sample | TEAC |
|---|---|
| (μM Trolox/mg of protein) | |
| WPC | 0.19 ± 0.01 |
| WPHF | 0.81 ± 0.04 |
| WPHA | 1.16 ± 0.05 |
| WPHC | 1.42 ± 0.12 |
The value expressed as means ± SEM, n = 3
Identification of peptides from whey protein hydrolysates
With the aim of identifying the peptides which are responsible for the biofunctional properties derived from WPHs, these WPHs were subjected to LC-MS/MS. Different whey protein and casein fractions have been identified from WPHs and presented in Table 4, 5, 6. Five peptides (β-lg(92–100), β-lg(9–14), β-lg(84–91), and β-lg(125–135), α-la(99–108)), eleven peptides (β-lg(146–150), β-lg(75–82), β-lg(24–32), β-lg123-131), β-lg(122–131), β-lg(123–134), β-lg(122–134), β-CN(170–175), β-CN(176–182), β-CN(183–190), and β-CN(166–175) and six peptides (β-lg(50–56), β-lg(43–49), β-lg(43–51), β-lg(123–135), β-CN(170–175) and β-CN(177–184)) from WPHF, WPHA and WPHC respectively. Some of these peptides or their fragments have been identified as antioxidant peptides by previous researchers such as β-lg(95–101), β-lg(87–91), β-lg(145–149), β-lg(72–79), β-lg(19–29), β-lg(42–46), α-la(101–104)), β-CN(169–176), β-CN(177–183), and β-CN(177–183) as mentioned in Table 4, 5, 6.
Table 4.
Identification of peptides from flavourzyme whey protein hydrolysate
| Peptide Identified from Whey protein hydrolysates | Peptides reported in literature | |||||
|---|---|---|---|---|---|---|
| Protein fragment | Peptide Sequence | Mass (m/z) | Protein fragment | Peptide Sequence | Activity | References |
| β-lg f(92–100) | VLVLDTDYK | 1065.65 | LDTDYKK | β-lg f(95–101) | Antioxidant activity | Contreras del Mar et al. (2011) |
| β- lg f(9–14) | GLDIQK | 672.97 | – | – | – | – |
| β- lg f(84–91) | IDALNEK | 915.31 | LNENK | β-lg f(87–91) | Antioxidant activity | Contreras del Mar et al. (2011) |
| β- lg f(125–135) | TPEVDDEALEK | 1245.0 | – | – | – | – |
| α- la f(99–108) | VGINYWLAHK | 1200.7 | INYW | α-la f(101–104) | Antioxidant activity | Sadat et al. (2011) |
Table 5.
Identification of peptides from alcalase whey protein hydrolysate
| Peptide Identified from Whey protein hydrolysates | Peptides reported in literature | |||||
|---|---|---|---|---|---|---|
| Protein fragment | Peptide sequence | Mass (m/z) | Protein fragment | Peptide sequence | Activity | References |
| β-lg f(146–150) | HIRLS | 624.4 | β-lg f(145–149) | MHIRL | Antioxidant activity | Hernandez-Ledesma et al. (2005a) |
| β-lg f(75–82) | KTKIPAVF | 902.9 | β-lg f(72–79) | IAEKTKIP | Antioxidant activity | Sadat et al. (2011) |
| β-lg f(24–32) | MAASDISLL | 935.5 | β-lg f(19–29) | WYSLAMAASDI | Antioxidant activity | Hernandez-Ledesma et al. (2005a) |
| β-lg f(123–131) | VRTPEVDDE | 1058.7 | – | – | – | – |
| β-lg f(122–131) | LVRTPEVDDE | 1171.8 | – | – | – | – |
| β-lg f(123–134) | VRTPEVDDEALE | 1371.9 | – | – | – | – |
| β-lg f(122–134) | LVRTPEVDDEALE | 1484.9 | – | – | – | – |
| β-CN f(170–175) |
VLPVPQ | 651.7 | β-CN f(169–176) | KVLPVPQK | Antioxidant activity | Rival et al. (2001) |
| β-CN f(176–182) | KAVPYPQ | 801.5 | β-CN f(177–183) | AVPYPQR | Antioxidant activity | Rival et al. (2001) |
| β-CN f(183–190) | RDMPIQAF | 992.8 | β-CN f(177–183) | AVPYPQR | Antioxidant activity | Rival et al. (2001) |
| β-CN f(166–175) | SQSKVLPVPQ | 1081.9 | β-CN f(169–176) | KVLPVPQK | Antioxidant activity | Rival et al. (2001) |
Table 6.
Identification of peptides from corolase whey protein hydrolysate
| Peptide Identified from Whey protein hydrolysates | Peptides reported in literature | |||||
|---|---|---|---|---|---|---|
| Protein fragment | Peptide sequence | Mass (m/z) | Protein fragment | Peptide Sequence | Activity | References |
| β-lg f(50–56) | PEGDLEI | 771.4 | – | – | – | – |
| β-lg f(43–49) | VEELKPT | 815.2 | β-lg f(42–46) | YVEEL | Antioxidant activity | Hernandez-Ledesma et al. (2005a) |
| β-lg f(43–51) | VEELKPTPE | 1040.6 | β-lg f(42–46) | YVEEL | Antioxidant activity | Hernandez-Ledesma et al. (2005a) |
| β-lg f(123–135) | TPEVDDEALEK | 1244.6 | – | – | – | – |
| β-CN f(170–175) | VLPVPQ | 651.4 | β-CN f(169–176) | KVLPVPQK | Antioxidant activity | Rival et al. (2001) |
| β-CN f(177–184) | AVPYPQ | 673.4 | β-CN f(177–183) | AVPYPQR | Antioxidant activity | Rival et al. (2001) |
The radical scavenging mechanisms of the peptides are related to the prevalence of hydrophobic amino acids, such as Ala (A), Pro (P), Val (V), Ile (I), Leu (L), Phe (F), Trp (W), Tyr (Y) and Met (M) (Alcaide-Hidalgo et al. 2000; Pena-Ramos et al. 2004; Cheison et al. 2007; Ren et al. 2008). As shown in Table 4, 5, 6 one or more of these amino acids are contained in all detected peptides. Specifically, Tyr (Y) and Trp (W) have been described by different authors as main responsible of antioxidant activity of peptides in the ORAC-FL model (Elias et al. 2006; Hernandez-Ledesma et al. 2005a). Tyr, Trp, Met, Lys, Cys and His are the examples of amino acids that contribute antioxidant activity (Wang and De Mejia 2005). Furthermore, Amino acids with aromatic residues can donate proton to electron deficient radicals. This property improves the radical scavenging property of the amino acid residues (Rajapakse et al. 2005). Chen et al. (1995) also reported that His and Pro play an important role in the antioxidant activity of peptides. Gupta et al. (2010) identified two antioxidant peptides fragments of β-casein f (98–105) and αS1-casein f (80–90) from cheddar cheese made with adjunct culture Lactobacillus casei ssp. casei NCDC 300. The antioxidant activity of these peptides has been related the Histidine and Proline in αS1-casein derived peptide and Methionine and proline in β-casein derived peptides. Other workers also indicated that antioxidant peptides isolated from κ-caseins (κ-CN f23-28), have four tyrosine and two proline residues (Hernandez-Ledesma et al. 2005a). Thus due to their size and characteristic amino acid composition, all the identified peptides from WPHs may contribute for the antioxidant activity.
Antioxidant property of flavoured milk
Antioxidant activity of chocolate flavor (CH) was much higher than strawberry (SB) and which could be due to polyphenol content in chocolate flavour. Antioxidant activity of flavoured milk was estimated for all groups. The antioxidant activity increased after addition of 1 % WPC, flavourzyme, alcalase and corolase WPHs upto 4.6, 12.5, 20.3 and 35.53 % respectively in strawberry flavoured milk and 3.33, 12.22, 23.33 and 26.67 % respectively in chocolate flavoured milk as compared to the control (Fig. 1).
Fig. 1.
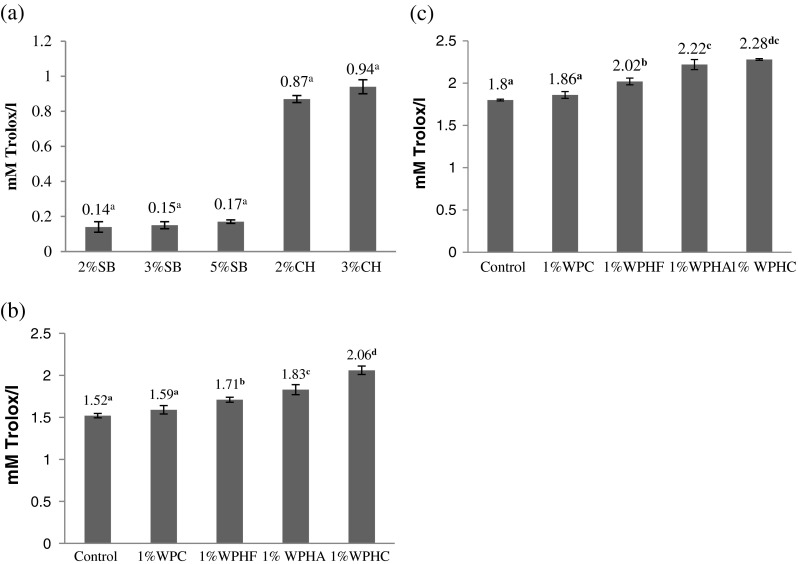
Antioxidant activity of (a) Flavour (b) strawberry flavoured milk with 1%WPHs (c) chocolate flavoured milk with 1%WPHs. SB, strawberry flavour; CH, chocolate flavour; WPHF, flavourzyme whey protein hydrolysate; WPHA, alcalase whey protein hydrolysate; WPHC, corolase whey protein hydrolysate. a,b,cMean values with unlike superscript letters within a column were significantly different (p < 0.05)
The antioxidant activity of beverages after addition of 2 % WPC, flavourzyme, alcalase and corolase WPHs were increased upto 12.5, 21.7, 37.5, 42.10 % respectively in strawberry flavoured milk and 10.0, 14.4, 43.3 and 42.7 % respectively in chocolate flavoured milk when compared with their respective control (Fig. 2). Kumari et al. (2013) also found an increase in antioxidant activity after addition of 1 % flavourzyme WPH and alcalase WPH upto 14.73 and 29.6 % in chocolate ice cream.
Fig. 2.
Antioxidant activity of (a) strawberry flavoured milk with 2 % WPHs (b) chocolate flavoured milk with 2 % WPHs. SB, strawberry flavour; CH, chocolate flavour; WPHF, flavourzyme whey protein hydrolysate; WPHA, alcalase whey protein hydrolysate; WPHC, corolase whey protein hydrolysate. a,b,cMean values with unlike superscript letters within a column were significantly different (p < 0.05)
When antioxidant activity [measured as Trolox equivalent antioxidant capacity (TEAC)] of these beverages were compared with the corresponding data of other beverages (Pellagrani et al.2003), the antioxidant activities of the flavoured milk added with alcalase and corolase WPHs are similar to that of apricot juice (2.48 mM of trolox/l) and peach juice (2.51 mM of trolox/l) while the antioxidant activities of the flavoured milk added with flavourzyme WPH are similar to the lemon juice (2.21 mM of trolox/l), apple juice (1.83 mM of trolox/l) and pineapple juice (1.50 mM of trolox/l).
Sensory evaluation
Concentration of WPHs was optimized on the basis of antioxidant activity. As the 2 % addition of WPHs has significantly more antioxidant activity than 1 % addition of WPHs, 2 % addition was selected for final product. The alcalase and corolase WPHs added flavoured milk was slightly bitter than other groups. The bitter taste of these beverages may be due to the presence of bitter peptides which may generate during hydrolysis. The lower bitterness in flavouzyme WPH could be due to the endo- and exopeptidase enzyme mixture which minimizes bitterness in the hydrolyzed products (Liaset et al. 2000). The optimized concentration of flavour was sufficient to mask the bitterness.
The sensory scores of WPHs added strawberry and chocolate flavoured milk was similar to that of control (Table 7, 8). Similar results were obtained by Kumari et al. (2013) who found a comparable sensory score after addition of 1 % flavourzyme and alcalase WPHs in ice cream.
Table 7.
Sensory evaluation of strawberry flavoured milk supplemented with WPC/WPHs at the rate of 2 %
| Parameters | Control | 2 %WPC | 2 % WPHF | 2 % WPHA | 2 % WPHC |
|---|---|---|---|---|---|
| Flavour addition | 2 % | 2 % | 2 % | 5 % | 5 % |
| Colour & appearance | 6.2 ± 0.49a | 6.6 ± 0.25a | 6.4 ± 0.25a | 7.4 ± 0.58b | 7.2 ± 0.40b |
| Mouthfeel | 6.6 ± 0.25a | 6.6 ± 0.25a | 6.6 ± 0.51a | 7.0 ± 0.49a | 6.8 ± 0.32a |
| Sweetness | 6.4 ± 0.25a | 6.8 ± 0.37a | 7.1 ± 0.25b | 7.1 ± 0.49b | 6.8 ± 0.25ab |
| Flavour | 6.4 ± 0.51a | 7.2 ± 0.20b | 6.8 ± 0.37ab | 6.8 ± 0.45ab | 6.5 ± 0.37a |
| Overall acceptibility | 7.2 ± 0.37a | 6.8 ± 0.20a | 6.6 ± 0.33ba | 7.1 ± 0.49a | 6.2 ± 0.25bc |
CD = 0.45
The values expressed as means ± SEM (n = 27) and values within columns (a, b, c) with different superscript letters significantly different (P < 0.05)
Table 8.
Sensory evaluation of chocolate flavoured milk supplemented with WPC/WPHs at the rate of 2 %
| Parameters | Control | 2 %WPC | 2 % WPHF | 2 % WPHA | 2 % WPHC |
|---|---|---|---|---|---|
| Flavour addition | 2 % | 2 % | 2 % | 3 % | 3 % |
| Colour & appearance | 7.2 ± 0.20a | 7.4 ± 0.40a | 7.2 ± 0.20a | 7.4 ± 0.25a | 7.4 ± 0.25a |
| Mouthfeel | 7.5 ± 0.45a | 7.0 ± 0.32b | 6.6 ± 0.25c | 7.2 ± 0.20db | 7.0 ± 0.32db |
| Sweetness | 7.2 ± 0.20a | 6.8 ± 0.37b | 6.4 ± 0.25c | 7.6 ± 0.25d | 7.4 ± 0.25da |
| Flavour | 6.8 ± 0.58ac | 6.6 ± 0.51a | 6.6 ± 0.25a | 7.4 ± 0.25b | 7.0 ± 0.32c |
| Overall acceptibility | 7.8 ± 0.20a | 7.2 ± 0.20b | 7.0 ± 0.32b | 7.6 ± 0.40c | 7.6 ± 0.40c |
CD = 0.29
The values expressed as means ± SEM (n = 27) and values within columns (a, b, c) with different superscript letters significantly different (P < 0.05)
Conclusion
The result revealed that WPH show a potent radical scavenging activity, which is attributed by several peptides contained in it. There is increase in antioxidant activity of WPH as compared to WPC which was sustained after incorporation into flavoured milk. Corolase WPH displayed the highest antioxidant activity amongst all and also offered higher degree of hydrolysis. Hence WPHs can be suggested for considering as an effective antioxidant in various food systems.
References
- Adler-Nissen J. In enzymatic hydrolysis of food proteins. London: Elsevier Applied Science Publishers; 1986. pp. 122–124. [Google Scholar]
- Adriena DK, Anne P, Pertti M, Ladislav C, Hannu JTK. Antioxidant properties of whey protein hydrolysates as measured by three methods. Eur Food Res Technol. 2010;230:865–874. doi: 10.1007/s00217-010-1231-9. [DOI] [Google Scholar]
- Alcaide-Hidalgo JM, Pueyo E, Polo MC, Martinez-Rodriguez AJ. Bioactive peptides released from Saccharomyces cerevisiae under accelerated autolysis in a wine model system. J Food Sci. 2000;72:M276–M279. doi: 10.1111/j.1750-3841.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. Association of official analytical chemists. 18. Maryland: North fredrick avenue gaithersburg; 2005. [Google Scholar]
- Athira S, Mann B, Sharma R, Kumar R. Ameliorative potential of whey protein hydrolysate against paracetamol-induced oxidative stress. J Dairy Sci. 2013;96(3):1431–1437. doi: 10.3168/jds.2012-6080. [DOI] [PubMed] [Google Scholar]
- Castro RJS, Sato H. Comparison and synergistic effects of intact proteins and their hydrolysates on the functional properties andantioxidant activities in a simultaneous process of enzymatic hydrolysis. Food Bioprod Process. 2014;92(1):80–88. doi: 10.1016/j.fbp.2013.07.004. [DOI] [Google Scholar]
- Cervato G, Cazzola R, Cestaro B. Studies on the antioxidant activity of milk casein. Int J Food Sci Nutr. 1999;50:291–296. doi: 10.1080/096374899101175. [DOI] [PubMed] [Google Scholar]
- Cheison SC, Wang Z, Xu SY. Preparation of whey protein hydrolysates using a single- and two stage enzymatic membrane reactor and their immunological and antioxidant properties: characterization by multivariate data analysis. J Agric Food Chem. 2007;55:3896–3904. doi: 10.1021/jf062936i. [DOI] [PubMed] [Google Scholar]
- Chen HM, Muramoto K, Yamauchi F. Structural analysis of antioxidative peptides from soybean-conglycinin. J Agric Food Chem. 1995;43:574–578. doi: 10.1021/jf00051a004. [DOI] [Google Scholar]
- Clare DA, Swaisgood HE. Bioactive milk peptides: a prospectus. J Dairy Sci. 2000;83:1187–1195. doi: 10.3168/jds.S0022-0302(00)74983-6. [DOI] [PubMed] [Google Scholar]
- Colbert LB, Decker EA. Antioxidant activity of an ultrafiltration permeate from acid whey. J Food Sci. 1991;56:1248–1250. doi: 10.1111/j.1365-2621.1991.tb04744.x. [DOI] [Google Scholar]
- Contreras del Mar M, Hernandez-Ledesma B, Amigo L, Martin-Alvarez PJ, Recio I. Production of of Antioxidant hydrolysates from whey protein hydrolysate with thermolysin: optimization by response surface methodology. LWT Food Sci Technol. 2011;44:9–15. doi: 10.1016/j.lwt.2010.06.017. [DOI] [Google Scholar]
- De S. Special milk. In Outlines of dairy technology. India: Oxford University Press; 2008. pp. 98–99. [Google Scholar]
- Elias RJ, Bridgewater JD, Vachet RW, Waraho T, McClements DJ, Decker EA. Antioxidant mechanisms of enzymatic hydrolysates of β-lactoglobulin in food lipid dispersions. J Agric Food Chem. 2006;54:9565–9572. doi: 10.1021/jf062178w. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mann B, Kumar R, Sagwan RB. Identification of antioxidant peptides in cheddar cheese made with adjunct culture lactobacillus casei ssp. casei 300. Milchwissenschaft. 2010;65(4):396–399. [Google Scholar]
- Hernandez-Ledesma B, Davalos A, Bartolome B, Amigo L. Preparation of antioxidant enzymatic hydrolysates from alphalactalbumin and beta-actoglobulin. Identification of active peptides by HPLC–MS/MS. J Agric Food Chem. 2005;53:588–593. doi: 10.1021/jf048626m. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ledesma B, Beatriz M, Lourdes A, Mercedes R, Isidra R. Identification of antioxidant and ACE-inhibitorypeptides in fermented milk. J Sci Food Agric. 2005;85:1041–1048. doi: 10.1002/jsfa.2063. [DOI] [Google Scholar]
- Korhonen H, Pihlanto-Leppala A. The functional and biological properties of whey proteins: prospects for the development of functional foods. AgrIC Food Sci Finl. 1998;7:283–296. [Google Scholar]
- Kumari A, Mann B, Prerna S, Singh RRB, Kumar R, Sharma R. Assesment of antioxidant and physico-chemical characteristics of ice cream added with whey protein hydrolysates. Indian J Dairy Sci. 2013;66(5):382–387. [Google Scholar]
- Liaset B, Lied E, Espe M. Enzymatic hydrolysis of by-products from the fish-filleting industry; chemical characterisation and nutritional evaluation. J Sci Food Agric. 2000;80:581–589. doi: 10.1002/(SICI)1097-0010(200004)80:5<581::AID-JSFA578>3.0.CO;2-I. [DOI] [Google Scholar]
- Meisel H, Schlimme E. Bioactive peptides derived from milk proteins: ingredients for functional foods. Kiel Milchw Forsch. 1996;48:343–357. [Google Scholar]
- Naik L, Mann B, Bajaj R, Sangwan RB, Sharma R. Process optimization for the production of bio-functional whey protein hydrolysates: adopting response surface methodology. Int J Pept Res Ther. 2013;19:231–237. doi: 10.1007/s10989-012-9340-x. [DOI] [Google Scholar]
- Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK. Purification and characterization of antioxidative peptides from lecithin-free egg yolk protein. J Am Oil Chem Soc. 2001;78:651–656. doi: 10.1007/s11746-001-0321-0. [DOI] [Google Scholar]
- Pellagrani N, Serafini M, Colombi B, Delrio D, Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in italy assayed by three different methods. J Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Pena-Ramos EA, Xiong Y. Antioxidative activity of whey protein hydrolysates in liposomal system. J Dairy Sci. 2001;84:2577–2583. doi: 10.3168/jds.S0022-0302(01)74711-X. [DOI] [PubMed] [Google Scholar]
- Pena-Ramos EA, Xiong YL, Arteaga GE. Fractionation and characterization for antioxidant activity of hydrolysed whey protein. J Sci Food Agric. 2004;84:1908–1918. doi: 10.1002/jsfa.1886. [DOI] [Google Scholar]
- Pihlanto Leppala A. Bioactive peptides derived from bovine whey proteins: opioid and ACE-inhibitory peptides. Trends Food Sci Tech. 2001;11:347–356. doi: 10.1016/S0924-2244(01)00003-6. [DOI] [Google Scholar]
- Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- Re R, Pellegrani N, Pannula A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolouration assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008;108:727–736. doi: 10.1016/j.foodchem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Rival SG, Fornaroli S, Boeriu CG, Wichers HJ. Caseins and casein hydrolysates. 1. Lipoxygenase inhibitory properties. J Agr Food Chem. 2001;49:287–294. doi: 10.1021/jf000392t. [DOI] [PubMed] [Google Scholar]
- Sadat L, Cakir-Kiefer C, Marie-Andree M, Gaillard J, Girardet J, Miclo L. Isolation and identification of antioxidative peptides from bovine α-lactalbumin. Int Dairy J. 2011;21:214–221. doi: 10.1016/j.idairyj.2010.11.011. [DOI] [Google Scholar]
- Steijins JM, Van HAC. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br J Nutr. 2000;84:S11–S17. doi: 10.1017/s0007114500002191. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Richardson T. Antioxidant oxidant of skim milk: effect of heat and resultant sulfhydryl groups. J Dairy Sci. 1980;63:1783–1795. doi: 10.3168/jds.S0022-0302(80)83140-7. [DOI] [PubMed] [Google Scholar]
- Tong LM, Sasaki S, McClements DJ, Decker EA. Mechanisms of the antioxidant activity of a high molecular weight fraction of whey. J Agric Food Chem. 2000;48:1473–1478. doi: 10.1021/jf991342v. [DOI] [PubMed] [Google Scholar]
- Wang WY, De Mejia EG. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr Rev Food Sci F. 2005;4:63–78. doi: 10.1111/j.1541-4337.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Xinyan P, Baohua K, Xiufang X, Qian L. Reducing and radical-scavenging activities of whey protein hydrolysates prepared with Alcalase. Int Dairy J. 2010;20(5):360–365. doi: 10.1016/j.idairyj.2009.11.019. [DOI] [Google Scholar]
- Yadav N, Mann B, Saini P, Kumar R. Antioxidant properties of whey protein hydrolysates prepared using heat denatured whey proteins. Milchwissenschaft. 2011;67:67–70. [Google Scholar]



