Abstract
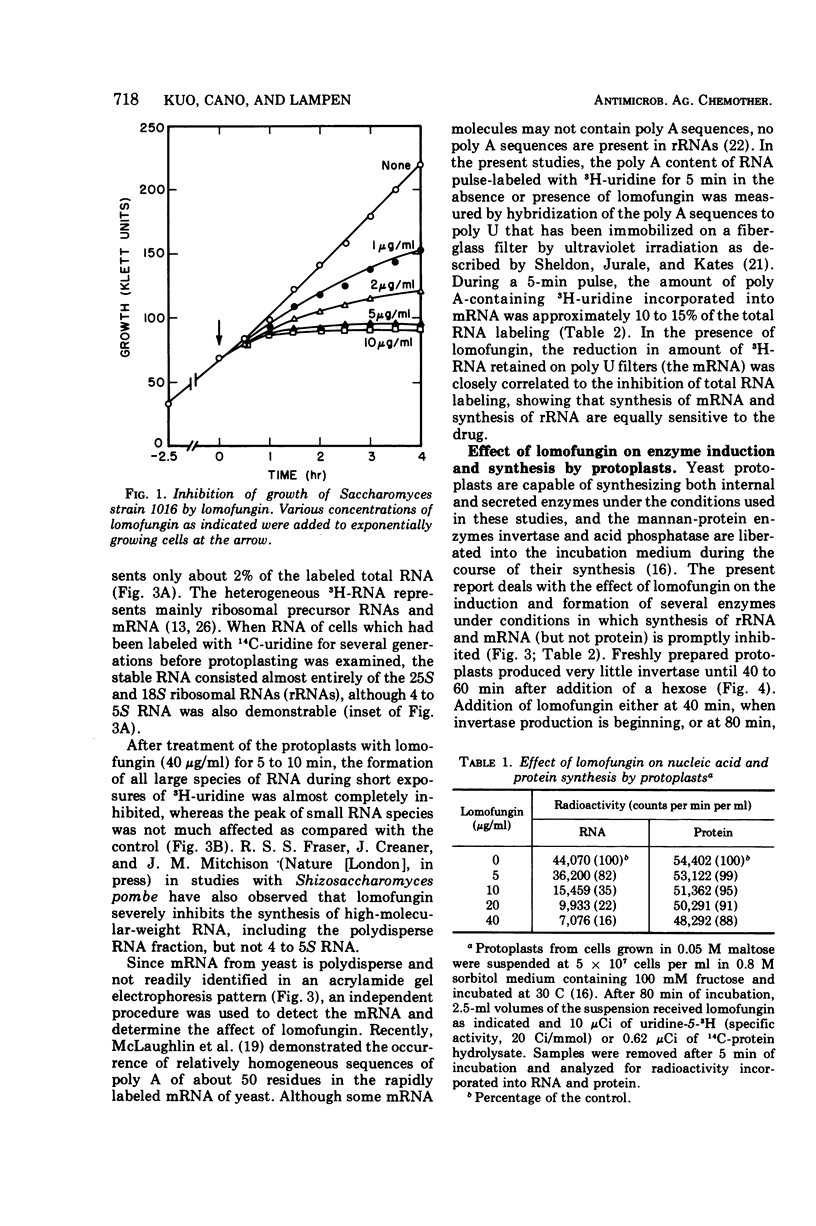
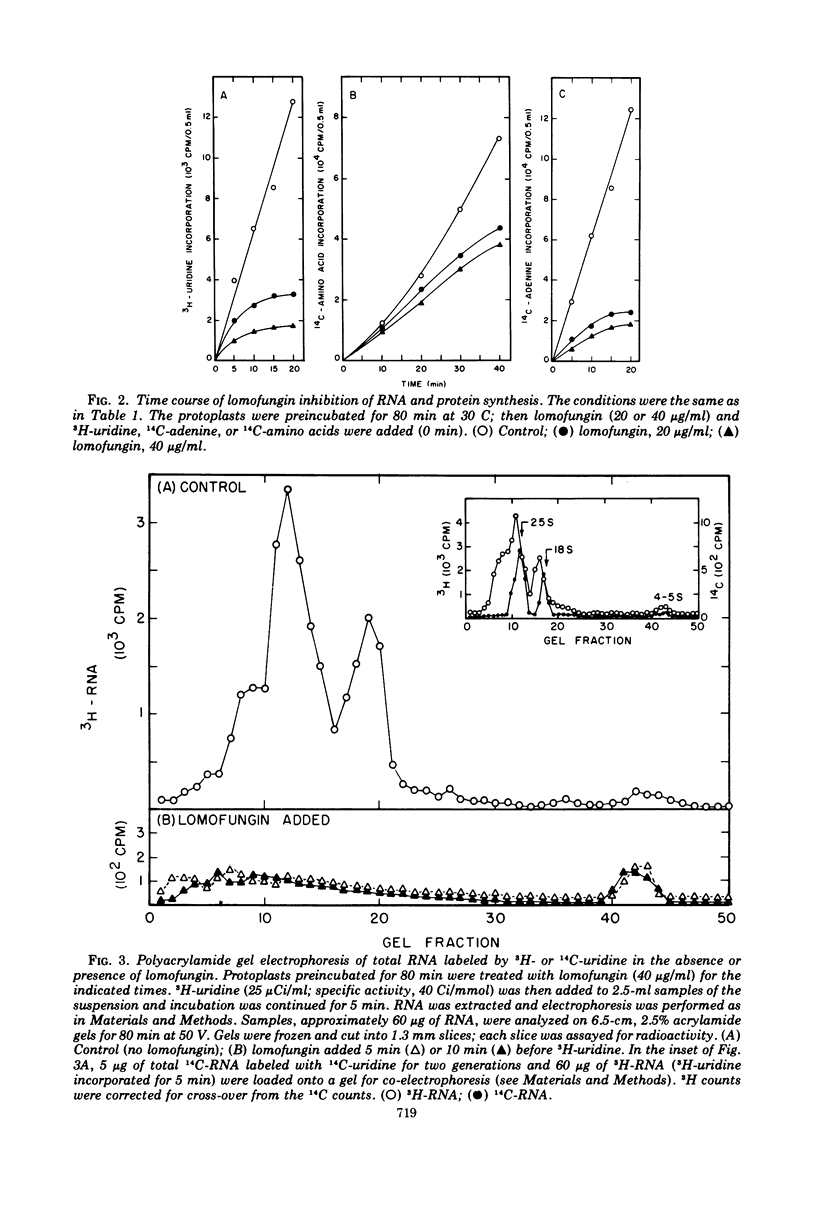
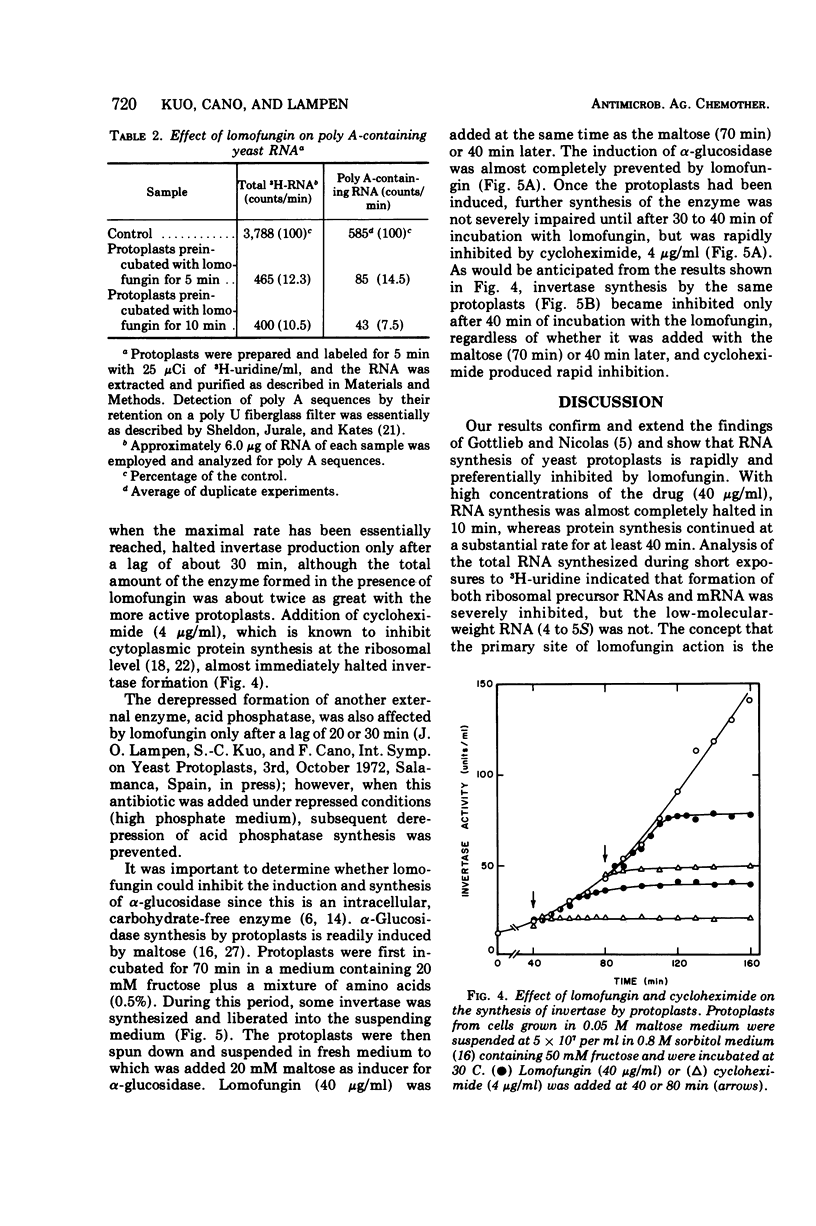
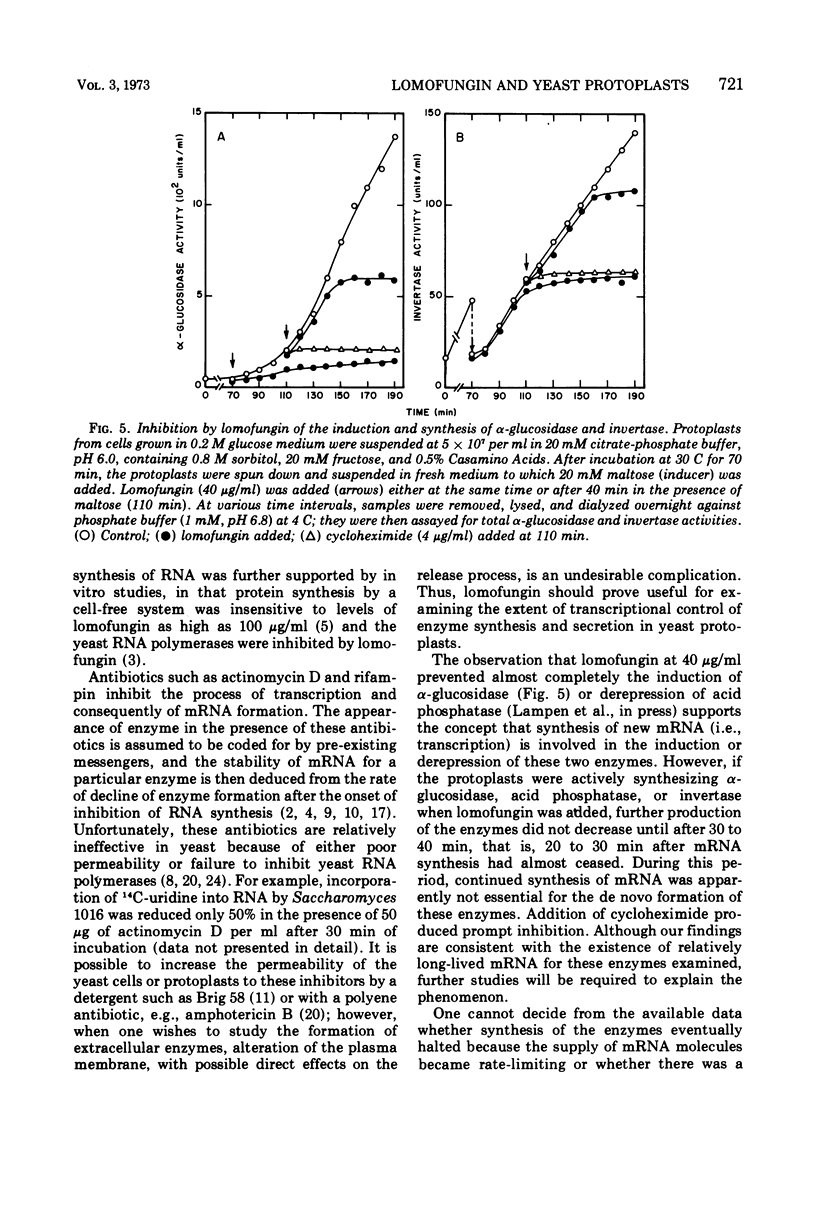
Lomofungin, an antibiotic active against fungi, yeasts, and bacteria, rapidly inhibits synthesis of ribonucleic acid (RNA) but not protein by protoplasts of Saccharomyces strain 1016. With 40 μg of lomofungin/ml, RNA synthesis was almost completely halted after 10 min of incubation; protein synthesis continued for at least 40 min. Since lomofungin inhibits isolated RNA polymerases from yeast, but not in vitro protein synthesis, it is concluded that the primary action of lomofungin in yeast protoplasts is on RNA synthesis. Examination of the pulse-labeled RNA indicated that biosynthesis of both ribosomal precursor RNAs and messenger RNAs was severely inhibited after the protoplasts were incubated with lomofungin for 5 min, whereas formation of small-molecular-weight RNA (4 to 5s) was only slightly affected. Under these conditions, lomofungin almost completely prevented induction of α-glucosidase. Once the protoplasts had been induced, further production of the enzyme was not impaired by lomofungin until after 30 min of incubation, but was rapidly halted by cycloheximide (4 μg/ml). Lomofungin inhibition of invertase formation by protoplasts actively synthesizing the enzyme also became evident only after a lag of about 30 to 40 min, although synthesis was promptly halted by cycloheximide. These observations suggest the existence of relatively long-lived specific messenger RNAs for these enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., May B. K., Elliott W. H. Insensitivity of Bacillus amyloliquefaciens extracellular protease formation to rifampicin and actinomycin D. Biochem Biophys Res Commun. 1971 Feb 19;42(4):750–757. doi: 10.1016/0006-291x(71)90551-1. [DOI] [PubMed] [Google Scholar]
- Cano F. R., Kuo S. C., Lampen J. O. Lomofungin, an inhibitor of deoxyribonucleic acid-dependent ribonucleic acid polymerases. Antimicrob Agents Chemother. 1973 Jun;3(6):723–728. doi: 10.1128/aac.3.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. W. The stability and cell content of penicillinase messenger RNA in Bacillus licheniformis. J Gen Microbiol. 1969 Dec;59(2):171–184. doi: 10.1099/00221287-59-2-171. [DOI] [PubMed] [Google Scholar]
- Gottlieb D., Nicolas G. Mode of action of lomofungin. Appl Microbiol. 1969 Jul;18(1):35–40. doi: 10.1128/am.18.1.35-40.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H., ELLIAS L. The purification and properties of an alpha-glucosidase of Saccharomyces italicus Y1225. Biochim Biophys Acta. 1958 Oct;30(1):28–40. doi: 10.1016/0006-3002(58)90237-3. [DOI] [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MECHANISM OF HISTIDASE INDUCTION AND FORMATION IN BACILLUS SUBTILIS. J Mol Biol. 1964 Oct;10:105–119. doi: 10.1016/s0022-2836(64)80031-0. [DOI] [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MOLECULAR BASIS OF HISTIDASE INDUCTION IN BACILLUS SUBTILIS. J Mol Biol. 1963 Oct;7:401–420. doi: 10.1016/s0022-2836(63)80033-9. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Defective DNA synthesis in permeabilized yeast mutants. Nat New Biol. 1971 Dec 8;234(49):171–172. doi: 10.1038/newbio234171a0. [DOI] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. E., Dietz A. Lomofungin, a new antibiotic produced by Streptomyces lomondensis sp. n. Appl Microbiol. 1969 May;17(5):755–759. doi: 10.1128/am.17.5.755-759.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. A., Eaton N. R. Purification and characterization of maltase and alpha-methyl glucosidase from yeast. Biochim Biophys Acta. 1967 Sep 12;146(1):173–180. doi: 10.1016/0005-2744(67)90084-8. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Osmotic regulation of invertase formation and secretion by protoplasts of Saccharomyces. J Bacteriol. 1971 Apr;106(1):183–191. doi: 10.1128/jb.106.1.183-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- McKeehan W., Hardesty B. The mechanism of cycloheximide inhibition of protein synthesis in rabbit reticulocytes. Biochem Biophys Res Commun. 1969 Aug 15;36(4):625–630. doi: 10.1016/0006-291x(69)90351-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S., Kwan C. N., Schlessinger D., Venkov P. Potentiation of rifampicin and 5-fluorocytosine as antifungal antibiotics by amphotericin B (yeast-membrane permeability-ribosomal RNA-eukaryotic cell-synergism). Proc Natl Acad Sci U S A. 1972 Jan;69(1):196–199. doi: 10.1073/pnas.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL M. R., SISLER H. D. SITE OF ACTION OF CYCLOHEXIMIDE IN CELLS OF SACCHAROMYCES PASTORIANUS. II. THE NATURE OF INHIBITION OF PROTEIN SYNTHESIS IN A CELL-FREE SYSTEM. Biochim Biophys Acta. 1964 May 18;87:83–89. [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Sosková L., Soska J., Svoboda A. The effect of 5-fluorouracil on cells and regenerating protoplsts of Saccharoyces cerevisiae. Folia Microbiol (Praha) 1970;15(6):442–447. doi: 10.1007/BF02880188. [DOI] [PubMed] [Google Scholar]
- Tipton C. D., Rinehart K. L., Jr Lomofungin. I. Degradative studies of a new phenazine antibiotic. J Am Chem Soc. 1970 Mar 11;92(5):1425–1426. doi: 10.1021/ja00708a066. [DOI] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- van Wijk R. Alpha-glucosidase synthesis, respiratory enzymes and catabolite repression in yeast. IV. B. Studies on the level of protein synthesis at which repression and induction of alpha-glucosidase synthesis occur. Proc K Ned Akad Wet C. 1968;71(3):300–313. [PubMed] [Google Scholar]



