Abstract
Antioxidant and sensory properties of Nile tilapia protein hydrolysates prepared by one- and two-step hydrolysis using commercial proteases were investigated. Hydrolysates prepared using single protease including Alcalase (HA), Flavourzyme (HF), Protamex (HPr) and papain (HPa) had increases in antioxidant activities as the degree of hydrolysis (DH) increased up to 40 % (P < 0.05). Amongst all hydrolysates, HA having 40 % DH showed the highest antioxidant activities. When HA was further hydrolysed by papain, the resulting hydrolysate (HAPa) exhibited the highest antioxidant activities for all assays tested (P < 0.05). ABTS radical scavenging activity and metal chelating of HAPa generally remained constant in a wide pH range (1–11) and during heating at 30–100 °C. Both activities increased in the simulated gastrointestinal tract model system, especially in intestine condition. HAPa (100–1,000 ppm) could retard lipid oxidation in β-carotene-linoleate and lecithin-liposome model systems in a dose dependent manner. Peptides in both HA and HAPa with molecular weight of 513 Da and 1,484 Da possessed the strongest ABTS radical scavenging activity and metal chelating activity, respectively. The amino acid profile of both HA and HAPa contained a high amount of hydrophobic amino acids (38.26–38.85 %) and had glutamic acid/glutamine, lysine and aspartic acid/asparagine as the dominant amino acids. However, HAPa showed a higher acceptability than did HA, owing to the lower bitterness. Therefore, the use of Alcalase in combination with papain for hydrolysis of protein isolate rendered the hydrolysate with antioxidant properties and reduced bitterness, which could serve as the functional supplement.
Keywords: Antioxidant activity, Protein hydrolysate, Nile tilapia, Two-step hydrolysis, Commercial proteases
Introduction
Antioxidants play an important role as health protecting factors and are used to preserve food products to retard off-odour/flavour and discolour caused by lipid oxidation (Decker et al. 2005). Lipid oxidation is of great concern for food industry and consumers, since it is associated with the development of undesirable off-flavours and potentially toxic products (Decker et al. 2005). Protein hydrolysates produced by the enzymatic digestion of aquatic processing byproducts have been proven to be a promising source of antioxidant peptides (Bougatef et al. 2010; Shahidi et al. 1995). Under the controlled enzymatic hydrolysis, the functional peptides could be released from the starting proteins, yielding the hydrolysate with varying properties. The substrate and protease employed as well as the degree of hydrolysis greatly affect physicochemical properties of the resulting hydrolysate. The type of enzyme generally dictates the cleavage patterns of the peptide bonds. Jun et al. (2004) reported that protein hydrolysates from yellowfin sole (Limanda aspera) frame prepared by Alcalase, Neutrase, papain, trypsin, pepsin, α-chymotrypsin, and Pronase E had different antioxidant activities.
Lean fish, such as Nile tilapia etc., are traditionally used for protein hydrolysate preparation. With an appropriate hydrolytic process, the hydrolysate might contain novel peptides with specific or multi-functional bioactivity (Khantaphant et al. 2011a). However, the presence of pro-oxidants, such as haem proteins and unstable lipid substrates associated with the muscle, is a drawback (Raghavan et al. 2008). These constituents contribute to undesirable characteristic and instability of hydrolysate (Raghavan et al. 2008). Recently, protein isolate (PI) from the pretreated muscle using pH-shifted method has been proven to be a potential substrate, yielding the hydrolysate with a negligible fishy odour (Yarnpakdee et al. 2012). Khantaphant et al. (2011a) reported that hydrolysate property from brownstripe red snapper (Lutjanus vita) PI was superior to that prepared directly from fish mince. Yarnpakdee et al. (2012) also found that Nile tilapia protein hydrolysate derived from its PI had a negligible fishy odour. However, the bitterness associated with hydrolysates is a critical problem for further applications, especially as the supplement for health or functional foods or drinks. To conquer the drawback, the use of several proteases in the step-wise process could remove the domain causing the bitterness in peptides. Nevertheless, there was no information regarding the step-wise hydrolysis of Nile tilapia muscle, especially from PI. Khantaphant et al. (2011b) reported that protein hydrolysates prepared from the muscle of brownstripe red snapper using Alcalase or Flavourzyme in the first step hydrolysis together with protease from its pyloric caeca for the second step contained the peptides with the highest antioxidant and ACE inhibitory activities. Thus, this work aimed to produce protein hydrolysate from Nile tilapia PI prepared by one-step and two-step hydrolysis using various types of commercial proteases and to investigate their antioxidant activities and sensory characteristic.
Material and methods
Chemical/enzymes
Alcalase 2.4 L (E.C. 3.4.21.62) (2.4 AU/g), Flavourzyme 500 L (E.C. 3.4.21.77) (500 LAPU/g) and ProtamexTM (EC. 3.4.21.14/3.4.24.28) (1.5 AU/g) were provided by Novozymes (Bagsvaerd, Denmark). Papain (E.C. 3.4.22.2) (≥3 AU/mg), 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-trinitrobenzenesulfonic acid (TNBS), 1,1,3,3-tetramethoxypropane, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt (ferrozine), 2,4,6-tripyridyl-triazine (TPTZ), l-α -phosphatidylcholine (lecithin) and linoleic acid were purchased from Sigma (St. Louis, MO, USA). Thiobarbituric acid (TBA), potassium persulphate, β-carotene and Tween 40 were obtained from Fluka (Buchs, Switzerland). Sodium sulphite was obtained from Riedel-deHaën (Seelze, Germany). All chemicals were of analytical grade.
Preparation of PI from Nile tilapia mince
PI was prepared from pre-washed Nile tilapia mince using alkaline solubilisation process as per the method of Yarnpakdee et al. (2012). Washed mince was homogenised with five volumes of cold distilled water (2–4 °C) using an IKA Labortechnik homogeniser (Selangor, Malaysia) at a speed of 11,000 rpm for 1 min. The homogenate was adjusted to pH 11 and placed on ice for 60 min with a continuous stirring. The mixture was then centrifuged at 5,000 × g for 10 min at 4 °C using an Avanti J-E centrifuge (Beckman Coulter, Inc., Fullerton, CA, USA). The alkaline soluble fraction obtained, referred to as ‘PI solution’, was used as substrate for hydrolysis.
Production of Nile tilapia protein hydrolysate with antioxidant activities
One-step hydrolysis using various single proteases
PI solution was mixed with distilled water to obtain a final protein concentration of 2 % (w/v) as determined by the Biuret method (Robinson and Hogden 1940). The hydrolysis was conducted for 1 h using 0.08–5.54 % (w/w) Alcalase (pH 8.0, 50 °C), 0.07–11.69 % (w/w) Flavourzyme (pH 7.0, 50 °C), 0.58–8.67 % (w/w) Protamex (pH 6.0, 40 °C) and 0.83–4.69 % (w/w) papain (pH 7.0, 40 °C) to obtain the desirable degree of hydrolysis (DH) (10, 20, 30 and 40 %) as described by Benjakul and Morrissey (1997). After 1 h of hydrolysis, the reactions were terminated by placing the mixtures in boiling water for 10 min. Thereafter, the mixture was centrifuged at 2,000 × g at 4 °C for 10 min. The supernatants were collected and then lyophilised using a Scanvac Model Coolsafe 55 freeze dryer (Coolsafe, Lynge, Denmark). The hydrolysates prepared using Alcalase, Flavourzyme, Protamex and papain were referred to as HA, HF, HPr and HPa, respectively. Those samples were subjected to analyses.
Hydrolysate possessing the highest antioxidant activities using the appropriate enzyme was selected for further hydrolysis.
Two-step hydrolysis using different proteases
After 1 h of the first hydrolysis, the selected hydrolysate (HA) was adjusted to the desirable pH using 2 M NaOH or HCl for proteases used for the second step. To initiate the second step of hydrolysis, different proteases at the same amount (5.54 % w/w) used in the first step were added into the pre-incubated mixture with optimal temperature of the corresponding proteases (50 °C for Flavourzyme and 40 °C for Protamex and papain). Reaction was conducted for 1 h and the mixture was submerged in boiling water for 10 min to inactivate the enzymes. Thereafter, the mixture was centrifuged at 2,000 × g at 4 °C for 10 min and the supernatant was collected, followed by lyophilisation to obtain hydrolysate powder. HA with further hydrolysis using Flavourzyme, Protamex and papain were referred to as HAF, HAPr and HAPa, respectively. All hydrolysates were subjected to analyses.
Analyses
Determination of degree of hydrolysis (DH)
DH of protein hydrolysate was determined according to the method of Benjakul and Morrissey (1997). Protein hydrolysate with an appropriate dilution (125 μL) was added with 2 mL of 0.2 M phosphate buffer (pH 8.2) and 1 mL of 0.01 % TNBS solution. The solutions were mixed thoroughly and placed in a temperature-controlled water bath at 50 °C for 30 min in the dark. The reaction was terminated by adding 2 mL of 0.1 M sodium sulphite. The mixtures were cooled at room temperature for 15 min. The absorbance was read at 420 nm and α-amino group was expressed in terms of L-leucine. The DH was defined as follows:
where LH is the amount of α-amino acid in hydrolysate. LPI is the amount of free amino group in original PI solution. Lmax is the total amino group content in the initial PI solution obtained after acid hydrolysis (6 M HCl at 100 °C for 24 h).
Determination of antioxidant activities
DPPH radical scavenging activity
DPPH radical scavenging activity was determined according to the method of Binsan et al. (2008). Sample solution (1.5 mL) was added with 1.5 mL of 0.1 mM DPPH in 95 % ethanol. The mixture was incubated at room temperature for 30 min in dark. The resulting solution was measured at 517 nm. The control was prepared in the same manner except that distilled water was used instead of the sample. The DPPH radical scavenging activity was calculated from Trolox standard curve (0–60 μM) and expressed as μmol Trolox equivalents (TE)/g solid.
ABTS radical scavenging activity.
ABTS radical scavenging activity was determined as described by Binsan et al. (2008). ABTS radical (ABTS•+) was produced by reacting 7.4 mM ABTS stock solution with 2.6 mM potassium persulphate at a ratio of 1:1 (v/v). The mixture was allowed to react in dark for 12 h at room temperature. Prior to assay, ABTS•+ solution was diluted with methanol to obtain an absorbance of 1.1 (±0.02) at 734 nm. To initiate the reaction, 150 μL of sample was mixed with 2.85 mL of ABTS•+ solution. The mixture was incubated at room temperature for 2 h in dark. The absorbance was then read at 734 nm. Trolox standard curve (0–600 μM) was prepared. Distilled water was used instead of the sample and prepared in the same manner to obtain the control. ABTS radical scavenging activity was expressed as μmol TE/g solid.
Ferric reducing antioxidant power (FRAP).
The ability of samples to reduce ferric ion (Fe3+) was evaluated as per the method of Benzie and Strain (1996). FRAP reagent (a freshly prepared mixture of 10 mM TPTZ solution in 40 mM HCl, 20 mM FeCl3.6H2O solution and 300 mM acetate buffer, pH 3.6 (1:1:10 v/v/v) (2.85 mL) was incubated at 37 °C for 30 min prior to mixing with 150 μL of sample. The reaction mixture was allowed to stand in dark for 30 min at room temperature. Absorbance at 593 nm was read and FRAP was calculated from the Trolox standard curve (0–600 μM) and expressed as μmol TE/g solid. The control was prepared in the same manner except that distilled water was used instead of the sample.
Metal chelating activity.
Chelating activity of samples towards ferrous ion (Fe2+) was measured by the method of Binsan et al. (2008) with a slight modification. Sample (200 μL) was mixed with 800 μL of distilled water. Thereafter, 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine were added. The mixture was allowed to react for 20 min at room temperature. The absorbance was then read at 562 nm. The standard curve of EDTA (0–30 μM) was prepared. The control was prepared in the same manner except that distilled water was used instead of the sample. Ferrous chelating activity was expressed as μmol EDTA equivalents (EE)/g solid.
The hydrolysate prepared using a two-step hydrolysis, showing the highest antioxidant activity, was used for further studies.
Stability of the selected protein hydrolysate
pH and thermal stability
The selected protein hydrolysate powder (25 mg) was dispersed in 8 mL of distilled water previously adjusted to different pHs (1, 3, 5, 7, 9 and 11) using 1 M HCl or NaOH. The final volume was made up to 10 mL with the water having the corresponding pH and allowed to stand at room temperature for 30 min. Thereafter, the pH of the mixtures was adjusted to 7.0 and their volume was made up to 25 mL with distilled water. The residual antioxidant activities were determined for ABTS radical scavenging activity and metal chelating activity. Activity (%) relative to that obtained without pH adjustment was reported.
To determine thermal stability, protein hydrolysate solution (1 mg hydrolysate/mL; 10 mL) was placed in a temperature controlled water bath at different temperatures (30, 40, 50, 60, 70, 80 90 and 100 °C) for 30 min. Thereafter, the solutions were suddenly cooled in iced water. The residual antioxidant activities were determined for ABTS radical scavenging activity and metal chelating activity and expressed as the activity (%) relative to those without heat treatment.
Stability in gastrointestinal tract model system (GIMs)
GIMs using an in vitro pepsin—pancreatin hydrolysis was carried out according to the method of You et al. (2010) with a slight modification. The pH of protein hydrolysate solution (1 mg hydrolysate/mL; 25 mL) was adjusted to pH 2.0 with 1 M HCl. Pepsin solution (E/S 1:35 w/w) was then added and the mixture was incubated with continuous shaking at 100 rpm for 1 h at 37 °C (stomach condition). The pH was then adjusted to 5.3 with 0.9 M NaHCO3 solution and further to pH 7.5 with 6 M NaOH. Pancreatin was added (E/S 1:35 w/w), and the mixture was further incubated with continuous shaking for 3 h at 37 °C (intestine condition). To terminate the digestion, the solution was submerged in boiling water for 10 min. During digestion, the mixture was sampled at 0, 20, 40, 60, 80, 100, 120, 150, 180, 210 and 240 min for measurement of ABTS radical scavenging activity and metal chelating activity. The residual activities were expressed as the activity (%) relative to that without digestion.
Antioxidant activity of the selected protein hydrolysate in different model systems
β-carotene linoleic acid emulsion model system
The antioxidant activity of the protein hydrolysate in the β-carotene linoleic acid emulsion model system was determined as described by Binsan et al. (2008). β-carotene (1 mg) was dissolved in 10 mL of chloroform. Thereafter, the solution (3 mL) was added to 20 mg linoleic acid and 200 mg Tween 40. Chloroform was then removed by purging with nitrogen. Fifty millilitres of oxygenated distilled water were added to the β-carotene emulsion and mixed well. Hydrolysate (200 μL) was then mixed with 3 mL of oxygenated β-carotene emulsion to obtain the final concentrations of 100, 500 and 1,000 ppm. The oxidation of β-carotene emulsion was monitored spectrophotometrically at 470 nm after 0, 10, 20, 30 40, 60, 90 and 120 min of incubation at 50 °C in dark. Trolox at a level of 100 ppm was also used as positive control. The control was prepared by using distilled water instead of protein hydrolysate in the assay system.
Lecithin liposome model system
The antioxidant activity of hydrolysates in a lecithin liposome system was determined according to the method of Frankel et al. (1997) as modified by Thiansilakul et al. (2007b). Lecithin liposome system was prepared by suspending lecithin in deionised water at a concentration of 8 mg/mL. The mixture was stirred with a glass rod, followed by sonication at room temperature (25–28 ºC) for 30 min in a sonicating bath (ElmaModel S30H, Singen, Germany). Hydrolysate solution (3 mL) was added to the lecithin liposome system (15 mL) to obtain final concentrations of 100, 500 and 1,000 ppm. The mixture was sonicated for 2 min. To initiate the reaction, 20 μL of 0.15 M cupric acetate were added. The mixture was shaken in dark at 120 rpm using a shaker (Heidolph Model Unimax 1010, Schwabach, Germany) at 37 °C. The system containing 100 ppm Trolox was also prepared. The control was prepared in the same manner, except that distilled water was used instead of hydrolysate. Oxidation in lecithin liposome systems was monitored at 6 h intervals for 48 h by determining the formation of TBARS (Buege and Aust 1978) and conjugated diene (Frankel et al. 1997).
Fractionation of antioxidant peptides from the selected protein hydrolysate
HA and HAPa were fractionated using a Sephadex G-25 column (1.6 × 63.5 cm). Sample (100 mg) was dissolved in distilled water (2 mL). The mixture was loaded onto a column and the elution was performed using distilled water at a flow rate of 0.5 mL/min. The 3 mL-fractions were collected and their absorbance was monitored at 220 and 280 nm. The standards, including insulin chain B (3,496 Da), vitamin B12 (1,356 Da), Gly-Tyr (238 Da) and tyrosine (181 Da) were used. Blue dextran (2,000 kDa) was used to measure the void volume of the column. The fractions were determined for their ABTS radical scavenging activity and chelating activity. The molecular weight of fraction exhibiting antioxidant activity was estimated from the plot between the available partition coefficient (Kav) against the logarithm of the molecular weight of the protein standards.
Amino acid analysis
HA and HAPa were hydrolysed with 4.0 M methanesulphonic acid under reduced pressure at 110 °C for 22 h to prevent the oxidation of tryptophan. The hydrolysates were neutralised with 3.5 M NaOH and diluted with 0.2 M citrate buffer (pH 2.2). An aliquot of 100 μl was applied to an amino acid analyser (MLC-703; Atto Co., Tokyo, Japan).
Sensory properties of the selected protein hydrolysate
Evaluation for fishy, muddy odour/flavour and bitter taste in protein hydrolysates was conducted by 10 trained panelists (7 female and 3 male) with the ages of 25–32. Prior to the evaluation, the panelists were trained three times a week for totally 1 month. Panelists were trained with standards for two sessions using a 15 cm line scale anchored from ‘none’ to ‘extremely strong’ for fishy-, muddy-odour/flavour and bitterness. The working standards included the stored fish protein hydrolysate (0–1 %), mixed solution of geosmin and 2-MIB with a ratio of 1:1 w/w (0–5 ppb) and caffeine solution (0–0.5 mg/mL) for fishy-, muddy-odour/flavour and bitterness, respectively. To evaluate the samples, hydrolysate solutions (1 % w/w) were placed in a sealable plastic cup and heated at 60 °C in a temperature controlled water bath for 5 min prior to serving. The panelists were asked to open the cup and sniff the headspace above the samples for determining odour. To evaluate the flavour, panelists were asked to taste the sample and rinse their mouth between different samples.
The evaluation of acceptance was performed by 30 untrained panelists who were familiar with fish consumption. The assessment was conducted for the odour, colour and overall likeness using a 9-point hedonic scale: 1, dislike extremely; 2, dislike very much; 3, dislike moderately; 4, dislike slightly; 5, neither like nor dislike; 6, like slightly; 7, like moderately; 8, like very much; 9, like extremely.
Statistical analysis
Experiments were run in triplicate using three lots of samples. Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by Duncan’s multiple range tests. The T-test was used for pair comparison (Steel and Torrie 1980). Statistical analysis was performed using the Statistical Package for Social Science (SPSS 11.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussions
Antioxidant activities of protein hydrolysate prepared using various single proteases with different DHs
DPPH radical scavenging activity
DPPH radical scavenging activities of Nile tilapia protein hydrolysates including HA, HF, HPr and HPa with different DHs are depicted in Fig. 1a. DPPH is a stable free radical that shows the maximal absorbance at 517 nm. When DPPH encounters a proton-donating substance, such as an antioxidant, the radical is scavenged. The colour changes from purple to yellow and the absorbance is reduced (Binsan et al. 2008). The effect of antioxidants on DPPH radical scavenging was thought to be due to their hydrogen-donating ability, thereby terminating the radical chain reaction. The differences in activities were observed amongst hydrolysates and the activity varied with DHs. As the DH increased, DPPH radical scavenging activities increased (P < 0.05). However, DPPH radical scavenging activity of HPa decreased when DH was above 30 %. The result was in agreement with Thiansilakul et al. (2007a) who reported that DPPH radical scavenging activity of protein hydrolysate from round scad muscle prepared using Flavourzyme and Alcalase increased as DH increased. Nevertheless, DPPH radical scavenging activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates using Protamex decreased with increasing DH (Theodore et al. 2008). At all DHs, HA showed the highest activity, compared to other hydrolysates (P < 0.05). The highest activity was observed in HA with 40 % DH (P < 0.05). Rajapakse et al. (2005) reported that high DPPH or other radical scavenging activities for the protein hydrolysates or peptides derived from giant squid muscle are usually associated with high hydrophobic amino acid or hydrophobicity. The results revealed that the efficiency in hydrogen donation of peptides in hydrolysate was governed by types of proteases used and DH.
Fig. 1.
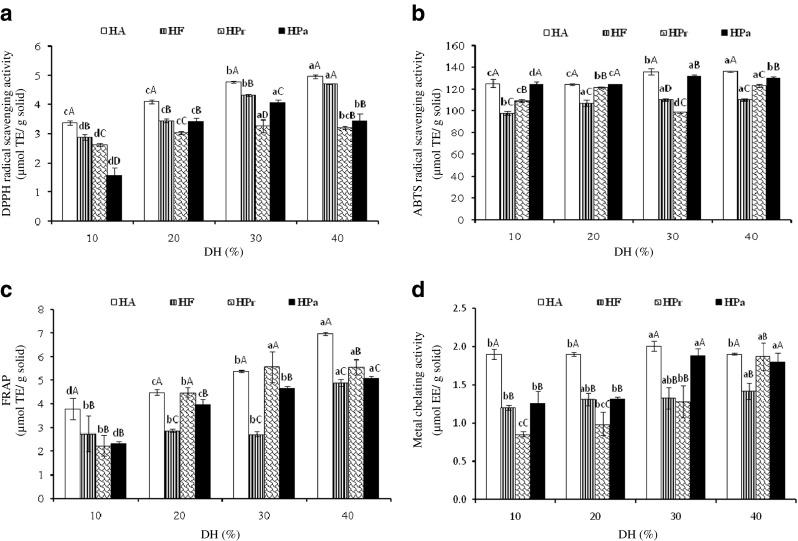
Antioxidant activities of Nile tilapia protein hydrolysate prepared using various single proteaseses with different DHs determined by DPPH radical scavenging activity (a), ABTS radical scavenging activity (b), FRAP (c) and metal chelating activity (d). HA, HF, HPr and HPa: hydrolysate prepared using Alcalase, Flavourzyme, Protamex and papain, respectively. Bar represent stand deviations (n = 3). Different lowercase letters within the same type of enzyme and different uppercase letters within the same DH indicate the significant differences (P < 0.05)
ABTS radical scavenging activity
Protein hydrolysates prepared using various proteases showed varying ABTS radical scavenging activities (Fig. 1b). The ABTS assay is a colourimetric assay that evaluates the potential of an antioxidant to inhibit the formation of a coloured radical cation ABTS•+, a blue-green chromophore with the characteristic absorption at 734 nm. The ABTS•+ is generated by the oxidation of ABTS with potassium persulphate and reduced in the presence of hydrogen-donating antioxidants or of chain breaking anitioxidant (Raghavan et al. 2008). The highest activity was observed in all hydrolysates with 40 % DH (P < 0.05), regardless of type of enzymes used. There were no differences in ABTS activity for HF with DH of 20–40 % (P > 0.05). However, the lowest activity was observed in HPr with 30 % DH, compared with HPr having other DHs (P < 0.05). Peptides produced might be different in terms of amino acid composition, sequence and chain length. The result suggested that the hydrolysates contained peptides or proteins, which served as hydrogen or electron donors to radicals by converting them to more stable products. Li et al. (2012) reported that grass carp hydrolysate prepared using Alcalase with DH ranging from 10 to 20 % had increased ABTS scavenging activity with increasing DH.
Ferric reducing antioxidant power (FRAP)
FRAP of Nile tilapia protein hydrolysates as affected by DH and types of proteases is shown in Fig. 1c. FRAP is generally used for measurement of the reducing ability (TPTZ-Fe3+ to TPTZ-Fe2+). All protein hydrolysates prepared using different proteases had the increases in FRAP when DH increased (P < 0.05). The result was in agreement with DPPH radical scavenging activity (Fig. 1b). However, FRAP of HF was not different as DH was in the range of 10–30 % (P > 0.05). At 40 % DH, HA showed the highest activity (6.98 μmol TE/mg solid), followed by HPr (5.56 μmol TE/mg solid), HPa (5.05 μmol TE/mg solid) and HF (4.90 μmol TE/mg solid), respectively. The reducing ability of hydrolysates was possibly due to the presence of peptides, which donated electrons to free radicals, leading to the prevention or retardation of propagation. Protein hydrolysates from yellow stripe trevally (Klompong et al. 2008) and alkaline-solubilised tilapia (Raghavan et al. 2008) have been reported to possess FRAP.
Metal chelating activity
The ability of protein hydrolysates prepared using various proteases with different DHs in metal chelating is depicted in Fig. 1d. Generally, the metal chelating activity of hydrolysates increased as the DHs increased (P < 0.05), except for HA and HF, in which no difference in activity was observed with increasing DHs (P > 0.05). At the same DH tested, HA exhibited the higher metal chelating activity than others (P < 0.05). Ferrous ion (Fe2+) is a pro-oxidant and can interact with hydrogen peroxide in a Fenton reaction to produce reactive oxygen species and hydroxyl (OH•) radicals, leading to the initiation and/or acceleration of lipid oxidation (Binsan et al. 2008). For the assay, ferrozine produces a violet complex with Fe2+. The formation of this complex is interrupted in the presence of a chelating agent, resulting in the decreased violet colour. Thus, protein hydrolysate containing peptides had the ability in chelation of metals. As a consequence, prooxidative metals could be sequestered, leading to the lowered oxidation.
Based on the results, enzymatic hydrolysis most likely increased the antioxidant activity of Nile tilapia PI to different degrees. The DH greatly influenced the peptide chain length as well as the exposure of terminal amino groups of products (Thiansilakul et al. 2007a). Wu et al. (2003) found that changes in size, level and composition of free amino acids of peptides affected the antioxidant activity. Furthermore, Shahidi et al. (1995) reported that the composition of protein hydrolysates from capelin depended on the type of enzyme used. Proteases from various types most likely cleaved the peptide bonds in protein structure at the different positions, leading to the different products with various antioxidant activities. Alcalase and Protamex are endopeptidase capable of hydrolysing proteins with broad specificity for peptide bonds and prefers the uncharged residue, whereas Flavourzyme is a mixture of endo-and exopeptidase, which can produce both free amino acids and peptides (Je et al. 2009). Papain has fairly broad specificity; it has endopeptidase, amidase, and esterase activities. It exhibits specific substrate preferences, primarily for bulky hydrophobic or aromatic residues (Tavano 2013). Amongst all hydrolysates, HA with 40 % DH showed the highest antioxidant activity and it was selected for further hydrolysis using other proteases.
Antioxidant activities of protein hydrolysate prepared using two-step process
Antioxidant activities of hydrolysates prepared by a two-step hydrolysis process using combined proteases including HAF, HAPr and HAPa with DHs of 48.9, 50.6 and 53.8 %, respectively (data not shown), in comparison with HA are depicted in Fig. 2.
Fig. 2.
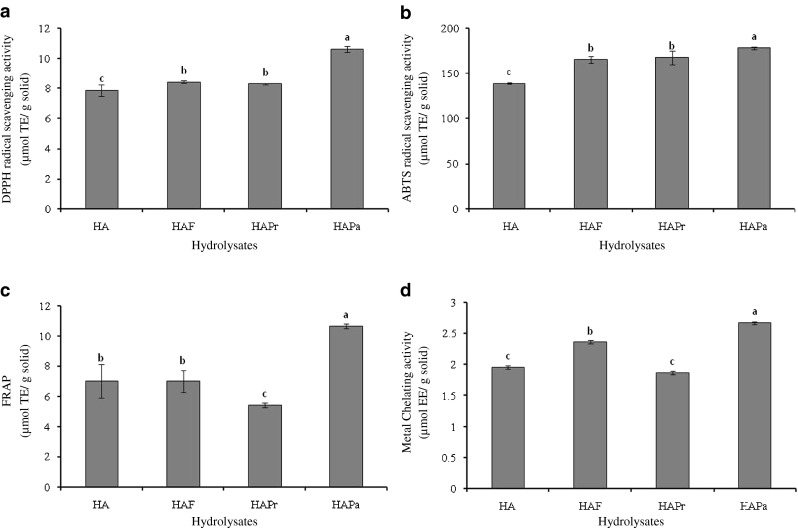
Antioxidant activities of Nile tilapia protein hydrolysate prepared by a two-step hydrolysis using various proteases as determined by DPPH radical scavenging activity (a), ABTS radical scavenging activity (b), FRAP (c) and metal chelating activity (d). HA: hydrolysate prepared using Alcalase. HAF, HAPr and HAPa: HA further hydrolyzed with Flavourzyme, Protamex and papain, respectively. Bar represent stand deviations (n = 3). Different lowercase letters in the same parameter indicate the significant differences (P < 0.05)
DPPH radical scavenging activity
DPPH radical scavenging activity of protein hydrolysates from Nile tilapia PI using a two-step hydrolysis with two different proteases is shown in Fig. 2a. The increases in DPPH radical scavenging activity were observed for all hydrolysates, when a two-step hydrolysis was employed (P < 0.05). Je et al. (2009) reported that the DPPH scavenging activity of hydrolysates from a two-step hydrolysis process was more potent than that of one-step hydrolysis. The present result indicated that the two-step hydrolysis plausibly enhanced the liberation of bioactive peptides from the hydrolysate prepared at the first step. The peptides generated more likely showed a greater ability to scavenge DPPH radicals or to donate hydrogen. However, the scavenging activity against DPPH radical was not different between HAF and HAPr (P > 0.05), whilst the highest activity was observed for HAPa (P < 0.05). Thus, DPPH radical scavenging activity increased when papain was used for the second hydrolysis.
ABTS radical scavenging activity
ABTS radical scavenging activity of protein hydrolysates prepared by a two-step hydrolysis in comparison with HA is depicted in Fig. 2b. The hydrolysate prepared by a two-step process showed higher ABTS radical scavenging activity, compared with HA (P < 0.05). However, the ability to scavenge the ABTS radicals varied with enzymes used. Amongst all hydrolysates, HAPa had the highest ABTS radical scavenging activity (P < 0.05). This was in accordance with DPPH radical scavenging activity, in which the highest activity was found in HAPa (Fig. 2a). HAPa with the highest DH had the active peptides, which could react with free radicals to form more stable products. Khantaphant et al. (2011b) reported that the increases in ABTS radical scavenging activity of brownstripe red snapper muscle hydroysate varied, depending on the hydrolysis time of the second hydrolysis step.
FRAP
Figure 2c shows FRAP of Nile tilapia protein hydrolysates prepared using a two-step hydrolysis process. The highest FRAP was noticeable in HAPa (P < 0.05) and its FRAP was much higher than that of HA (P < 0.05). On the other hand, HAPr had a decrease in FRAP by 22.4 %, compared with that of HA. There was no difference in FRAP between HAF and HA (P > 0.05). The differences in FRAP might be governed by peptides in the hydrolysates. Protamex used in the second step might generate peptides with the lower FRAP. A number of studies have shown that the antioxidant activity of hydrolysates was dependent on their molecular weight distribution (Wu et al. 2003). The highest reducing power of protein hydrolysate from whole anchovy sprat prepared using bromelain and Promod® was observed, whilst the lowest activity was found in those prepared by autolysis and Flavourzyme (Ovissipour et al. 2013).
Metal chelating activity
Metal chelating activity of Nile tilapia protein hydrolysate as affected by a two-step hydrolysis process is shown in Fig. 2d. In general, the increases in metal chelating ability of all hydrolysates prepared by two-step process were observed, except for HAPr. This might be attributed to more exposure of effective sites capable of chelating ferrous ion. HAPa showed the strongest metal cheating activity and the activity increased by 36.4 %, compared with that found in HA (P < 0.05). Thus, metal chelating activity of hydrolysate was affected by types of protease used in the second step of hydrolysis.
According to antioxidant activities tested by different assays, HAPa exhibited the highest activity and it was used for stability study.
Stability of the selected protein hydrolysate (HAPa)
pH and thermal stabilities
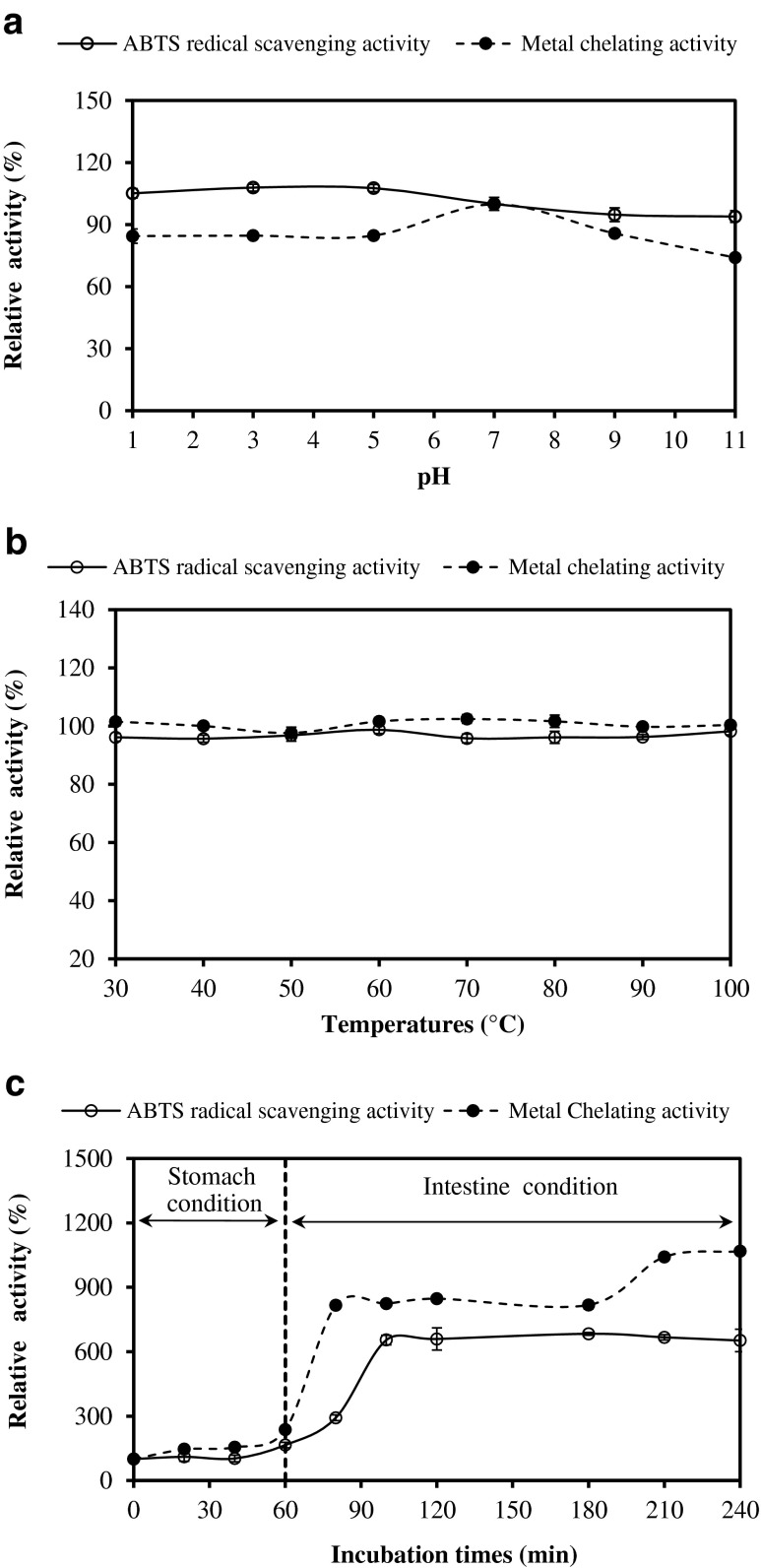
The influence of pH on antioxidant activity of HAPa as monitored by ABTS radical scavenging and metal chelating activities is shown in Fig. 3a. ABTS radical scavenging activity of HAPa was quite stable over the pH range of 1–11 (P > 0.05). Nevertheless, metal chelating activity of HAPa decreased by 15.3–25.9 % after pH adjustment to acidic and alkaline pH. This was possibly due to the changes of charge in peptides, particularly at N- and C-terminal, mediated by pH adjustment. Nalinanon et al. (2011) reported that the antioxidant peptides derived from ornate threadfin bream muscle possessing ABTS radical-scavenging activity lost its activity to some extent at high pH. Due to the stability over a wide pH range, antioxidant peptides of protein hydrolysate from Nile tilapia PI had the potential for applications in any food system in wide pH ranges.
Fig. 3.
Antioxidant stability of the selected Nile tilapia protein hydrolysate prepared using two-step hydrolysis process (HAPa) as affected by pH (a), heating (b) and GIMs (c) as monitored by ABTS radical scavenging activity and metal chelating activity. Bars represent standard deviation (n = 3)
Thermal stability of HAPa is depicted in Fig. 3b. Both ABTS radical scavenging and metal chelating activities of HAPa remained constant when subjected to the heating at 30–100 °C for 30 min. An activity of more than 98 % was retained after heat treatment. A slight decrease in ABTS radical scavenging activity might be due to either degradation or aggregation of some antioxidant peptides, caused by heat treatment. Peptides with smaller sizes were more stable to aggregation at high temperature (Nalinanon et al. 2011). In general, proteins were vulnerable to heat treatment, leading to the aggregation of protein and the exposure of hydrophobic domain. Peptides derived from many protein sources with increased hydrophobicity have been reported to correlate with antioxidant properties (Klompong et al. 2008). The result was in accordance with Binsan et al. (2008) who reported that antioxidant in the water extract from Mungoong showed high stability when temperature increased up to 100 °C, in which an activity of more than 80 % was retained. Thus, peptides in HAPa were stable to heating process. As a consequence, HAPa could be used or supplemented as a source of natural antioxidants in thermally processed foods.
Stability in gastrointestinal tract model system (GIMs)
Antioxidant activities of HAPa in GIMs were monitored (Fig. 3c). GIMs have been used to simulate the ingestion system of human body. HAPa showed the slight increase in ABTS radical scavenging activity and metal chelating activity during pepsin digestion (P < 0.05). With further hydrolysis in intestinal simulated system, the sharp increases in ABTS radical scavenging were obtained within the first 40 min under the intestine condition (P < 0.05). Thereafter, no difference in activity was found up to 240 min (P > 0.05). For metal chelating activity, the marked increase was found in the first 20 min under intestine condition and remained constant up to 180 min, followed by the increase at the end of digestion (240 min). Nalinanon et al. (2011) also found the increase in antioxidant activity of protein hydrolysate from ornate threadfin bream muscle after being ingested in the simulated model system. The result suggested that pancreatin might cleave the peptides to some degrees, leading to the release of new antioxidant peptides. This could enhance the antioxidant activities of hydrolysates in gastrointestinal tract after ingestion.
Antioxidant activities of the selected hydrolysate (HAPa) in different model systems
β-carotene linoleate model system
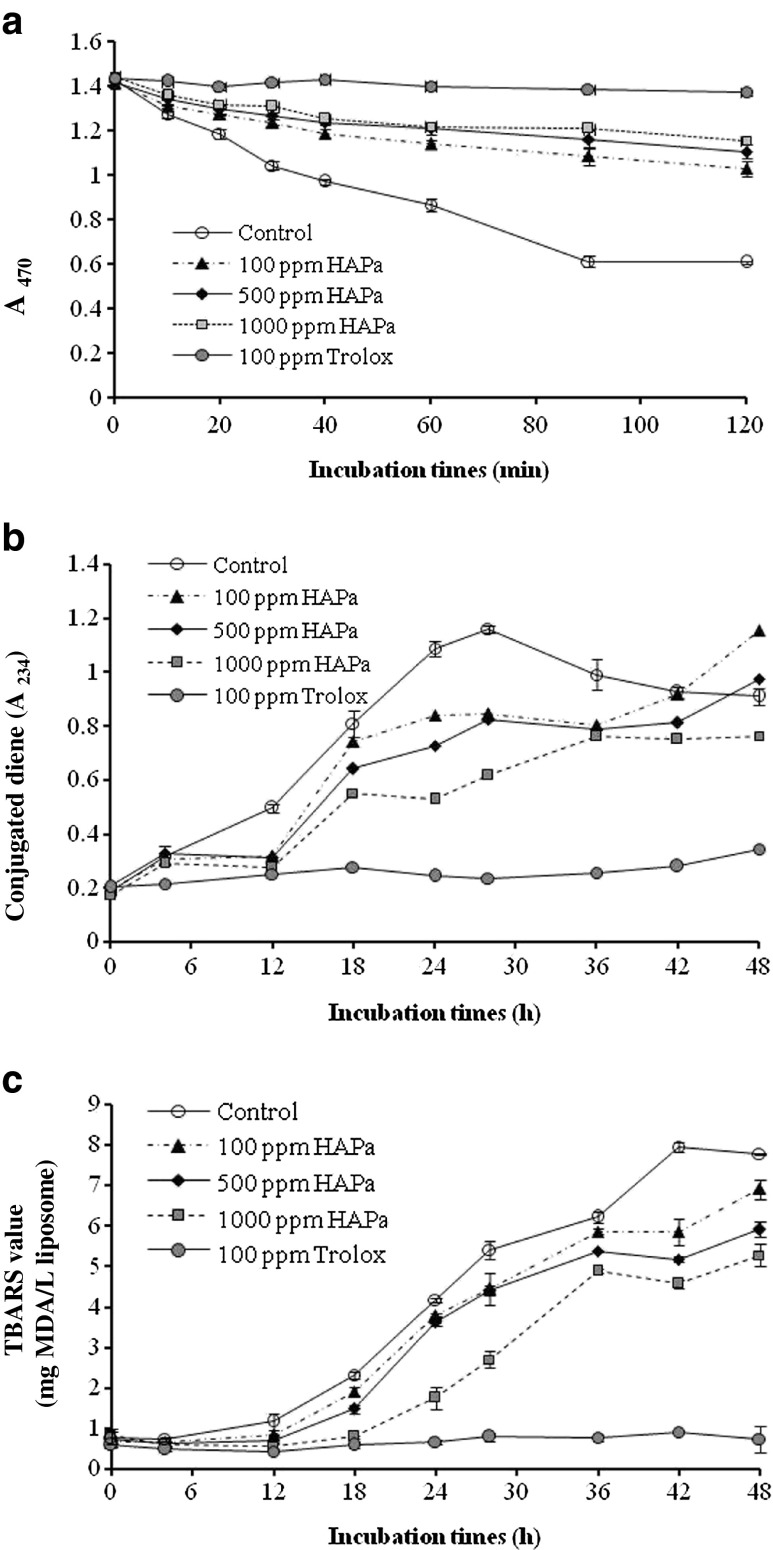
The antioxidant activity of HAPa at different concentrations in a β-carotene-linoleate model system in comparison with Trolox (100 ppm) is shown in Fig. 4a. The decrease in A470 indicates the oxidation of β-carotene in the system caused by free radical from oxidation of linoleic acid (Chandrasekara and Shahidi 2010). Free radicals formed are able to attack the highly unsaturated β-carotene molecules, leading to the losses in chromophore and characteristic orange colour of β-carotene (Binsan et al. 2008). A sharp decrease in A470 was noticeable in system without antioxidant (control), whilst systems containing HAPa retarded the decrease in A470 in a dose dependent manner. Nevertheless, no difference in A470 between the systems with HAPa at levels of 500 and 1,000 ppm was observed up to 120 min (P > 0.05). The antioxidant activity of HAPa was generally lower than Trolox. This indicated that β-carotene bleaching was retarded mainly due to the elimination of free radicals by HAPa. The difference in antioxidant activity between HAPa and Trolox might be caused by the difference in their polarity. The hydrophobic antioxidants have higher efficiency than hydrophilic antioxidants in preventing oxidation in oil-in-water emulsion systems by preferably orienting at the oil-water interface (Chandrasekara and Shahidi 2010). Khantaphant et al. (2011b) reported that the ability of hydrolysates to prevent the bleaching of β-carotene was governed by the amphiphilic properties of amino acid compositions. The use of HAPa was thus able to retard lipid oxidation in oil-in-water emulsions.
Fig. 4.
Changes in A470 of β-carotene linoleic acid system (a) and the formation of CD (b) and TBARS (c) in lecithin liposome system containing the selected Nile tilapia protein hydrolysate (HAPa) at different levels. Bars represent standard deviation (n = 3)
Lecithin liposome model system
HAPa at different levels affected the oxidation of the lecithin liposome system differently as indicated by different conjugated dienes and TBARS values during the incubation at 37 °C for 48 h (Fig. 4b and c). The oxidation of all systems generally increased throughout 48 h of incubation. However, a slight decrease was observed in the control after 30 h of incubation (P < 0.05). The formation of CD occurs during the early stages of lipid oxidation (Frankel et al. 1997). The decrease in CD was probably due to the transformation of CD into hydroperoxide or the secondary products. The system containing HAPa had lower increases in CD, compared with the control. However, the rate of changes varied with the concentration used. System containing HAPa at a level of 1,000 ppm showed the lowest CD formation, compared with other concentrations (P < 0.05). The result indicated that HAPa containing antioxidant peptide could retard the formation of CD. However, no marked changes in CD of system containing 100 ppm Trolox were observed during incubation of 48 h. The result indicated that HAPa had a lower efficacy in prevention of oxidation at the early stage than did Trolox.
Changes in TBARS of lecithin-liposome system containing HAPa at various concentrations during incubation of 48 h are shown in Fig. 4c. TBARS values increased as the incubation time increased (P < 0.05). The increase in TBARS indicated the formation of the secondary lipid oxidation products. TBARS in the control (without any additive) increased markedly after incubation for 4 h, whilst those containing HAPa showed the increases in TBARS after 12 h (P < 0.05). The longer induction period indicates a stronger antioxidant activity (Wu et al. 2003). The antioxidant effect of HAPa toward TBARS formation was dose-dependent. The system added with 1,000 ppm HAPa showed the lower TBARS formation than those added with other levels (P < 0.05). The similar changes in TBARS value were observed within the first 28 h of incubation when the HAPa at levels of 100 and 500 ppm were added to liposome system (P > 0.05). Nevertheless, Trolox exhibited higher antioxidant activity than HAPa. This was evidenced by no changes in TBARS during incubation of 48 h (P > 0.05). Therefore, HAPa, especially at 1,000 ppm, could retard lipid oxidation in lecithin-liposome system, however the efficiency was lower than Trolox.
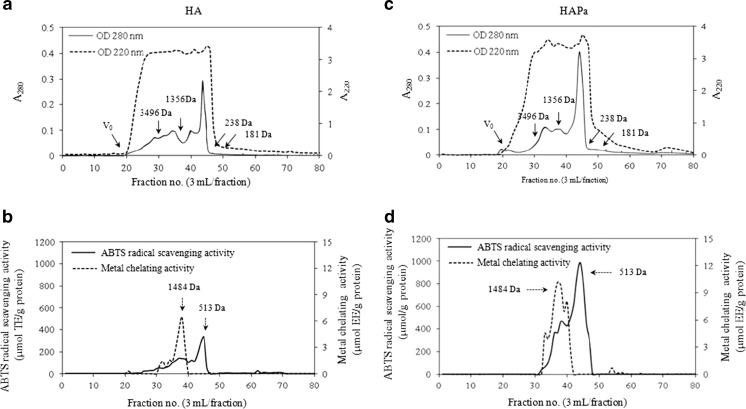
Molecular weight distribution of the selected protein hydrolysates
Nile tilapia protein hydrolysates, HA and HAPa, prepared using one-step and two-step hydrolysis were fractionated using a Sephadex G-25 gel filtration chromatography as shown in Fig. 5. A220 was used to monitor peptide bonds, whereas A280 was the parameter representing peptides, proteins or amino acids with aromatic rings. The different peaks of A280 were observed between HA and HAPa, indicating the presence of proteins or peptides containing aromatic amino acids with varying MW. However, A220 was greater than A280, indicating the prevalence of peptide bonds in hydrolysate (Klompong et al. 2009). Both hydrolysates showed a distinct peak of A280 with the fraction no. 44. However, several small A280 peaks were found in both samples. HAPa contained a larger proportion of the low MW peptides than did HA. The result was in accordance with a higher DH obtained in HAPa. Pre-hydrolysis of cod protein using Alcalase with subsequent hydrolysis by Kojizyme yielded the hydrolysate with a higher proportion of peptide below 455 Da (Liaset et al. 2000). Wu et al. (2003) reported that three major antioxidant peptides with MW of 1,400, 900 and 200 Da were fractionated from mackerel protein hydrolysate derived by autolysis, whilst the higher proportion of all peaks were observed when Pronase E was used in combination with autolysis.
Fig. 5.
Elution profiles of antioxidant peptides from the selected Nile tilapia protein hydrolysates, HA and HAPa, by a Sephadex G-25 column as monitored by A220 and A280 (a and c). Elution was performed using distilled water with a flow rate of 0.5 ml/min. Fractions (3 ml) were determined for ABTS radical scavenging activity and metal chelating activity (b and d). Solid arrows in (a) and (c) indicate the standard peaks. Dash arrows in (b) and (d) indicate the peaks with antioxidant activities
When the obtained fractions were measured for ABTS radical scavenging activity and metal chelating activity (Fig. 5b and d), different fractions exhibited varying antioxidant activities. The fraction containing peptides with MW of 1,484 Da exhibited the highest metal chelating activity, whilst peptide with MW of 513 Da showed remarkable ABTS radical scavenging activity for both HA and HAPa. However, stronger activities were noticeable in HAPa than those in HA. The proteases used for hydrolysis showed a pronounced impact on the size and antioxidant activity of peptides formed. Low MW peptides possessing metal chelating activity and ABTS radical scavenging activity were generated by enzymatic hydrolysis. The enhanced exposure of the functional groups of peptides could favour their antioxidant activities. Several reports suggested that phenolic hydroxyl group present in aromatic amino acids contributed substantially to scavenging of radicals via acting as electron donors Wu et al. (2003). Peptides containing tyrosine residues at the C-terminus, lysine or phenylalanine residues at the N-terminus and tyrosine in their sequence had strong free radical scavenging activity (Wang et al. 2008). Hydrolysates from yellow stripe trevally muscle with MW of 2.4 kDa exhibited higher antioxidant properties than those with MW of 35, 0.47 and 57 kDa, respectively (Klompong et al. 2009). The result suggested that Nile tilapia protein hydrolysate mostly contained certain peptides with antioxidant activities. The use of two-step hydrolysis process, particularly using Alcalase, followed by papain, could enhance the antioxidant activity of resulting hydrolysate.
Amino acids composition of the selected protein hydrolysates
Amino acid compositions of HA and HAPa are presented in Table 1. The major amino acids of both samples were glutamic acid/glutamine, lysine and aspartic acid/asparagines, which accounted for 17.87–18.35 %, 10.68–10.90 % and 10.49–10.64 % of the total amino acids, respectively. The result was in accordance with Foh et al. (2010) who reported that the hydrolysates from tilapia mince using Alcalase, Flavourzyme and Neutrase were rich in glutamic acid, aspartic acid and lysine. Khantaphant et al. (2011b) also found glutamic acid/glutamine, aspartic acid/asparagines, alanine, lysine and leucine as the most abundant amino acids in hydrolysates from brownstripe red snapper using Alcalase or Flavourzyme in conjunction with pyloric caeca protease. However, negligible contents of cysteine (0.02–0.03 %), hydroxylysine (0.03 %) and tryptophan (0.64–0.72 %) were observed in both HA and HAPa. Shahidi et al. (1995) reported that the sensitive amino acids, such as methionine and tryptophan were presented at smaller amount after hydrolysis of capelin proteins. Based on total amino acids, essential amino acids constituted 43.30 and 43.01 % of total amino acids for HA and HAPa, respectively. Therefore, they could be a dietary protein supplement to poorly balanced dietary proteins. Although both HA and HAPa showed the similar amino acid composition, HAPa had the higher antioxidant activity. The difference was plausibly determined by several factors including the sequence of amino acid, chain length, etc. The higher antioxidant activity of HAPa might be due to a higher content of peptides with a shorter chain than did HA. The result was evidenced by a higher %DH obtained in HAPa. In addition, the hydrophobic amino acid of HA and HAPa were 38.26 and 38.85 %, respectively. The slight differences in amino acid composition between HA and HAPa depended on the existing differences in enzyme specificity and hydrolysis conditions (Klompong et al. 2009). Peptides derived from many materials with increased hydrophobicity were reported to relate with antioxidant activity (Rajapakse et al. 2005). Kim et al. (2001) indicated that some amino acids including histidine, proline, alanine, and leucine can scavenge free radicals. Suetsuna et al. (2000) suggested that phenolic hydroxyl groups present in aromatic amino acids contribute substantially to radical scavenging by acting as potent electron donors. As a result, the higher antioxidant activity of HAPa (Fig. 2) was possibly associated with a higher content of hydrophobic amino acids, compared with HA. Nevertheless, the hydrophobicity has been reported to play a major role in bitter taste (FitzGerald and O’cuinn 2006). Bitter taste intensity of a peptide might be governed by several factors, such as degree of hydrolysis, concentration and location of bitter taste residues and number of carbons on the R group of branched chain amino acids (Leksrisompong et al. 2012). The results suggested that both HA and HAPa could serve as an excellent source of useful nutrient, based on their amino acid profiles.
Table 1.
Amino acid composition of selected protein hydrolysate derived from Nile tilapia PI (HA and HAPa)
| Amino acids (%) | HA | HAPa |
|---|---|---|
| Alanine† | 6.26 | 6.10 |
| Arginine | 6.52 | 6.49 |
| Aspartic Acid/Aspargine | 10.49 | 10.64 |
| Cysteine† | 0.02 | 0.03 |
| Glutamic Acid/Glutamine | 18.35 | 17.87 |
| Glycine | 3.90 | 3.90 |
| Histidine* | 2.67 | 2.65 |
| Hydroxylysine | 0.03 | 0.03 |
| Isoleucine*,† | 4.58 | 4.63 |
| Leucine*,† | 8.63 | 8.49 |
| Lysine* | 10.90 | 10.68 |
| Methionine*,† | 3.29 | 3.25 |
| Phenylalanine*,† | 3.63 | 3.74 |
| Proline† | 2.96 | 3.32 |
| Serine | 4.10 | 4.19 |
| Threonine* | 4.77 | 4.71 |
| Tryptophan† | 0.64 | 0.72 |
| Tyrosine† | 3.42 | 3.71 |
| Valine*,† | 4.83 | 4.86 |
| Hydrophobic amino acids | 38.26 | 38.85 |
| Essential amino acids | 43.30 | 43.01 |
| Non-essential amino acids | 56.60 | 57.00 |
* Essential amino acids
†Hydrophobic amino acids
Sensory properties of the selected protein hydrolysates
The intensity of fishy-, muddy-odour/flavour and bitterness of HA and HAPa are presented in Table 2. Fishy- and muddy- odour/flavour were negligible in both samples. However, no differences in those attributes were observed between HA and HAPa (P > 0.05). Fishy odour/flavour development associated with Nile tilapia protein hydrolysate was mainly caused by lipid oxidation, whereas muddy taint was related to muddy compounds known as geosmin (Yarnpakdee et al. 2014). During the preparation of PI, the undesirable materials including lipid or phospholipid were removed. HA showed a higher bitterness score than did HAPa (P < 0.05). This might be associated with the formation of peptides containing bulky hydrophobic groups toward their C-terminal such as valine, isoleucine, phenylalanine, tryptophan, leucine and tyrosine (Wu et al. 2003). Hydrolysis process with Alcalase might induce the exposure of the buried hydrophobic peptides, resulting in detection of bitter taste by human taste buds. Peptides containing hydrophobicity (Q) values >1,400 cal/mole and molecular masses <6 kDa are bitter in taste (Ney 1979). Additionally, the presence of internally sited Pro residues was also shown to be a major and distinct contributor to peptide bitterness (Ney 1979). Further hydrolysis of HA by papain was probably produced peptide with less bitterness by the removal of hydrophobic amino acid side chain to some extent. Due to the similar content of hydrophobic amino acids (Table 1), the differences in bitterness between HA and HAPa were plausibly governed by amino acid sequences of peptides in both hydrolysates. The result was in accordance with Wróblewska and Troszyñska (2005) who reported that the application of two-step hydrolysis with Alcalase, followed by papain improved the sensory quality of whey protein hydolysate via lowering the bitterness as compared to those produced using Alcalase, Alcalase + Pepsin or Alcalase + Alcalase. Liaset et al. (2000) found that the combination of Alcalase and Kojizyme effectively yielded a non-bitter cod byproduct hydrolysate.
Table 2.
Sensory properties of the selected protein hydrolysates derived from Nile tilapia PI (HA and HAPa)
| Tests | Attributes | HA | HAPa |
|---|---|---|---|
| Intensity † | Fishy - odour Fishy - flavour |
1.00 ± 0.82a
2.19 ± 1.35a |
1.45 ± 1.03a,§
2.96 ± 2.01a |
| Muddy - odour Muddy - flavour |
0.60 ± 0.41a
1.20 ± 0.72a |
0.96 ± 0.80a
1.46 ± 1.24a |
|
| Bitterness | 10.14 ± 1.03a | 6.24 ± 1.87b | |
| Acceptance †† | Colour Odour Taste Overall |
8.00 ± 0.55a
6.92 ± 0.90a 6.25 ± 1.14b 6.14 ± 0.79b |
7.83 ± 0.72a
6.73 ± 1.42a 7.08 ± 0.73a 6.75 ± 0.75a |
Values are given as mean ± SD (n = 3)
§Different superscripts in the same row indicate significant differences (P < 0.05)
†Score are based on 15 cm-line scales (0: none and 15: extremely strong odour/flavour)
††Score are based on 9-point hedonic scales (1: dislike extremely, 5: Neither like nor dislike, 9: Like extremely)
Colour, odour, taste and overall likeness scores of HA and HAPa are shown in Table 2. There was no difference in likeness scores of colour and odour between HA and HAPa (P > 0.05). According to the criterion for acceptability limit, the score greater than 5 indicates acceptability (Meilgaard et al. 2007). Pre-washing prior to alkaline solubilisation of fish mince yielded the resulting PI with a lower water soluble pigment, such as haem proteins (Yarnpakdee et al. 2012). As a result, the lighter colour was obtained with both hydrolysates, leading to high acceptability. A high likeness score of odour was in agreement with a low intensity of fishy- or muddy- odour. However, a slightly lower score in odour likeness was probably governed by the offensive smell associated with enzymes used. For taste and overall likeness, HA exhibited the lower likeness scores, compared with HAPa (P < 0.05). This was more likely due to the lower bitterness in HAPa. Saha and Hayashi (2001) noted that the formation of bitter peptide is the most serious problem in the practical use of food protein hydrolysates. Thus, the application of a two-step hydrolysis process using Alcalase followed by papain could be a promising mean for production of muscle protein hydrolysate with improved sensory quality via the reduction of bitterness.
Conclusion
Protein hydrolysate from Nile tilapia PI prepared using different single proteases exhibited various antioxidant activities, depending on DH and types of enzyme used. Hydrolysate prepared using Alcalase with 40 % DH possessed the highest antioxidant activities. The application of a two-step hydrolysis process using Alcalase and papain for the first and the second steps, respectively yielded the hydrolysate with antioxidant activities, both in vitro and oxidation model systems. The activities were stable in a wide range of pH (1–11) and during heating (30–100 °C). The increase in activity was found after digestion in GIMs. The antioxidant peptides in both HA and HAPa were characterised to have MW of 513 and 1,484 Da, capable of scavenging free radicals and metal chelation, respectively. Additionally, both hydrolysates were rich in essential amino acids. However, HAPa showed a stronger antioxidant activity together with a higher sensorial acceptability than did HA. Therefore, hydrolysate from Nile tilapia PI prepared using two-step hydrolysis could serve as the natural antioxidant for food preservation or as functional foods.
Acknowledgments
This research was supported by the Thailand Research Fund under the Royal Golden Jubilee Ph.D. Program to SuthasineeYarnpakdee (PHD/0226/2552) and the Grant-in-Aid for dissertation from Graduate School, Prince of Songkla University, Thailand. TRF senior research scholar program was also acknowledged for financial support.
References
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45(9):3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106(1):185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010;118(3):559–565. doi: 10.1016/j.foodchem.2009.05.021. [DOI] [Google Scholar]
- Buege J, Aust S. Microsomal lipid peroxidation. Method Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Chandrasekara A, Shahidi F. Inhibitory activities of soluble and bound millet seed phenolics on free radicals and reactive oxygen species. J Agric Food Chem. 2010;59(1):428–436. doi: 10.1021/jf103896z. [DOI] [PubMed] [Google Scholar]
- Decker EA, Warner K, Richards MP, Shahidi F. Measuring antioxidant effectiveness in food. J Agric Food Chem. 2005;53(10):4303–4310. doi: 10.1021/jf058012x. [DOI] [PubMed] [Google Scholar]
- FitzGerald R, O’cuinn G. Enzymatic debittering of food protein hydrolysates. Biotechnol Adv. 2006;24(2):234–237. doi: 10.1016/j.biotechadv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Foh MBK, Amadou I, Foh BM, Kamara MT, Xia W. Functionality and antioxidant properties of tilapia (Oreochromis niloticus) as influenced by the degree of hydrolysis. Int J Mol Sci. 2010;11(4):1851–1869. doi: 10.3390/ijms11041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel EN, Huang S-W, Aeschbach R. Antioxidant activity of green teas in different lipid systems. J Am Oil Chem Soc. 1997;74(10):1309–1315. doi: 10.1007/s11746-997-0062-8. [DOI] [Google Scholar]
- Je J-Y, Lee K-H, Lee MH, Ahn C-B. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res Int. 2009;42(9):1266–1272. doi: 10.1016/j.foodres.2009.06.013. [DOI] [Google Scholar]
- Jun S-Y, Park P-J, Jung W-K, Kim S-K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur Food Res Technol. 2004;219(1):20–26. doi: 10.1007/s00217-004-0882-9. [DOI] [Google Scholar]
- Khantaphant S, Benjakul S, Ghomi MR. The effects of pretreatments on antioxidative activities of protein hydrolysate from the muscle of brownstripe red snapper (Lutjanus vitta) LWT Food Sci Technol. 2011;44(4):1139–1148. doi: 10.1016/j.lwt.2010.10.009. [DOI] [Google Scholar]
- Khantaphant S, Benjakul S, Kishimura H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process Biochem. 2011;46(1):318–327. doi: 10.1016/j.procbio.2010.09.005. [DOI] [Google Scholar]
- Kim S-K, Kim Y-T, Byun H-G, Nam K-S, Joo D-S, Shahidi F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J Agric Food Chem. 2001;49(4):1984–1989. doi: 10.1021/jf000494j. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Hayes KD, Shahidi F. Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme. Int J Food Sci Technol. 2008;43(6):1019–1026. doi: 10.1111/j.1365-2621.2007.01555.x. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, Hayes K. Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis) J Food Sci. 2009;74(2):C126–C133. doi: 10.1111/j.1750-3841.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- Leksrisompong P, Gerard P, Lopetcharat K, Drake M. Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. J Food Sci. 2012;77(8):S282–S287. doi: 10.1111/j.1750-3841.2012.02800.x. [DOI] [PubMed] [Google Scholar]
- Li X, Luo Y, Shen H, You J. Antioxidant activities and functional properties of grass carp (Ctenopharyngodon idellus) protein hydrolysates. J Sci Food Agric. 2012;92(2):292–298. doi: 10.1002/jsfa.4574. [DOI] [PubMed] [Google Scholar]
- Liaset B, Lied E, Espe M. Enzymatic hydrolysis of by-products from the fish-filleting industry; chemical characterisation and nutritional evaluation. J Sci Food Agric. 2000;80(5):581–589. doi: 10.1002/(SICI)1097-0010(200004)80:5<581::AID-JSFA578>3.0.CO;2-I. [DOI] [Google Scholar]
- Meilgaard M, Civille GV, Carr BT. Sensory evaluation techniques. Boca Raton: CRC Press; 2007. [Google Scholar]
- Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124(4):1354–1362. doi: 10.1016/j.foodchem.2010.07.089. [DOI] [Google Scholar]
- Ney KH (1979) Bitterness of peptides: amino acid composition and chain length. In: Boudreau JC (ed) Food Taste Chemistry. ACS Washington DC, pp 149–173
- Ovissipour M, Rasco B, Shiroodi SG, Modanlow M, Gholami S, Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J Sci Food Agric. 2013;93(7):1718–1726. doi: 10.1002/jsfa.5957. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Kristinsson HG, Leeuwenburgh C. Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J Agric Food Chem. 2008;56(21):10359–10367. doi: 10.1021/jf8017194. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Mendis E, Byun H-G, Kim S-K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem. 2005;16(9):562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Robinson HW, Hogden CG. The biuret reaction in the deter serum proteins. J Biol Chem. 1940;135(2):727. [Google Scholar]
- Saha BC, Hayashi K. Debittering of protein hydrolyzates. Biotechnol Adv. 2001;19(5):355–370. doi: 10.1016/S0734-9750(01)00070-2. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Han X, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53(3):285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. New York: McGraw-Hill; 1980. [Google Scholar]
- Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem. 2000;11(3):128–131. doi: 10.1016/S0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Tavano OL. Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym. 2013;90:1–11. doi: 10.1016/j.molcatb.2013.01.011. [DOI] [Google Scholar]
- Theodore AE, Raghavan S, Kristinsson HG. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J Agric Food Chem. 2008;56(16):7459–7466. doi: 10.1021/jf800185f. [DOI] [PubMed] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J Food Biochem. 2007;31(2):266–287. doi: 10.1111/j.1745-4514.2007.00111.x. [DOI] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103(4):1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- Wang Y, Zhu F, Han F, Wang H. Purification and characterization of antioxidative peptides from salmon protamine hydrolysate. J Food Biochem. 2008;32(5):654–671. doi: 10.1111/j.1745-4514.2008.00190.x. [DOI] [Google Scholar]
- Wróblewska B, Troszyñska A. Enzymatic hydrolysis of cow’s whey milk proteins in the aspect of their utilization for the production of hypoallergenic formulas. Pol J Food Nutr Sci. 2005;14(4):349. [Google Scholar]
- Wu H-C, Chen H-M, Shiau C-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36(9):949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Yarnpakdee S, Benjakul S, Kristinsson HG. Effect of pretreatments on chemical compositions of mince from Nile tilapia (Oreochromis niloticus) and fishy odor development in protein hydrolysate. Int Aquat Res. 2012;4(1):7. doi: 10.1186/2008-6970-4-7. [DOI] [Google Scholar]
- Yarnpakdee S, Benjakul S, Penjamras P, Kristinsson HG. Chemical compositions and muddy flavour/odour of protein hydrolysate from Nile tilapia and broadhead catfish mince and protein isolate. Food Chem. 2014;142(1):210–216. doi: 10.1016/j.foodchem.2013.07.043. [DOI] [PubMed] [Google Scholar]
- You L, Zhao M, Regenstein JM, Ren J. Changes in the antioxidant activity of loach (Misgumus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010;120(3):810–816. doi: 10.1016/j.foodchem.2009.11.018. [DOI] [Google Scholar]