Abstract
Several lines of evidences have established a lineage between Oxidised LDL (Ox-LDL) to apoptosis of macrophages in which the high level of intracellular cholesterol play a crucial role. This study assesses the potency of Murraya koenigii (MK) leaf extract in alleviating LDL oxidation and Ox-LDL induced lipotoxicity in murine macrophage (RAW 264.7) cells. Results indicated that presence of MK extract prevented oxidation of LDL as evidenced by its oxidation kinetics and formation of LDL oxidation products. Also, MK extract accounted for improvement in cell viability and mitochondrial membrane potential of Ox-LDL treated cells. The Ox-LDL induced increment in intracellular oxidative stress, nuclear condensation and apoptosis was effectively prevented by MK extract possibly due to their established anti-oxidant and free radical scavenging potentials which may be attributed to the presence of flavonoids present in the extract. Prevention of oxidative modification of LDL, free radical induced damage and Ox-LDL induced death of RAW 264.7 cells provide preliminary evidences of its anti-atherosclerotic potential and warrants further elucidation and validation for its use in-vivo and may be useful as a functional food supplement and an alternative medicine to prevent LDL oxidation and oxidized LDL induced toxicity.
Keywords: Ox-LDL, Atherosclerosis, RAW 264.7 macrophage cells, Oxidative stress, Murraya koenigii
Introduction
Cardiovascular diseases (CVDs) are among the prime causes of morbidity and mortality in developed as well as developing nations (Stachura and Pierzynowski 2009). Low density lipoproteins (LDL) are an integral part of cholesterol homeostasis and play a crucial role in onset and progression of CVDs including atherosclerosis.
Oxidatively modified LDL (Ox-LDL) has a chemo-attractant-like activity that triggers an immune response viarecruitment of monocytes that differentiate into macrophages for clearing Ox-LDL particles deposited inside the endothelial layer of arterial wall. Such lipid laden foam cells account for formation of an atheromatous plaque. An unstable plaque results in occurrence of cardiovascular ailments including myocardial infarction or stroke (Berliner and Heinecke 1996; Aviram and Fuhrman 1998; Heinecke 1998; Steinbrecher 1999).
Natural antioxidants and lipid lowering compounds originating from functional foods, spices, and herbs find extensive application in prevention of atherosclerosis because of their ability to prevent LDL oxidation (Chang et al. 2006) and plaque formation (Ho et al. 2010). Murraya koenigii (family-Rutaceae; subfamily-Aurantoidae; curry leaf plant; MK) is traditionally used as a flavoring agent in Asian delicacies and as a condiment. Aerial parts of MK have been used in traditional system of medicine for the treatment of indigestion, influenza, rheumatism, traumatic injury and as an anti-inflammatory agent (Kong et al. 1986).
Dietary co-supplementation of MK has been reported to lower plasma lipid profile, prevent obesity and improve insulin resistance in streptozotocin induced diabetic rats (Birari et al. 2010; Tembhurne and Sakarkar 2010). MK extract has been reported to be rich in phenolic acids such as tannic acid, gallic acid, caffeic acid, cinnamic acid, chlorogenic acid, ferulic acid, and vanillic acid (Singh et al. 2004). A pilot study in our lab had established hepatoprotective potential of MK extract in carbon tetrachloride (CCl4) induced hepatotoxic rat model due to high contents of flavonoids and polyphenols present in the extract (Desai et al. 2012).
Oxidative modification of LDL and its subsequent uptake by macrophages and their transformation into foam cells are one of the key events toward formation of atheromatous plaque and progression of atherosclerosis (Libby 2002; Skalen et al. 2002). Since, MK leaf extract has been reported to be a potent anti-oxidant (Tachibana et al. 2001), it was thought pertinent to assess the potency of this extract in alleviating LDL oxidation and Ox-LDL induced lipotoxicity in RAW 264.7 cells.
Materials and methods
Materials
Sodium carbonate, folin’s reagent,, potassium acetate, sodium chloride, copper sulphate, ethylene di-amine tetrachloro-acetic acid (EDTA), thiobarbituric acid (TBA), trichloro-aceticacid (TCA), Butylated hydroxyanisole (BHT), sodium dodecyl sulphate (SDS), di-nitrophenyl hydrazine (DNPH), heptane, ethyl acetate, glycerol, bromophenol blue and coomassie brilliant blue were purchased from Sisco Research Laboratories, Mumbai, India. Hydrochloric acid (HCl), sulphuric acid (H2SO4), glacial acetic acid and were purchased from Suvidhanath Laboratories, Baroda, India. Barbituric acid and Na-barbiturate were purchased from National Chemicals, Baroda, India. Acridine orange (AO), ethidium bromide (EtBr), phosphate buffer saline (PBS), Phosphomolybdic acid (PMA), RPMI-1640, fetal bovine serum, Dimethyl sulfoxide (DMSO), 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl tetrazolium bromide (MTT), Dulbecco’s modified eagles medium (DMEM) and antimicrobial-antimycotic solution were purchased from Himedia Laboratories, Mumbai, India.
Aluminium chloride, Dichloro-dihydro-fluorescein diacetate (DCF-DA), rhodamine-123 (RHO), 4′,6-diamidino-2-phenylindole (DAPI), were purchased from Sigma-Aldrich, USA.
Methods
Collection of plant material and preparation of extract
Fresh leaves of Murraya koenigii (MK) leaves were collected in the month of December-2011 from the local market and shade dried to obtain powder. Flavonoid rich extract was obtained by extracting the powder with 80 % methanol in a soxhlet apparatus. Briefly, leaves were defatted overnight using 70 % petroleum ether and extracted using 80 % methanol and concentrated in a rotary evaporator. The resultant extract was hydrolysed in a water bath (H2O at 60 °C) for 24 h that yielded two phases. Quantitative determination of flavonoids was performed in the organic phase and the same was used for further investigation (Krug and Borkowski 1965).
Total flavonoid estimation
The amount of total flavonoid content was determined by Aluminium chloride method (Chang et al. 2002). Briefly, 1.0 ml of extract, 0.5 ml of 1.2 % aluminium chloride and 0.5 ml of 120 mM potassium acetate was incubated at room temperature for 30 min and absorbance was measured at 415 nm. Quercetin was used as a standard. The concentration of flavonoids was expressed in terms of μg/mg equivalent- quercetin/100 mg plant extract.
Isolation of LDL and LDL oxidation kinetics
LDL was isolated according to the method of Wieland and Seidel (1983). Briefly, LDL was isolated from healthy normolipidemic adult volunteer, who had fasted overnight and collected by venepuncture into clean sterile glass tubes and allowed to stand for 45 min at room temperature. Serum was recovered by centrifuging tubes at 3,000 rpm for 15 min at 4 °C. Briefly 0.1 ml of serum sample was mixed with 1 ml of heparin citrate buffer (0.064 M trisodium citrate, pH 5.04 adjusted using 5 N HCl with 50,000 IU/L of heparin), vortexed and allowed to stand for 15 min at room temperature. The contents were centrifuged at 1,000 g for 10 min and the resultant pellet was dissolved in 0.1 ml of PBS and used for further analysis. Every time, a fresh LDL sample was isolated and used for analysis.
For studying LDL oxidation, 100 μg/ml of LDL was with or without of MK extract (10, 25, 50, 100, 200, 300 μg/ml) at 37 °C for 15 min. At the end of incubation period, oxidation was initiated by adding 10 μl of freshly prepared 0.167 mM CuSO4 and LDL oxidation kinetics were determined by monitoring the change in absorbance at every 10 min interval for a total period of 180 min. Readings were recorded at 234 nm in a UV–VIS Perkin Elmer spectrophotometer. The Lag time was determined from the intercepts of lines through the linear portions of the lag phase and propagation phase and the rate of oxidation was determined from the slope of the propagation phase. The concentration of CD in the samples was calculated by using a molar extinction coefficient of 2.95 × 104 M−1 cm−1. Maximum concentration of CD formed was calculated from the difference in the concentration of CD at zero time and at absorption maxima. (Esterbauer et al. 1989).
Estimation of LDL oxidation end products
All the sample tubes were taken in triplicates. The oxidation of LDL was mediated by copper sulphate in presence or absence of MK extract (10, 25, 50, 100, 200, 300 μg/ml) for 24 h. Later, 10 μl of 7 mM BHT was added in each tube to stop the oxidation process and samples malondialdehyde (MDA), lipid hydroperoxides (LHP) and protein carbonyls (PC) was assayed in the samples by following methods.
For MDA estimation, 100 μl aliquot of sample was mixed with 1 ml TBA reagent (0.37%TBA, 15 % TCA in 0.25 N HCl) and placed in water bath at 100 °C for 60 min. The reaction mixture was brought to room temperature and the supernatant was collected by centrifuging tubes at 3,000 rpm for 10 min. The absorbance of the supernatant was measured at 532 nm with UV/VIS Perkin-Elmer spectrophotometer and MDA was calculated using a molar absorption coefficient of 1.56 × 105 M−1 cm−1 Buege and Aust (1978).
For LHP estimation, 100 μl aliquot of sample was mixed with 0.9 ml of Fox reagent (250 μM ammonium sulfate, 100 μM xylenol orange, 25 mM H2SO4 and 4 mM BHT in 90 % (v/v) HPLC-grade methanol) and incubated at 37 °C for 45 min to develop a colour. The reaction mixture was read at 560 nm and LHP content was determined using the molar absorption coefficient of 4.3 × 104 M−1 cm−1 (Nourooz-Zadeh et al. 1996).
For PC estimation, 100 μl aliquot of sample was taken in a tube and mixed with 200 μl of DNPH and incubated for 1 h at room temperature. After incubation, 0.6 ml denaturing buffer (0.15 M sodium phosphate buffer containing 3 % SDS) was added and mixed followed by addition of ethanol and heptane (1:1). The reaction mixture was mixed and centrifuged at 3,500 rpm to precipitate protein. The protein was washed three times with 1 ml ethylacetate/ethanol and dissolved in 1 ml denaturing buffer and read at 360 nm in a spectrophotometer. The carbonyl content was calculated from the absorbance using an absorption coefficient e of 22,000 M−1 cm−1 Reznick and Packer (1994).
ApoB fragmentation analysis
The LDL samples were treated as indicated above with or without MK and later were diluted using sample buffer (0.6 M Tris–HCl, pH 6.8,1 % SDS, 10 % Sucrose, β-Mercaptoethanol and 0.5 % Bromophenol blue) and incubated at 95 °C for 5 min. Later, all of these were subjected to electrophoretic separation (using 10 % SDS polyacrylamide gel) at 100 V for 6 h. The gel was subsequently stained with Coomassie brilliant blue R250 overnight and destained using a solution containing methanol: glacial acetic acid: water followed by its imaging using Bio-Rad gel documentation system (Miura et al. 1994).
Culture of RAW 264.7 murine macrophage cells
The RAW 264.7 cell line was procured from NCCS (National Centre for Cell Sciences) Pune, India. Cells were cultured using Dulbecco’s Modified Eagles Medium (DMEM) with 10 % FBS in a humidified incubator at 5 % CO2 and 37 °C, later passaged using a scraper at split ratio of 1:3 in a 6 well plate (0.5 × 106 cells). Thereafter, cells were seeded in 96 well plates (0.5 × 106 cells) for cytotoxicity assays. Cells were also grown on sterile glass cover slips in 6-well plates and the same were used for apoptosis, nuclear condensation, ROS generation and mitochondrial membrane potential analysis.
Ox-LDL induced cytotoxicity assay
The murine macrophage RAW 264.7 cells were seeded in a tissue culture 96 well plate as mentioned above and was exposed to 100 μg/ml of Ox-LDL for 24 h with and without indicated concentrations of MK in a humidified CO2 incubator with 5 % CO2. After 24 h, cells were incubated with 0.5 mg/mL MTT in culture medium for 4 h. The resultant purple formazan crystals were dissolved in DMSO and read at 563 nm (using Bio-Tek instruments, Inc., Winooski, VT) (Ferrari et al. 1990).
Mitochondrial dysfunction test
Mitochondrial membrane potential (ΔΨm) was assessed using a lipophilic cationic probe Rhodamine123 (RHO 123). After the treatment with Ox-LDL for 24 h in the presence or absence of MK (10, 25, 50,100,200 and 300 μg/mL) in a humidified CO2 incubator with 5 % CO2, cells were washed incubated with 0.001 mM RHO123 for 10 min at 37 °C and the fluorescence was determined (485 and 530 nm exCitation and emission respectively) using spectroflurometer (Jasco FP-6350). After incubation at 37 °C for 20 min, cells were washed with cold PBS, lysed using 0.5 % triton X-100 and centrifuged at 2,500 rpm. Later, supernatant was collected and read using a spectrofluorimeter (Jasco FP-6350) Pereira and Oliveira (2000).
Intracellular oxidative stress assay
2′,7′-Dichlorofluorescin diacetate (DCFH-DA), a potent ROS detection dye was used as a probe for the presence of intracellular oxidative stress. RAW 264.7cells were pre-incubated with or without 200 μg/ml of MK for 30 min and later with 100 μg/ml Ox-LDL in a 6 well plate for 24 h in a humidified CO2 incubator with 5 % CO2. Later, cells were washed and incubated with 2.5 μM/ml DCFH-DA for 10 min. The cells were then thoroughly washed 3 times with PBS and photographed immediately under Leica fluorescent microscope (Degli-Esposti 2001).
Nuclear condensation assay
RAW 264.7 cells were grown on glass cover slips in 6 well tissue culture plates as mentioned above and treated with Ox-LDL (100 μg/ml) for 24 h in presence or absence of 200 μg/ml of MK in a humidified CO2 incubator with 5 % CO2. Later, cells were washed with PBS twice for 10 min at room temperature. Cells were stained with 0.6 μg/mL of DAPI for 5 min and washed thrice to remove unbound and excess stain and nuclear characteristics were observed under a fluorescence microscope. Apoptotic cells were morphologically defined on the basis of nuclear shrinkage and chromatin condensation (Chen et al. 2005).
Apoptosis assay
RAW 264.7 cells were grown 6 well culture plate treated with Ox-LDL (100 μg/ml) for 24 h in presence or absence of MK extract (200 μg/ml) in a humidified CO2 incubator with 5 % CO2. Cells were stained with 5 μl of Acridine Orange (AO) and Ethidium Bromide (EB) dyes (100 μg/ml) in PBS for 5 min in dark and immediately washed thrice with PBS. Cells were re-suspended in PBS and observed under fluorescence microscope (Leica DMRB fluorescence microscope) and photographed (Arunkumar et al. 2005).
Statistical analysis
All analysis were run in triplicate and were expressed as means ± S.E.M., except the results of oxidative stress, nuclear condensation and apoptosis which were carried out as two independent experiments performed in triplicate. Statistical analysis was done using Graph Pad Prism version 3.0 for Windows (Graph Pad Software, San Diego, California, USA) and significant differences were calculated by ANOVA and Bonferroni’s multiple comparison test taking consideration by least significant difference test (P < 0.05), unless noted otherwise.
Results and discussion
According to the method published earlier by (Chang et al. 2002), the total flavonoid content in MK extract was found to be 3.1 ± 0.5 μg/mg using quercetin as a standard.
Oxidative modification of LDL in atherosclerotic inflammation has been reported to be the causative agent in its covalent modification and the production of harmful intermediary oxidation products (Steinberg 1997). The key event to the onset of atherosclerosis involves reactive oxygen species mediated oxidative modification of LDL particle that leads to the formation of conjugated dienes (CD). In subsequent events, the CD undergoes several molecular interactions and rearrangements leading to the formation of end products such as lipid hydroperoxides (LHP) and malondialdehydes (MDA) that oxidatively modify apolipoprotein B100 of LDL particle. Uptake of these oxidized LDL particles transforms macrophages into foam cell that subsequently undergo apoptosis (Young and McEneny 2001).
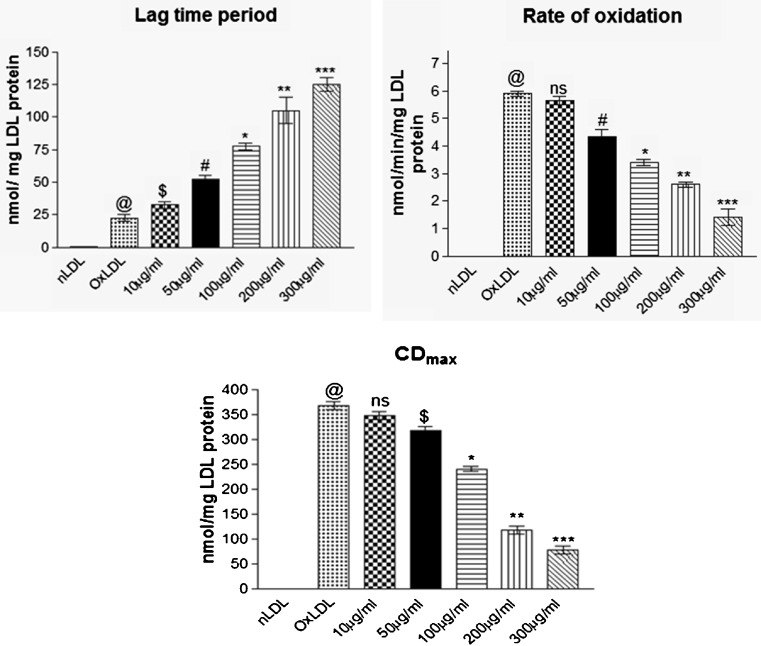
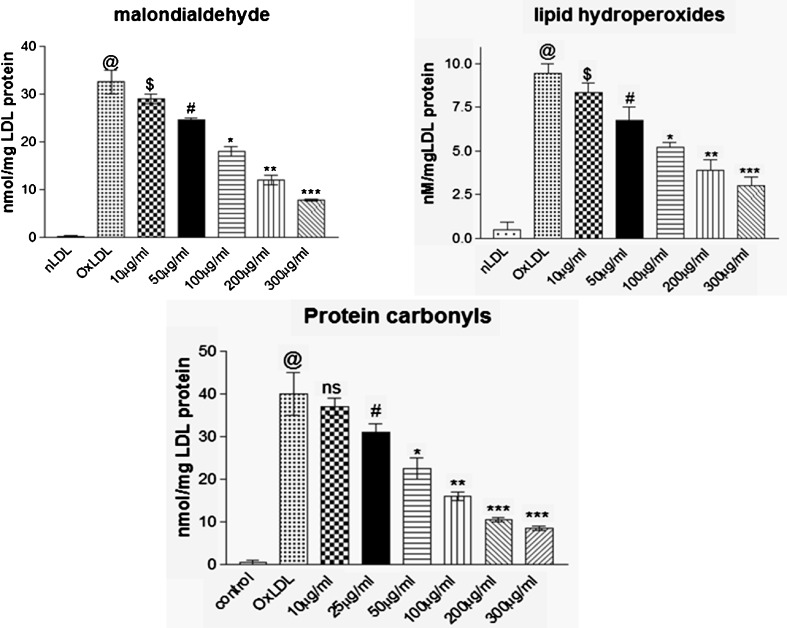
In the present study, co-supplementation of MK extract significantly increased (p < 0.041) the lag phase of LDL oxidation and reduced rate of oxidation of CDs compared to groups without co-supplementation of MK extract which reveals that MK extract prevented oxidative stress generated due to copper sulphate mediated free radicals compared to control group (Fig. 1). Figure 2 represents Cu2+ mediated LDL oxidation which is characterized by elevated indices of TBARS, LHP and PC whereas, presence of MK extract significantly (p < 0.024) minimized the production of TBARS, LHP and PC during LDL oxidation These results are attributable to the established free radical scavenging and metal chelating properties of MK leaves that prevents oxidative modification of LDL. High contents of total flavonoids present in MK leaf extract possibly favour prevention of LDL oxidation which is in agreement with other flavonoid rich extracts with potent anti-oxidant and free radical scavenging potentials (Chograni et al. 2013; Kamiyama and Shibamoto 2012; Bitis et al. 2010).
Fig. 1.
Effect of MK extract on LDL oxidation kinetics (lag time period, rate of oxidation and maximum conjugated diene formation; CDmax) in Cu+2 induced LDL oxidation. Data expressed as mean ± S.E.M. for n = 3. @p < 0.001 compared to nLDL, ns non-significant, $p > 0.05, * p < 0.048, ** p < 0.021, *** p < 0.015 compared to Ox-LDL
Fig. 2.
Effect of MK extract on LDL oxidation end products (malondialdehyde, lipid hydroperoxides and protein carbonyls) in Cu+2 induced LDL oxidation. Data expressed as mean ± S.E.M. for n = 3. @p < 0.001 compared to nLDL, $p > 0.05, #p < 0.05, * p < 0.042, ** p < 0.028, *** p < 0.001 compared to Ox-LDL
Free radicals and oxidative stress induces derivatization of lysine residue of apolipoprotein B (ApoB) protein in LDL particles leads to its fragmentation. This is one of a key event in the onset and progression of atherosclerosis Young and McEneny (2001). In our study, Ox-LDL showed absence of ApoB protein band indicating at its free radical induced fragmentation and loss of integrity. However MK co-supplementation showed a dose dependent re-appearance of the ApoB band suggesting that presence of MK extract accounted for prevention of ApoB fragmentation and preserved its integrity (Fig. 3). These observations are in accordance with our previous studies wherein, flavonoid and polyphenol rich herbal extracts have been reported to prevent ApoB fragmentation of Ox-LDL samples (Thounaojam et al. 2011; Jadeja et al. 2011, 2012).
Fig. 3.
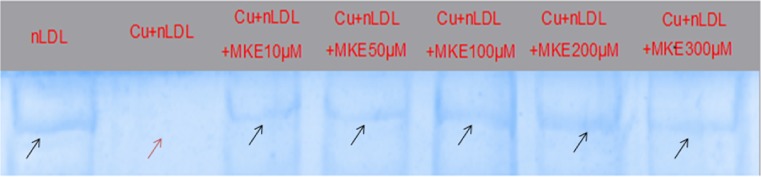
Effect of MK extract on ApoB fragmentation in Cu+2 induced LDL oxidation. Black arrow indicated presence of ApoB protein band in SDS-Polyacryl amide gel while Cu+2 induced oxidative modification which led to fragmentation of the protein and disappearance of protein band in the gel (red arrow). However, MK extract successfully prevented ApoB fragmentation which is indicated by black arrow in respective wells
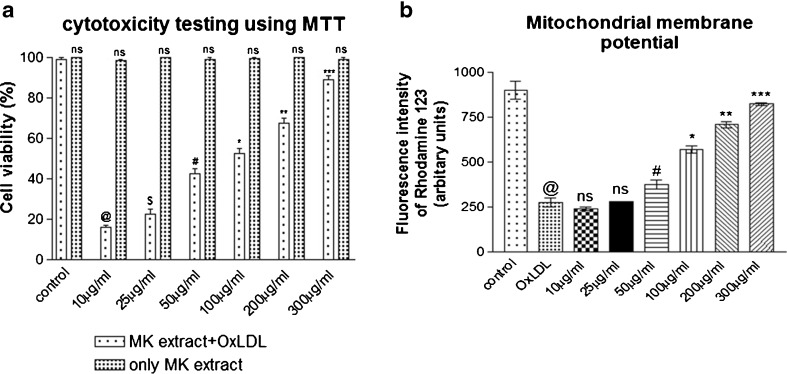
The ox-LDL induces pro-inflammatory changes in vascular endothelium and macrophage mediated uptake of ox-LDL transforms them into “lipid laden” foam cells (Steinberg 1997). Hence, in the second phase of the study, murine macrophages RAW 264.7 cells cultured in presence of Ox-LDL showed significant decrement in cell viability as evidenced by MTT based cytotoxicity assay. However, presence of 200 μg/ml MK extracts lead to a dose dependent improvement in cell viability (Fig.4a). Also, MK extract was found to be non-toxic up to 1,000 μg/ml (data not shown). Mitochondrial dysfunction is attributable to peroxyl radicals generated by Ox-LDL Madamanchi and Runge (2007) and hence, in the present study, mitochondrial membrane potential was examined using a lipophilic cationic dye Rhodamine 123 (Hoye et al. 2008). Treatment with Ox-LDL significantly decreased mitochondrial membrane potential as compared to the control cells. However, co-supplementation of MK extract was able to alleviate ox-LDL mediated mitochondrial dysfunction as evidenced by results comparable to the control (Fig. 4b). These observations further indicate at the ability of MK extract in preventing ox-LDL induced mitochondrial dysfunction and warrants further study to decipher the underlying mechanism of MK extract mediated alleviation of ox-LDL induced cytotoxicity of macrophages.
Fig. 4.
a Effect of MK extract on Ox-LDL induced cytotoxicity in RAW 264.7 cells. Data expressed as mean ± S.E.M. for n = 3. ns; non-significant, @p < 0.001 compared to control cells, $p > 0.05, #p < 0.05, * p < 0.02, ** p < 0.01, *** p < 0.001 compared to Ox-LDL treated cells b Effect of MK extract on Ox-LDL induced alterations in mitochondrial membrane potential in RAW 264.7 cells. Data expressed as mean ± S.E.M. for n = 3. @p < 0.001 compared to control cells, ns non-significant, #p < 0.05, * p < 0.02, ** p < 0.01, *** p < 0.001 compared to Ox-LDL treated cells
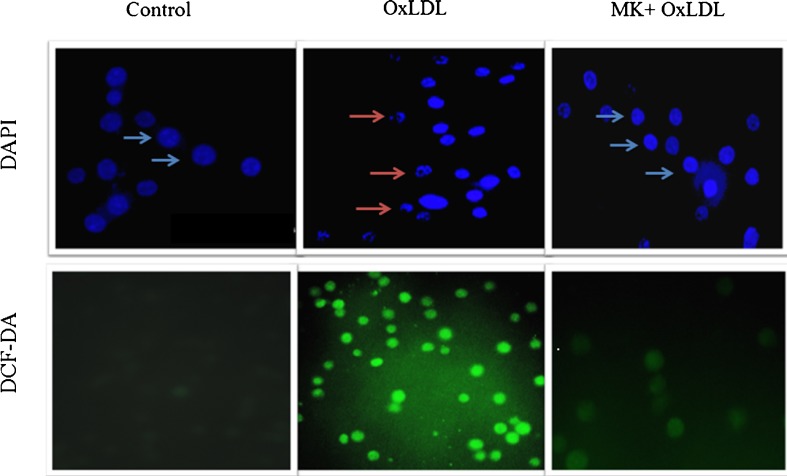
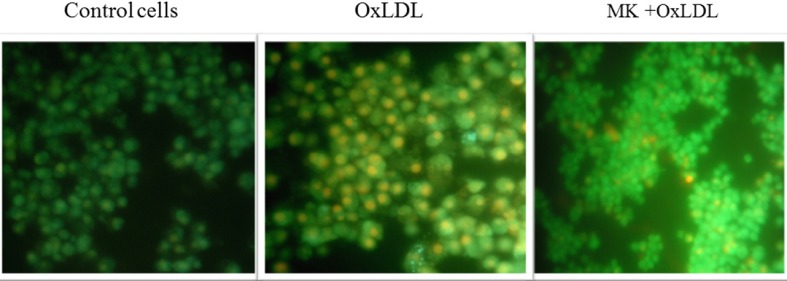
Macrophages mediated uptake of Ox-LDL produces heightened levels of intracellular ROS which in turn have a more subtle impact on the atherogenic process by modulating intracellular oxidative pathways in vascular tissues to affect cell adhesion, migration, proliferation and differentiation culminating in apoptosis (Patel et al. 2000). In the present study, a lipophilic and non-fluorescent compound; H2DCF-DA (dihydrodichlorofluoresceindiacetate) stain was used to evaluate intracellular oxidative stress (Wang and Joseph (1999) wherein; Ox-LDL treated RAW 264.7 cells showed prominent green fluorescence compared to control cells. Co-supplementation of 200 μg/ml of MK extract resulted in significant decrement in oxidative stress as evidenced by weak green fluorescence which was comparable with control cells (Fig. 5). These results are in agreement with reports of other research groups (Ningappa et al. 2008) that have attributed the same to the anti-oxidant and free radical scavenging potential of MK leaf extract.
Fig. 5.
Effect of MK extract on Ox-LDL induced nuclear condensation and oxidative stress in RAW 264.7 cells. Control; cells grown in normal condition, Ox-LDL; cells grown in presence of Ox-LDL and MK + Ox-LDL; cells grown with Ox-LDL and MK extract (200 μg/ml). Blue arrow indicates nuclei with normal chromatin structure and red arrow indicates condensed nuclei of cells
The term apoptosis refers to a peculiar morphology of cell death. It is of special interest because it can be triggered physiologically (for the removal of damaged or unwanted cells) and pathologically (foam cells in atherosclerosis) which is regulated by the actions of specific gene products resulting in the removal of damaged or unwanted cells (Squier and Cohen 2001). In our study, nuclear morphology was assessed using a fluorescent dye, DAPI (4′,6-Diamidine-2′-phenylindole dihydrochloride) to assess possible apoptotic changes (Gonzalez-Juanatey et al. 2004). Ox-LDL treatment provided visual evidence of nuclear condensation as compared to control cells. However, Ox-LDL + MK co-supplemented group showed nuclear characteristics comparable to that of control cells possibly due to the ability of MK extract in preventing apoptotic changes (Fig. 5). The same was further confirmed using AO-EB staining that accounted for a large population of EtBr positive cells compared to AO positive cells. These results in agreement with ox-LDL induced nuclear condensation and oxidative stress also observed herein. However, MK treated cells showed more number of AO positive viable cells (Fig. 6). These results provide valuable supportive evidence to the MK mediated prevention of Ox-LDL induced apoptosis of RAW 264.7 cells.
Fig. 6.
Effect of MK extract on Ox-LDL induced apoptosis in RAW 264.7 cells. Control; cells grown in normal condition, Ox-LDL; cells grown in presence of Ox-LDL and MK + Ox-LDL; cells grown with Ox-LDL and MK extract (200 μg/ml)
Several lines of evidences have established a lineage between Ox-LDL and apoptosis of macrophages in which the high intracellular oxidative stress and apoptosis resulting due to elevated levels of ox-LDL play a crucial role (Steinberg 1997). In this regard, the present study is a preliminary investigation that reports on anti-atherosclerotic potential MK leaf extract by preventing ox-LDL induced macrophage apoptosis by lowering intracellular oxidative stress. The same warrants a detailed study at organismal level to establish its in-vivo anti-atherogenic potential and validation for its use as an alternative medicine.
Conclusion
MK leaf extract shows remarkable potential in preventing oxidative modification of LDL, ox-LDL induced cell death and subsequent free radical induced damages to the murine macrophage cells. The same is attributable to their anti-oxidant and free radical scavenging potentials due to high contents of flavonoids and polyphenols. These attributes also provide compelling preliminary evidence on anti-atherosclerotic potential of MK leaf extract.
Acknowledgments
Authors acknowledge Gujarat State Biotechnology Mission (GSBTM), Gandhinagar, Gujarat, India for funding the study through FAP-2010 and Co-ordinator, DBT-ILSPARE for infrastructure and technical support.
References
- Arunkumar A, Vijayababu MR, Kanagaraj P, Balasubramanian K, Aruldhas MM, Arunakaran J. Growth suppressing effect of garlic compound diallyldisulfide onprostate cancer cell line (pc-3) in vitro. Biol Pharm Bull. 2005;28:740–743. doi: 10.1248/bpb.28.740. [DOI] [PubMed] [Google Scholar]
- Aviram M, Fuhrman B. LDL oxidation by arterial wall macrophages depends on the oxidative status in the lipoprotein in the cells: role of pro-oxidants vs antioxidants. Mol Cell Biochem. 1998;188:149–159. doi: 10.1023/A:1006841011201. [DOI] [PubMed] [Google Scholar]
- Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- Birari R, Javia V, Bhutani KK. Antiobesity lipid lowering effects of Murrayakoenigii (l) spring leaves extracts mahanimbine on high fat diet induced obese rats. Fitoterapia. 2010;81:1129–1133. doi: 10.1016/j.fitote.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Bitis L, Kultur S, Melıkoglu G, Ozsoy N, Can A. Flavonoids and antioxidant activity of Rosa agrestis leaves. Nat Prod Res. 2010;24:580–589. doi: 10.1080/14786410903075507. [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chang YC, Huang KX, Huang AC, Ho YC, Wang CJ. Hibiscus anthocyanins- rich extract inhibited LDL oxidation OxLDL-mediated macrophages apoptosis. Food Chem Toxicol. 2006;44:1015–1023. doi: 10.1016/j.fct.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chen PN, Chu SC, Chiou HL, Chiang CL, Yang SF, Hsieh YS. Cyanidin 3-glucoside peonidin 3-glucoside inhibit tumor cell growth induce apoptosis in-vitro suppress tumour growth in-vivo. Nutr Cancer. 2005;53:232–243. doi: 10.1207/s15327914nc5302_12. [DOI] [PubMed] [Google Scholar]
- Chograni H, Riahi L, Zaouali Y, Boussaid M. Polyphenols, flavonoids, antioxidant activity in leaves and flowers of Tunisian globulariaalypum L. (Globulariaceae) Afr J Ecol. 2013;51:343–347. doi: 10.1111/aje.12041. [DOI] [Google Scholar]
- Degli EM. Assessing functional integrity of mitochondria in vitro in vivo. In: Pon LA, Schon EA, editors. Methods in cell biology, vol.65. California: Academic; 2001. pp. 75–96. [DOI] [PubMed] [Google Scholar]
- Desai SN, Patel DK, Devkar RV, Patel PV, Ramachandran AV. Hepatoprotective potential of polyphenol rich extract of Murrayakoenigii L:an in vivo study. Food Chem Toxicol. 2012;50:310–314. doi: 10.1016/j.fct.2011.10.063. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Striegl G, Puhl H, Rotheneder M. Continuous monitoring of in-vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6:67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165–172. doi: 10.1016/0022-1759(90)90187-Z. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juanatey JR, Pineiro R, Iglesias MJ, Gualillo O, Kelly PA, Dieguez C, Lago F. GH prevents apoptosis in cardiomyocytes cultured in-vitro through a calcineurin-dependent mechanism. J Endocrinol. 2004;180:325–335. doi: 10.1677/joe.0.1800325. [DOI] [PubMed] [Google Scholar]
- Heinecke JW. Oxidants antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low-density lipoprotein hypothesis. Atherosclerosis. 1998;141:1–15. doi: 10.1016/S0021-9150(98)00173-7. [DOI] [PubMed] [Google Scholar]
- Ho HH, Hsu LS, Chan KC, Chen HM, Wu CH, Wang CJ. Extract from the leaf of Nucifera, reduced the development of atherosclerosis via inhibition of vascular smooth muscle cell proliferation and migration. Food Chem Toxicol. 2010;48:159–168. doi: 10.1016/j.fct.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Hoye AT, Davoren JE, WipfP FMP, Kagan VE. Targeting mitochondria. Acc Chem Res. 2008;41:87–97. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- Jadeja RN, Thounaojam MC, Devkar RV, Ramachandran AV. Clerodendronglandulosum (coleb) extract prevents in vitro human LDL oxidation oxidized LDL induced apoptosis in human monocyte derived macrophages. Food Chem Toxicol. 2011;49:1195–1202. doi: 10.1016/j.fct.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Jadeja RN, Thounaojam MC, Sankhari JM, Jain M, Devkar RV, Ramachandran AV. Standardized flavonoid-rich Eugeniajambolana seed extract retards in-vitro in-vivo LDL oxidation expression of VCAM-1 p-selectin in atherogenicrats. Cardiovasc Toxicol. 2012;12:73–82. doi: 10.1007/s12012-011-9140-0. [DOI] [PubMed] [Google Scholar]
- Kamiyama M, Shibamoto T. Flavonoids with potent antioxidant activity found in young green barley leaves. J Agric Food Chem. 2012;60:6260–6267. doi: 10.1021/jf301700j. [DOI] [PubMed] [Google Scholar]
- Kong YC, Ng KH, But PP, Li Q, Yu SX, Zhang HT, Cheng KF, Soejarto DD, Kan WS, Waterman PG. Sources of the anti-implantation alkaloid yuehchukene in the genus Murraya. J Ethnopharmacol. 1986;15:195–200. doi: 10.1016/0378-8741(86)90155-8. [DOI] [PubMed] [Google Scholar]
- Krug H, Borkowski N. Neue flavonoid-glykosideaus den blättern von peumusboldus. Die pharmazie. 1965;20:692–698. [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- Miura S, Watanabe J, Tomita T, Sano M, Tomita I. The inhibitory effects of tea polyphenols (flavon-3-ol derivatives) on Cu2+ mediated oxidative modification of low-density lipoprotein. Biol Pharm Bull. 1994;17:1567–1572. doi: 10.1248/bpb.17.1567. [DOI] [PubMed] [Google Scholar]
- Ningappa MB, Dinesha R, Srinivas L. Antioxidant free radical scavenging activities of polyphenol-enriched curry leaf (MurrayakoenigiiL) Extracts. Food Chem. 2008;106:720–728. doi: 10.1016/j.foodchem.2007.06.057. [DOI] [Google Scholar]
- Nourooz-zadeh J, Tajaddini-sarmadi J, Ling KL, Wolff SP. Low-density lipoprotein is the major carrier of lipid hydroperoxides in plasma relevance to determination of total plasma lipid hydroperoxide concentrations. Biochem J. 1996;313:781–786. doi: 10.1042/bj3130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-usmar VM. Cell signalling by reactive nitrogen species and oxygen species in atherosclerosis. Free Radic Biol Med. 2000;28:1780–1794. doi: 10.1016/S0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- Pereira CF, Oliveira CR. Oxidative glutamate toxicity involves mitochondrial dysfunction, perturbation of intracellular Ca2+ homeostasis. Neurosci Res. 2000;37:227–236. doi: 10.1016/S0168-0102(00)00124-3. [DOI] [PubMed] [Google Scholar]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh DP, Maurya S, Maheshwari R, Singh M, Dubey RS, Singh RB. Investigation on the phenolics of some spices having pharmacotherapeutic properties. J Herb Pharmacother. 2004;4:27–42. doi: 10.1080/J157v04n04_03. [DOI] [PubMed] [Google Scholar]
- Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- Squier MKT, Cohen JJ. Standard quantitative analysis for apoptosis. Mol Biotechnol. 2001;19:305–312. doi: 10.1385/MB:19:3:305. [DOI] [PubMed] [Google Scholar]
- Stachura M, Pierzynowski SG. Atherosclerosis and mitochondrial dysfunction - possible links. J Pre Clin Res. 2009;3:091–094. [Google Scholar]
- Steinberg D. Low density lipoprotein oxidation: its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- Steinbrecher UP. Receptors for oxidized low-density lipoproteins. Biochem Biophys Acta. 1999;1436:279–298. doi: 10.1016/s0005-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Kikuzaki H, LajisNH NN. Antioxidative Activity of Carbazoles from Murrayakoenigii Leaves. J Agric Food Chem. 2001;49:5589–5594. doi: 10.1021/jf010621r. [DOI] [PubMed] [Google Scholar]
- Tembhurne SV, Sakarkar DM. Protective effect of Murrayakoenigii (l) leaves extract instreptozotocin induced diabetic rats involving possible antioxidant mechanism. J Med Plants Res. 2010;4:2418–2423. [Google Scholar]
- Thounaojam MC, Jadeja RN, Devkar RV, Ramachandran AV. In-vitro evidence for the protective role of Sidarhomboidea (Roxb) extract against LDLoxidation oxidizedLDL-induced apoptosis in human monocyte-derived macrophages. Cardiovasc Toxicol. 2011;11:168–179. doi: 10.1007/s12012-011-9110-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. J Lipid Res. 1983;24:904–909. [PubMed] [Google Scholar]
- Young IS, McEneny J. Lipoprotein oxidation atherosclerosis. Biochem Soc Trans. 2001;29:358–362. doi: 10.1042/BST0290358. [DOI] [PubMed] [Google Scholar]