Abstract
Syzygium aromaticum (L.) (clove) is one of the most widely cultivated spices in many tropical countries. The aim of this study was to determine the phytochemical content, the antioxidant properties and the antiglycation properties of aqueous extract of clove against fructose-mediated protein glycation and oxidation. The result showed that the content of total phenolics and flavonoids in clove extract was 239.58 ± 0.70 mg gallic acid equivalents/g dried extract and 65.67 ± 0.01 mg catechin equivalents/g dried extract, respectively. In addition, clove exhibited antioxidant properties including DPPH radical scavenging activity (IC50 = 0.29 ± 0.01 mg/ml), Trolox equivalent antioxidant capacity (4.69 ± 0.03 μmol Trolox equivalents/mg dried extract), ferric reducing antioxidant power (20.55 ± 0.11 μmol ascorbic acid equivalents/mg dried extract), Oxygen radical absorbance capacity (31.12 ± 0.21 μmol Trolox equivalents/mg dried extract), hydroxyl radical scavenging activity (0.15 ± 0.04 mg Trolox equivalents/mg dried extract), and superoxide radical scavenging activity (18.82 ± 0.50 mg Trolox equivalents/mg dried extract). The aqueous extract of clove (0.25–1.00 mg/ml) significantly inhibited the formation of fluorescent advanced glycation end products (AGEs) and non-fluorescent AGEs (Nɛ-(carboxymethyl) lysine (CML)) in glycated BSA during 4 weeks of incubation. The extract also markedly prevented oxidation-induced protein damage by decreasing protein carbonyl formation and protecting against the loss of protein thiol group. These results clearly demonstrated that a polyphenol enriched clove extract, owing to its antioxidant, was capable to inhibit the formation of AGEs and protein glycation. The findings might lead to the possibility of using the clove extract for targeting diabetic complications.
Keywords: Advanced glycation end-products, Antiglycation, Antioxidant, Clove, Syzygium aromaticum (L.)
Introduction
Chronic hyperglycemia facilitates the formation of non-enzymatic protein glycation, which leads to a series of cascade reactions between the carbonyl group of reducing sugars (glucose and fructose) and the amino group of protein; consequently, a reversible structure called an unstable Schiff's base is produced. It is well established that the initial Schiff's base undergoes repeated condensation and rearrangement to form a relatively stable Amadori product, which induces further oxidative modifications to generate advanced glycation end products (AGEs), including cross-linking fluorescent adducts (pentosidine) and non-fluorescent adducts (Nɛ-(carboxymethyl) lysine (CML)) (Singh et al. 2001; Stitt 2001; Vlassara and Palace 2002). In the presence of transition metal ions, reducing sugars and protein dicarbonyl compounds can auto-oxidize to form superoxide radical (O2−) that can then be converted to the highly toxic hydroxyl radical (.OH) via the Fenton reaction. Subsequently, reactive oxygen species (ROS) also accelerate the formation of AGEs through the oxidation reactions (Ahmed 2005). Emerging evidence has shown a clinical correlation between accumulation of AGEs in body tissues and the progression of several age-related diseases (Basta et al. 2004; Sutherland et al. 2013). There is considerable recent interest in edible plants with antiglycation activity that have been a key strategy for prevention and amelioration of AGE-mediated diabetic complications (Elosta et al. 2012; Morrone et al. 2013).
Syzygium aromaticum (L.) (clove), an aromatic flower bud, is one of the most widely cultivated spices in many tropical countries. It has been used in traditional medicine since ancient times to treat respiratory and digestive ailments. Previous studies have shown a variety of pharmacological properties such as anti-inflammatory (Tapsell et al. 2006), antibacterial (Dorman and Deans 2000), anti-fungal (Park et al. 2007), and antioxidant activities (Abdel‐Wahhab and Ayl 2005). However, there have been no previous studies that address the inhibitory effect of aqueous extract of clove against fructose-mediated protein glycation and oxidation. Therefore, the aim of the present study was to investigate the effect of clove extract against bovine serum albumin (BSA) in fructose-mediated non-enzymatic glycation. Moreover, the study also examined the effect of clove extract on glycation-induced protein oxidative damages. In addition, the phytochemical contents and bioactivity of clove related to antioxidants were assessed.
Materials and methods
Chemicals
Bovine serum albumin (BSA, fraction V), aminoguanidine hydrochloride, guanidine hydrochloride, 5,5′-Dithiobis (2-nitrobenzoic acid) (DTNB), hydroxyl–2,5,7,8-tetramethylchromane−2-carboxylic acid (trolox), nitroblue-tetrazolium and L-cysteine were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Fructose and 2, 4-dinitrophenyl hydrazine were purchased from Ajax Finechem (Taren Point, Australia). Trichloroacetic acid was purchased from Merck (Darmstadt, FR, Germany). OxiSelect™ Nε-(carboxymethyl) lysine (CML) ELISA kit was obtained from Cell Biolabs (San Diego, CA, USA). All other chemicals and solvents used in this study were of analytical grade.
Plant material and preparation of extract
The dried buds of clove were purchased from a specific herbal drugstore, Bangkok, Thailand. The plant has been authenticated at the Princess Sirindhorn Plant Herbarium, Plant Varieties Protection Division, Department of Agriculture, Thailand, Voucher specimen: BKU066459. The dried buds of clove, 20 g, were boiled in distilled water (800 ml) for 3 h at 95 °C. After boiling, the plant residue was filtered through Whatman No. 1 filter paper. Thereafter, the extraction was lyophilized with a freeze drier. The lyophilized powder was stored at 4 °C in a dark bottle until analysis. The powder of clove extract was resuspended in distilled water before experiments.
Determination of total phenolic content
The total phenolic content of clove was determined using the Folin-Ciocalteu method, as described by Sriplang et al., with minor modifications (Sriplang et al. 2007). The extract was freshly dissolved in distilled water before use. Briefly, 50 μl of sample solution (0.50 mg/ml) was mixed with 50 μl of Folin-Ciocalteu reagent (10-fold dilution in distilled water before use). After incubation for 5 min at room temperature, then 50 μl of sodium bicarbonate solution was added, and the mixture was allowed to stand for 90 min at room temperature before the absorbance of the reaction mixture was measured at 725 nm. The concentration of gallic acid (0–150 μg/ml) was used for a standard curve. The total phenolic contents were calculated using a standard curve and expressed as milligrams of gallic acid equivalents (GAE) per gram of extract.
Determination of total flavonoid content
The total flavonoid content in the extract was measured according to the colorimetric method described by Hajimahmoodi et al., with slight modifications (Hajimahmoodi et al. 2008). Briefly, 100 μl of sample solution (1.00 mg/ml) was added to 400 μl of distilled water. 30 μl of 5 % NaNO2 was added to the mixture and allowed to stand for 5 min before adding 30 μl of 10 % AlCl3. After incubation for 1 min, 200 μl of 1 M NaOH was added and the total volume was adjusted to 1 ml by distilled water before the absorbance of the reaction mixture was read at 510 nm. The concentration of catechin (0–240 μg/ml) was used for a standard curve. The total flavonoids were determined using a standard curve and expressed as milligrams of catechin equivalents (CE) per gram of sample extract.
DPPH radical scavenging activity
The ability of clove to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radicals was determined according to a previously described method with minor modifications (Schlesier et al. 2002). Concisely, 10 μl of the extract (0.50 mg/ml) was added to 90 μl ethanol solution of 0.2 mM DPPH• radicals. The mixture solution was mixed and left at room temperature for 30 min in the dark. The absorbance was measured at 517 nm after incubation, using a spectrophotometer. The DPPH radical scavenging activity was calculated from a standard curve using ascorbic acid (0–1.00 mg/ml). The DPPH radical scavenging activity was expressed as percentage inhibition and calculated according to the following equation:% Inhibition = (Ac-As)/Ac × 100
Where Ac was the absorbance of the control (without extract) and As was the absorbance in the presence of the extract and the IC50 value was calculated and expressed as mg/ml.
Trolox equivalent antioxidant capacity (TEAC) assay
The TEAC assay was performed according to the method described by Madhujith et al., with some modifications (Madhujith et al. 2006). Concisely, the radical ABTS•+ was generated by mixing 7 mM ABTS in 0.1 M phosphate buffer saline (pH 7.4) with 2.45 mM K2S2O4 in distilled water. The mixture solution was allowed to stand for 16 h at room temperature in the dark to produce a dark green solution. The working solution was diluted with 0.1 M phosphate buffer saline to an absorbance of about 0.900 to 1.000 at 734 nm. An aliquot of 10 μl of the extract (0.50 mg/ml) was mixed with 90 μl of working ABTS•- solution and the decrease of absorbance was measured at 734 nm after 6 min in the dark. TEAC value was calculated from a standard curve using a Trolox (0–1.00 mg/ml). The TEAC value was expressed as micromole of Trolox equivalents per milligram of extract.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was done using a modified method of Benzie and Strain (Benzie and Strain 1996). The fresh FRAP reagent contained 0.3 M sodium acetate buffer solution (pH 3.6), 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3 in the ratio of 10:1:1. Briefly, an aliquot of 10 μl of the sample solution (0.50 mg/ml) was added to 90 μl of freshly working FRAP solution. The mixture solution was mixed and left at room temperature for 30 min in the dark. The absorbance was measured at 595 after incubation using a spectrophotometer. FRAP value was calculated from a standard curve using ascorbic acid (0–2.00 mg/ml). FRAP value was expressed as micromoles of ascorbic acid equivalents per milligram of extract.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was determined according to a previously described method (Van Hoyweghen et al. 2010). Briefly, 25 μl of sample solution (0.25 mg/ml) was mixed with 150 μl of 48 nM sodium fluorescein in the wells of a microtiter plate. After incubation for 10 min at room temperature, 25 μl of 2,2′-azo-bis (2-amidinopropane) dihydrochloride (APPH), a free radical generator solution, was added and mixed well. The fluorescence intensity at excitation wavelength 485 nm and emission wavelength 535 nm was recorded every 2 min for 60 min. A standard curve was generated with a Trolox concentration range from 0 to 48 μM. The ORAC value was calculated as the area under the curve (AUC) and expressed as micromole of Trolox equivalents per milligram of extract.
Hydroxyl radical scavenging activity (HRSA)
The HRSA was measured according to a previously described method, with minor modifications (Halliwell et al. 1987). The hydroxyl radical in the working solution was generated by adding 0.3 mM FeCl3, 0.6 mM ascorbic acid, 1.2 mM EDTA, 34 mM H2O2 and 17 mM 2-deoxy-D-ribose in the ratio 2:2:1:1:1. The fresh reaction mixture contained 210 μl of the working solution and 30 μl of the sample solution (0.50 mg/ml). The reaction was performed at 37 °C for 30 min in an overhead shaker. Thereafter, 300 μl of 2.8 % TCA and 150 μl of 1 % TBA (in 0.05 M NaOH) were added into the reaction mixture, which was then incubated at 100 °C for 5 min. After cooling, the absorbance was measured at 532 nm. The HRSA value was calculated from a standard curve using a Trolox (0–2.00 mg/ml). The HRSA value was expressed as milligrams of Trolox equivalents per milligram of extract.
Superoxide radical scavenging activity (SRSA)
The SRSA was measured according to a previously described report, with slight modifications (Kweon et al. 2001). The superoxide radicals in the mixture solution were generated by adding 75 μl of 0.3 mM xanthine, 50 μl of 0.15 mM NBT, 50 μl of 0.6 mM EDTA and 1 % of 5 unit/ml xanthine oxidase. In brief, 7.5 μl of sample solution (0.10 mg/ml) was mixed with mixture solution in a microtiter plate. After incubation for 40 min at 37 °C, the absorbance was determined at 560 nm. The SRSA value was calculated from a standard curve using a Trolox (0–2.00 mg/ml). The SRSA value was expressed as milligrams of Trolox equivalents per milligram of extract.
In vitro glycation of bovine serum albumin
The glycated BSA formation was determined according to a previously described method (Adisakwattana et al. 2012). Briefly, BSA (10 mg/ml) was incubated with 500 mM fructose in 0.1 M phosphate buffer saline (PBS) (pH 7.4), containing 0.02 % sodium azide in the dark at 37 °C for 1, 2, 3, and 4 weeks. Before incubation, the solution containing clove extract (0.25–1.00 mg/ml) dissolved in PBS was added to the mixtures. The glycated BSA formation was measured by using fluorescent intensity at an excitation wavelength of 355 nm and an emission wavelength of 460 nm. Aminoguanidine (AG) was used as a positive control for the study.
Determination of Nɛ-(carboxymethyl) lysine (CML)
The concentration of Nɛ-CML, a major non-fluorescence and non-crosslinking AGEs, was performed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions. The concentration of Nɛ-CML was calculated by using the calibration curve of CML-BSA.
Determination of protein carbonyl content
The level of carbonyl group in glycated BSA was slightly modified according to Levine’s method (Levine 2002). Concisely, 100 μl of glycated BSA was mixed with 400 μl of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2.5 M HCl. After incubation for 60 min at room temperature, glycated BSA was then precipitated using 500 μl of 20 % (w/v) trichloroacetic acid (TCA), left on ice for 5 min, and centrifuged at 10,000 g for 10 min at 4 °C. The protein pellet was washed 3 times by 500 μl of 1:1 (v/v) ethanol: ethyl acetate solution. The final protein pellet was resuspended in 250 μL of 6 M guanidine hydrochloride. The absorbance was read at 370 nm. The protein carbonyl group of each sample was calculated by using absorption coefficient (ɛ = 22,000 M−1.cm−1). The protein carbonyl content was expressed as nmol carbonyl/mg protein.
Determination of thiol group
The level of thiol group in glycated BSA was determined using the Ellman’s assay, with minor modifications (Sedlak and Lindsay 1968). 10 μl of glycated BSA was mixed with 90 μl of 5 mM DTNB in 0.1 M PBS (pH 7.4) and the absorbance was measured at 410 nm after incubation at room temperature for 15 min. The level of thiol group was calculated from a standard curve using L-cysteine (0–2.00 mg/ml). The level of thiol group was expressed as nmol/mg protein.
Statistical analysis
Data were expressed as means ± standard error of mean (S.E.M), N = 3. Data were analyzed using One way ANOVA followed by Tukey’s HSD post hoc test. P-value <0.05 was considered to be statistically significant.
Results
Phytochemical analysis
The result showed that the total phenolic content of clove was 239.58 ± 0.70 mg gallic acid equivalents/g dried extract. In addition, the content of total flavonoid in clove was 65.67 ± 0.01 mg catechin equivalents/g dried extract.
Antioxidant activity of clove
Antioxidant activity of clove including DPPH radical activity, TEAC, FRAP, ORAC, HRSA, and SRSA is shown in Table 1. In the DPPH assay, clove had the IC50 value of 0.29 ± 0.01 mg/ml. In the TEAC assay, clove had antioxidant activity of 4.69 ± 0.03 μmol Trolox equivalents/mg dried extract, whereas it had FRAP value of 20.55 ± 0.11 μmol ascorbic acid equivalents/mg dried extract. In addition, clove had the ORAC value of 31.12 ± 0.21 μmol Trolox equivalents/mg dried extract. The HRSA and SRSA values of clove were 0.15 ± 0.04 mg Trolox equivalent/mg dried extract and 18.82 ± 0.50 mg Trolox equivalents/mg dried extract, respectively.
Table. 1.
Antioxidant activity of clove extract including DPPH radical scavenging activity, TEAC, FRAP, ORAC, HRSA and SRSA
| Antioxidant activity | ||||||
|---|---|---|---|---|---|---|
| DPPH | TEAC | FRAP | ORAC | HRSA | SRSA | |
| Clove extract | 0.29 ± 0.01 | 4.69 ± 0.03 | 20.55 ± 0.11 | 31.12 ± 0.21 | 0.15 ± 0.04 | 18.82 ± 0.50 |
Data are expressed as mean ± S.E.M, n = 3. DPPH radical scavenging activity was expressed as the IC50 (mg/ml). TEAC was expressed as micromole trolox/milligram dried extract. FRAP was expressed as micromole ascorbic acid/milligram dried extract. ORAC was expressed as micromole trolox/milligram dried extract. Hydroxyl radical scavenging activity (HRSA) was expressed as milligram trolox/milligram dried extract. Superoxide radical scavenging activity (SRSA) was expressed as milligram trolox/milligram dried extract.
The effects of clove on AGEs formation
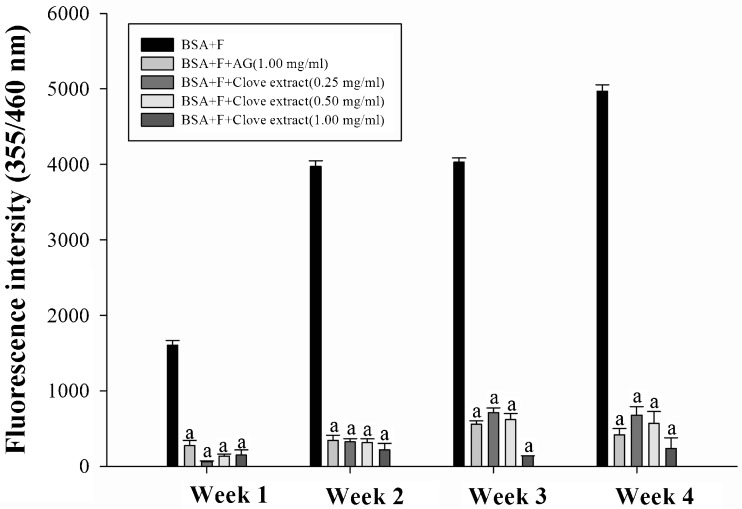
The formation of AGEs in BSA was measured by the fluorescence intensity. As shown in Fig. 1, the fluorescent intensity of fructose-mediated glycation of BSA substantially increased during 4 weeks of the experiment. The results demonstrated that clove (0.25–1.00 mg/ml) significantly reduced the formation of AGEs during weeks 1 to 4 of incubation. At the end of the study, clove at concentrations of 0.25, 0.50, and 1.00 mg/ml inhibited the formation of AGEs by 86.3 %, 88.6 %, and 95.2 %, respectively. Meanwhile, AG at concentration 1.00 mg/ml also reduced the formation of AGEs by 91.5 %.
Fig. 1.
The effects of clove extract on fluorescence AGE formation in fructose-glycated BSA. Each value represents the mean ± S.E.M (n = 3). a p < 0.05 compared to BSA + F (Fructose) at the same week
The effects of clove on Nɛ-CML formation
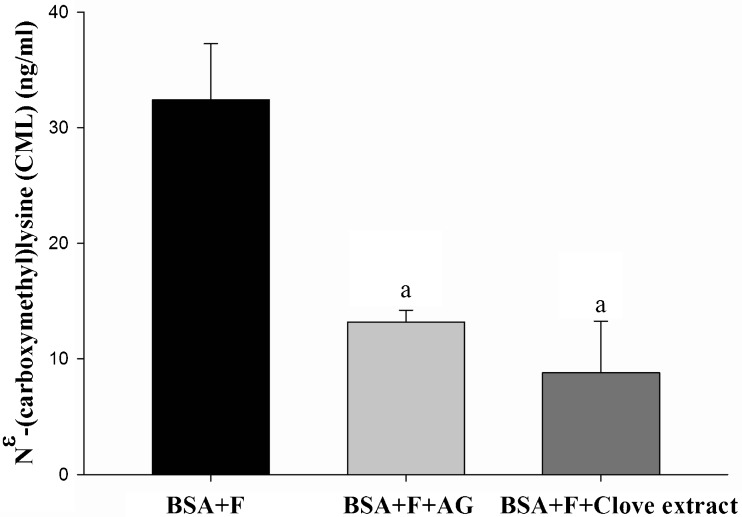
The level of Nɛ-CML has been used as a biomarker for the formation of the non-fluorescent AGEs. The results, as presented in Fig. 2, showed that the level of Nɛ-CML in glycated BSA was 32.39 ± 4.86 ng/ml after 4 weeks of incubation. When clove (1.00 mg/ml) was incubated with fructose and BSA, it could markedly decrease the level of Nɛ-CML (8.80 ± 4.47 ng/ml), with a percentage reduction of 72.8 %. In the presence of AG (1.00 mg/ml), the level of Nɛ-CML was 13.20 ± 1.01 ng/ml. This compound could inhibit the formation of Nɛ-CML by approximately 59.2 %.
Fig. 2.
The effects of clove extract (1.00 mg/ml) and AG (1 mg/ml) on the level of Nɛ-(carboxymethyl) lysine (CML) in fructose-glycated BSA system at week 4. Each value represents the mean ± S.E.M (n = 3). a p < 0.05 compared to BSA + F (Fructose)
The effects of clove on glycation-induced protein oxidation
The measurements of carbonyl content and free thiol group were used to evaluate the process of glycation-induced protein oxidation. The carbonyl content of glycated BSA was 1.15 ± 0.09 and 1.62 ± 0.21 nmol carbonyl/mg protein at weeks 2 and 4, respectively. As shown in Table 2, at week 4 a significant decrease in carbonyl content was observed in glycated BSA plus clove. The results showed that clove at concentrations of 0.50 and 1.00 mg/ml exhibited the percentage reduction of protein carbonyl formation by 73.7 % and 66.1 %, as compared with glycated BSA. It is important to note that clove at concentration 0.25 mg/ml did not seem to suppress the protein carbonyl content, whereas AG at concentration 1.00 mg/ml suppressed protein carbonyl formation approximately 60.0 % at the same week. The effects of clove on glycation-induced oxidation of protein thiol are shown in Table 2. At week 2 and week 4, the protein thiol group of BSA/fructose was 1.03 ± 0.01 and 1.18 ± 0.03 nmol/mg protein, respectively. As shown in Table 2, at week 4 a significant increase in protein thiol group was observed in glycated BSA together with clove. When clove (0.50 and 1.00 mg/ml) was introduced for incubation with BSA and fructose, the level of thiol group was significantly increased by 41.6 % and 87.0 %, respectively. However, there was no significant protective effect of clove at concentration 0.25 mg/ml. In addition, AG at concentration (1.00 mg/ml) increased protein thiol group by 56.5 %.
Table 2.
The effect of clove extract and aminoguanidine (AG) on the formation of carbonyl content and the level of thiol group in fructose-glycated BSA at week 2 and 4
| Experimental groups | Carbonyl content (nmol carbonyl/mg protein) | Thiol group (nmol/mg protein) | ||
|---|---|---|---|---|
| Week 2 | Week 4 | Week 2 | Week 4 | |
| BSA + F | 1.15 ± 0.09 | 1.62 ± 0.21 | 1.03 ± 0.01 | 1.18 ± 0.03 |
| BSA + F + Clove (0.25 mg/ml) | 0.61 ± 0.03a | 1.40 ± 0.07 | 1.35 ± 0.13 | 1.26 ± 0.01 |
| BSA + F + Clove (0.50 mg/ml) | 0.55 ± 0.04a | 0.43 ± 0.07a | 1.56 ± 0.09a | 1.67 ± 0.01a |
| BSA + F + Clove (1.00 mg/ml) | 0.49 ± 0.05a | 0.55 ± 0.04a | 2.13 ± 0.01a | 2.20 ± 0.03a |
| BSA + F + AG (1.00 mg/ml) | 0.59 ± 0.03a | 0.65 ± 0.07a | 1.54 ± 0.03a | 1.84 ± 0.02a |
a p < 0.05 compared to BSA + F at the same week.
Discussion
The induction of AGEs formation causes the modification of crucial physiological proteins, which is an important factor in the development of age-related diseases such as atherosclerosis, Alzheimer's disease, end-stage renal disease, rheumatoid arthritis, and diabetes and its complications. In recent decades, attention has been focused on the over-consumption of high-fructose diets that contributes to obesity, non-alcoholic fatty liver, insulin resistance, and diabetes and its complications. Likewise, consumption of high dietary fructose has been apparently associated with increased production of advanced glycation end products (Gaby 2005; Livesey and Taylor 2008). Previous study has shown that fructose has a greater rate of AGE formation than glucose (Suarez et al. 1989). In the early stages of glycation, after the reaction between the carbonyl group from reducing sugar and free amino group form protein, freely reversible Schiff’s bases are rearranged to more stable ketoamine or Amadori products such as fructosamine. In this stage, reducing sugars (glucose and fructose) itself can cause autoxidation in the present of transition metals to form superoxide radical and hydroxyl radical. The harmful radicals can participate in accelerating the protein glycation and AGEs formation. In addition, the Amadori products react with the amino acids to form Nɛ-CML, which is one of the most abundant compounds of AGEs. Aside from this pathway, Nɛ-CML is generated by oxidative breakdown of polyol pathway mediated by α-oxoaldehydes such as glyoxal, methylglyoxal, and 3-deoxyglucosone (Goldin et al. 2006). Furthermore, reactive carbonyl intermediaries and protein carbonyl derivatives generate both AGE formation and protein modification that are prone to oxidative reaction to amino acids such as cysteine, particularly the thiol side chain. The reactive oxygen species and reactive nitrogen species are generated during glycation and glycoxidation. In the meantime, they also are able to oxidize side chains of amino acid residues of protein to form a carbonyl derivative and diminish the oxidative defense of protein by eliminating the thiol groups (Miyata et al. 2000; Zeng et al. 2006). As mentioned above, the alterations are reflective of protein oxidative damage, with high oxidative stress and formation of AGEs, which are the consideration of excessive free radical generation. A large number of antiglycation agents have been recently reported (Rahbar and Figarola 2003). In this study, we examined the influence of clove against fructose-medicated non-enzymatic glycation and oxidation-dependent damage to BSA. According to the fluorescence property of AGEs, clove efficiently inhibited AGE formation. Moreover, clove also efficiently reduced the formation of Nɛ-CML in glycated BSA associated with decreased formation of AGEs. In addition, the reduction of protein carbonyl content and oxidation of thiol group of BSA/fructose was affected by clove.
Recent investigations have shown that many natural antioxidant-rich foods have the potential to prevent reducing sugar-mediated protein glycation (Elosta et al. 2012; Ramkissoon et al. 2013). In the present study, various methods of examining antioxidant capacities of clove, including DPPH radical scavenging assay, TEAC assay, FRAP assay, ORAC assay, HRSA assay, and SRSA assay, have been used. The results of this study provide supporting evidence that clove exhibits potent antioxidant activity by scavenging free radicals. These results are consistent with those obtained by other researchers, indicating that clove extract acts as hydroxyl and superoxide radical scavenger (Gülçin et al. 2004; Adefegha and Oboh 2012). Currently, several biochemical mechanisms of antiglycation reaction that can delay or prevent the glycation process have been proposed (Wu et al. 2011). Especially, antiglycation strategies are involved in scavenging of free radicals at the early stages of glycation. It is well established that superoxide anions are generated from early glycation products, including 1,2- to 2,3-enolization of the Schiff's base and oxidation of the enolate anion (Smith and Thornalley 1992). Consequently, the Amadori product or Schiff's base undergoes fragmentation through reactive oxygen species-mediated reactions to generate short-chain carbohydrate intermediates which alter lysine and arginine residues to produce AGEs. Furthermore, hydroxyl radicals generated by the reaction of Fe2+with H2O2 mediate the formation of Nɛ-CML from Amadori compounds (Nagai et al. 1997). Additionally, metal chelators also inhibit the process of AGEs by retarding further oxidation of Amadori products and metal-ion-catalysis glucose oxidation (Rahbar and Figarola 2003; Cho et al. 2007). It is worth mentioning that clove may inhibit AGE formation by its ROS scavenging capacity on hydroxyl and superoxide radicals during the auto-oxidation of sugar and/or oxidative degradation of Amadori products, leading to reduced protein oxidation and Nɛ-CML and dicarbonyl intermediate formation. Other mechanisms of antiglycation, particularly for inhibiting the formation of late-stage Amadori products, breaking the cross-linking structures in the intracellular formed AGEs, and blocking the receptor for advanced glycation end products (RAGEs), have been proposed (Wu et al. 2011). Further comprehensive studies of clove are required to determine the antiglycation mechanisms described above.
Several edible plants including berries (Wang et al. 2011), red grape skin (Jariyapamornkoon et al. 2013), pomelo (Caengprasath et al. 2013), and Cyperus rotundus (Ardestani and Yazdanparast 2007), have shown antiglycation activities in BSA-fructose models. The major components of edible plants contain high phenolic compounds and flavonoids such as catechin, epicatechin, quercetin, rutin, kaempferol, proanthocyanidins, hesperidin, neohesperidin dihydrochalcone, ellagic acid, gallic acid, and p-coumaric acid. These phytochemicals have provoked considerable interest because of their beneficial impact on human health by free radical scavenging and metal ion chelating abilities (Rice-Evans et al. 1996; Mira et al. 2002; de Souza and de Giovani 2004; Perron and Brumaghim 2009). The phytochemicals analysis of clove demonstrates the presence of phenolic compounds such as gallic acid, quercetin glucoside, ellagic acid, ellagic acid derivatives, and some other unidentified phenolic compounds (Atawodi et al. 2011; Milind and Deepa 2011). Notably, these phytochemicals showed consistent inhibition of protein glycation both in vitro and in vivo models. For example, gallic acid and quercetin glucoside have been reported to inhibit α-dicarbonyl compounds generation in the glycated BSA (Wu et al. 2009). Ellagic acid, a new antiglycating agent, has been reported to inhibit AGE formation in diabetic mice by decreasing the level of Nɛ-CML and the accumulation of pentosidine (Chao et al. 2010; Muthenna et al. 2012). In this regard, it might be explained that these polyphenols and flavonoids in clove may contribute to their antioxidant and antiglycation properties.
In conclusion, the results obtained in the current study shown that a polyphenol-enriched extract of clove has antioxidant activity with suppressing effect on AGE formation and protein oxidation. Additional studies are needed to investigate the bioactive compounds responsible for the observed activities.
Acknowledgments
This work was fully supported by Thai Government Research Budget in year 2013. The authors would like to thank the Research Group of Herbal Medicine for Prevention and Therapeutic of Metabolic diseases, Chulalongkorn University.
Conflicts of Interests
The authors declare that there is no conflict of interests.
References
- Abdel‐Wahhab M, Ayl S. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25:218–223. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- Adefegha SA, Oboh G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pac J Trop Biomed. 2012;2:774–781. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisakwattana S, Sompong W, Meeprom A, Ngamukote S, Yibchok-anun S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int J Mol Sci. 2012;13:1778–1789. doi: 10.3390/ijms13021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ardestani A, Yazdanparast R. Cyperus rotundus suppresses AGE formation and protein oxidation in a model of fructose-mediated protein glycoxidation. Int J Biol Macromol. 2007;41:572–578. doi: 10.1016/j.ijbiomac.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Atawodi S, Atawodi J, Pfundstein B, Spiegelhalder B, Bartsch H, Owen R. Assessment of the polyphenol components and in vitro antioxidant properties of syzygium aromaticum (L.) Merr. & Perry. Elec J Env Agric Food Chem. 2011;10:1970–1978. [Google Scholar]
- Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Caengprasath N, Ngamukote S, Mäkynen K, Adisakwattana S. The protective effect of pomelo extract (citrus grandis L. osbeck) against fructose-mediated protein oxidation and glycation. EXCLI J. 2013;12:491–502. [PMC free article] [PubMed] [Google Scholar]
- Chao CY, Mong MC, Chan KC, Yin MC. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol Nutr Food Res. 2010;54:388–395. doi: 10.1002/mnfr.200900087. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Roman G, Yeboah F, Konishi Y. The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem. 2007;14:1653–1671. doi: 10.2174/092986707780830989. [DOI] [PubMed] [Google Scholar]
- de Souza RF, De Giovani WF. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004;9:97–104. doi: 10.1179/135100004225003897. [DOI] [PubMed] [Google Scholar]
- Dorman H, Deans S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Elosta A, Ghous T, Ahmed N. Natural products as anti-glycation agents: possible therapeutic potential for diabetic complications. Curr Diabetes Rev. 2012;8:92–108. doi: 10.2174/157339912799424528. [DOI] [PubMed] [Google Scholar]
- Gaby AR. Adverse effects of dietary fructose. Altern Med Rev. 2005;10:294–306. [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- Gülçin Ì, Güngör Şat İ, Beydemir Ş, Elmastaş M, İrfan Küfrevioǧlu Ö. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.) Food Chem. 2004;87:393–400. doi: 10.1016/j.foodchem.2003.12.008. [DOI] [Google Scholar]
- Hajimahmoodi M, Oveisi MR, Sadeghi N, Jannat B, Hadjibabaie M, Farahani E, et al. Antioxidant properties of peel and pulp hydro extract in ten Persian pomegranate cultivars. Pak J Biol Sci. 2008;11:1600–1604. doi: 10.3923/pjbs.2008.1600.1604. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J, Aruoma OI. The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med. 2013;13:171. doi: 10.1186/1472-6882-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon MH, Hwang HJ, Sung HC. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (phyllostachys edulis) J Agric Food Chem. 2001;49:4646–4655. doi: 10.1021/jf010514x. [DOI] [PubMed] [Google Scholar]
- Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/S0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr. 2008;88:1419–1437. doi: 10.3945/ajcn.2007.25700. [DOI] [PubMed] [Google Scholar]
- Madhujith T, Izydorczyk M, Shahidi F. Antioxidant properties of pearled barley fractions. J Agric Food Chem. 2006;54:3283–3289. doi: 10.1021/jf0527504. [DOI] [PubMed] [Google Scholar]
- Milind P, Deepa K. Clove: a champion spice. IJRAP. 2011;2:47–54. [Google Scholar]
- Mira L, Tereza Fernandez M, Santos M, Rocha R, Helena Florêncio M, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kurokawa K, Van Ypersele De Strihou C (2000) Advanced glycation and lipoxidation end products role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J Am Soc Nephrol 11:1744–1752 [DOI] [PubMed]
- Morrone S, de Assis AM, da Rocha RF, Gasparotto J, Gazola AC, Costa GM, et al. Passiflora manicata (Juss.) aqueous leaf extract protects against reactive oxygen species and protein glycation in vitro and ex vivo models. Food Chem Toxicol. 2013;60:45–51. doi: 10.1016/j.fct.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Muthenna P, Akileshwari C, Reddy GB. Ellagic acid, a new antiglycating agent: its inhibition of N-(carboxymethyl)lysine. Biochem J. 2012;442:221–230. doi: 10.1042/BJ20110846. [DOI] [PubMed] [Google Scholar]
- Nagai R, Ikeda K, Higashi T, Sano H, Jinnouchi Y, Araki T, et al. Hydroxyl radical mediates N epsilon-(carboxymethyl)lysine formation from amadori product. Biochem Biophys Res Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- Park MJ, Gwak KS, Yang I, Choi WS, Jo HJ, Chang JW, et al. Antifungal activities of the essential oils in syzygium aromaticum (L.) Merr. Et Perry and leptospermum petersonii bailey and their constituents against various dermatophytes. J Microbiol. 2007;45:460–465. [PubMed] [Google Scholar]
- Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:63–79. doi: 10.1016/j.abb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Ramkissoon J, Mahomoodally M, Ahmed N, Subratty A. Antioxidant and anti–glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med. 2013;6:561–569. doi: 10.1016/S1995-7645(13)60097-8. [DOI] [PubMed] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- Schlesier K, Harwat M, Böhm V, Bitsch R. Assessment of antioxidant activity by using different in vitro methods. Free Radic Res. 2002;36:177–187. doi: 10.1080/10715760290006411. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Smith PR, Thornalley PJ. Mechanism of the degradation of non-enzymatically glycated proteins under physiological conditions. studies with the model fructosamine, N epsilon-(1-deoxy-D-fructos-1-yl)hippuryl-lysine. Eur J Biochem. 1992;210:729–739. doi: 10.1111/j.1432-1033.1992.tb17474.x. [DOI] [PubMed] [Google Scholar]
- Sriplang K, Adisakwattana S, Rungsipipat A, Yibchok-Anun S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;109:510–514. doi: 10.1016/j.jep.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–753. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez G, Rajaram R, Oronsky A, Gawinowicz M. Nonenzymatic glycation of bovine serum albumin by fructose (fructation) comparison with the Maillard reaction initiated by glucose. J Biol Chem. 1989;264:3674–3679. [PubMed] [Google Scholar]
- Sutherland GT, Chami B, Youssef P, Witting PK. Oxidative stress in Alzheimer's disease: Primary villain or physiological by-product? Redox Rep. 2013;18:134–141. doi: 10.1179/1351000213Y.0000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, et al. Health benefits of herbs and spices: the past, the present, the future. Med J Aust. 2006;185:S4–24. doi: 10.5694/j.1326-5377.2006.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Van Hoyweghen L, Karalic I, Van Calenbergh S, Deforce D, Heyerick A. Antioxidant flavone glycosides from the leaves of fargesia robusta. J Nat Prod. 2010;73:1573–1577. doi: 10.1021/np100220g. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Palace M. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Yagiz Y, Buran TJ, Nunes CN, Gu L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end‐products by scavenging reactive carbonyls. Food Res Int. 2011;44:2666–2673. doi: 10.1016/j.foodres.2011.05.022. [DOI] [Google Scholar]
- Wu CH, Huang SM, Lin JA, Yen GC. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011;2:224–234. doi: 10.1039/c1fo10026b. [DOI] [PubMed] [Google Scholar]
- Wu JW, Hsieh CL, Wang HY, Chen HY. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009;113:78–84. doi: 10.1016/j.foodchem.2008.07.025. [DOI] [Google Scholar]
- Zeng J, Dunlop R, Rodgers K, Davies M. Evidence for inactivation of cysteine proteases by reactive carbonyls via glycation of active site thiols. Biochem J. 2006;398:197–206. doi: 10.1042/BJ20060019. [DOI] [PMC free article] [PubMed] [Google Scholar]