Abstract
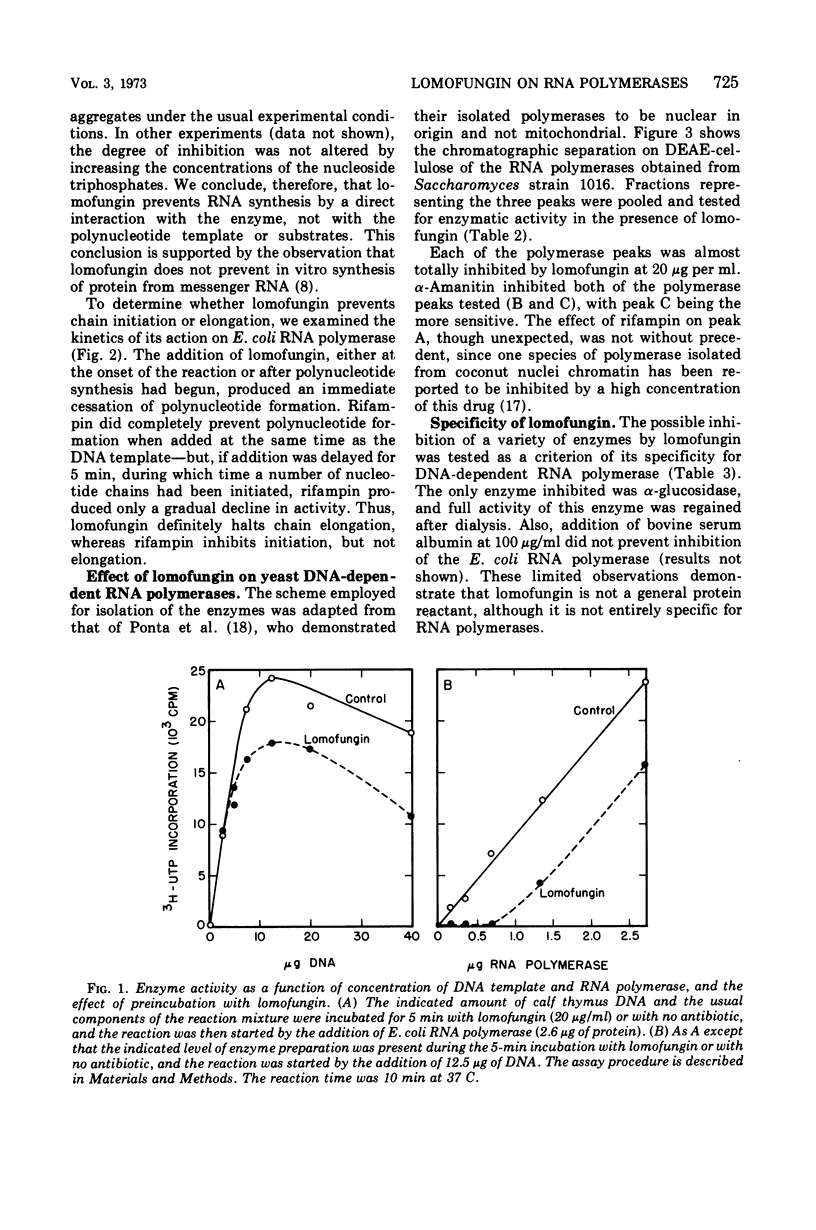
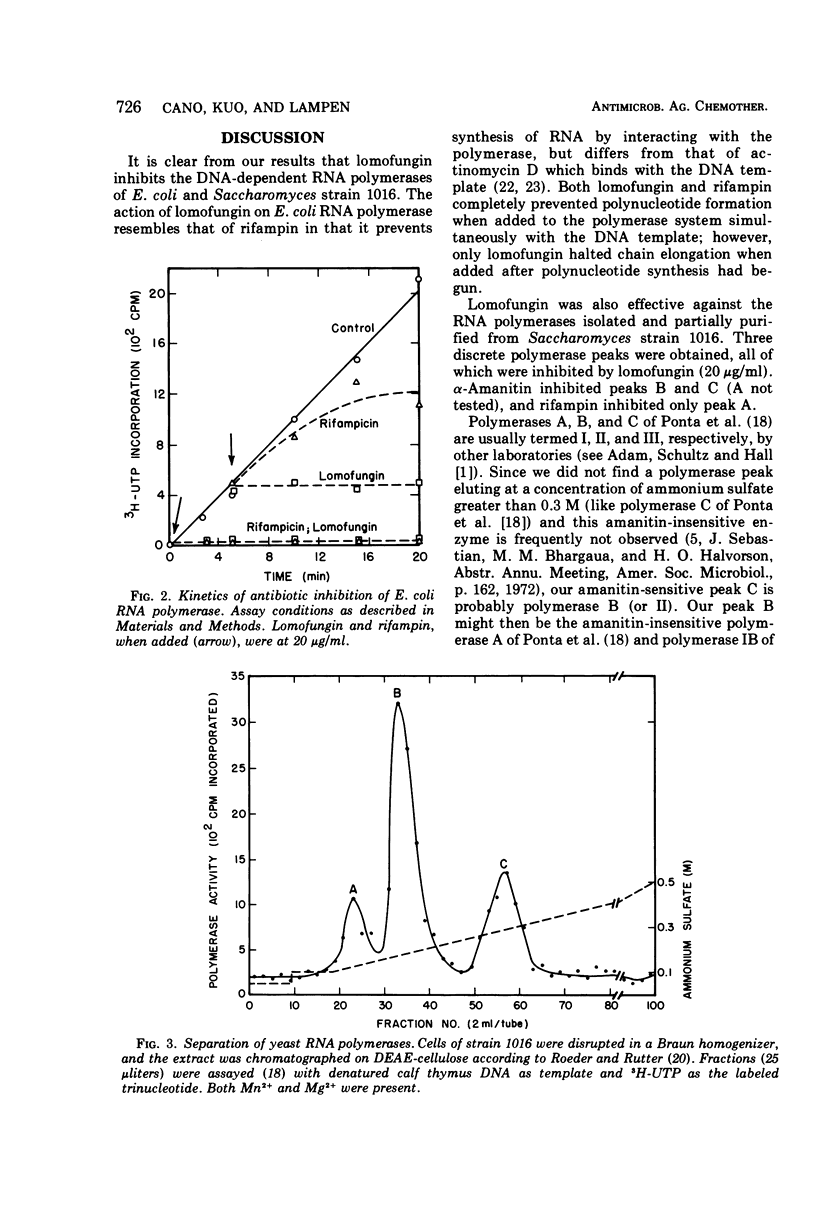
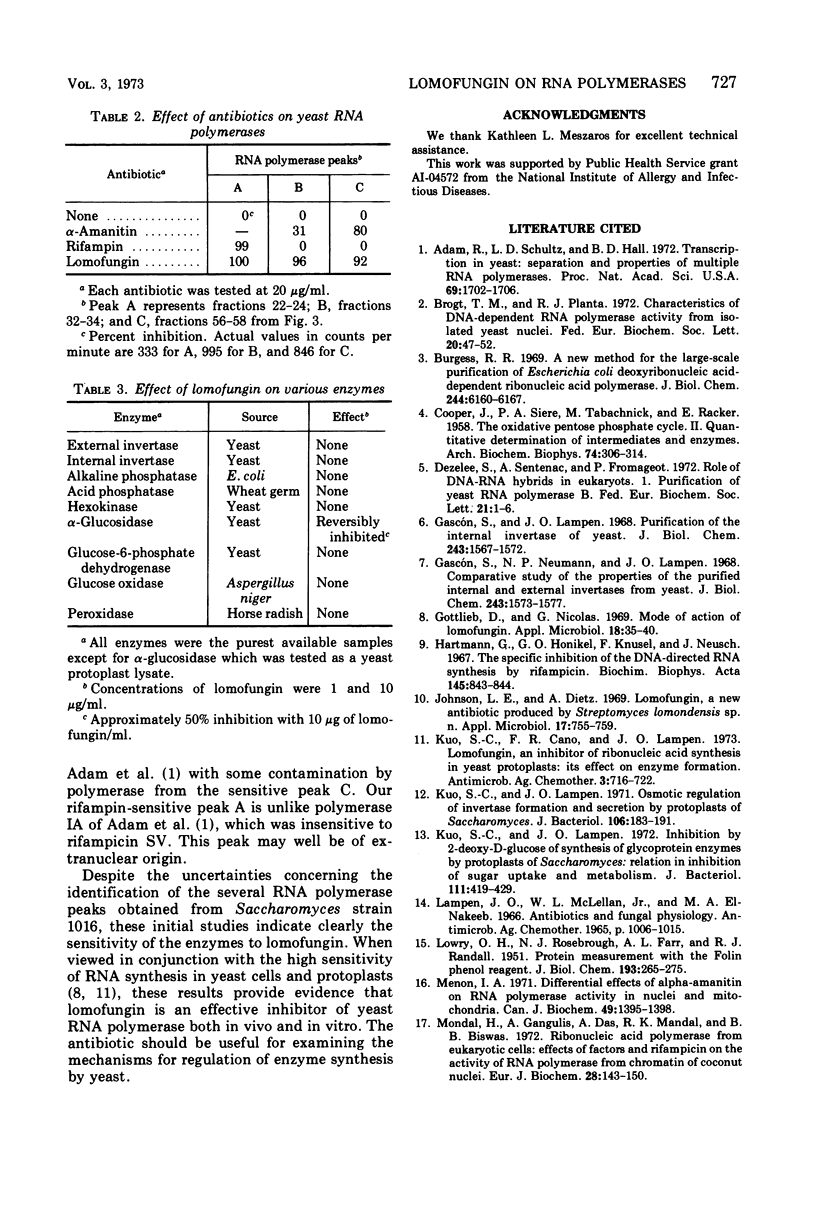
Lomofungin, an antibiotic active against fungi, yeasts, and bacteria, was found to be a potent inhibitor of purified Escherichia coli deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase. It prevents RNA synthesis by a direct interaction with the polymerase and not with the template or substrate; chain elongation is halted promptly. Three DNA-dependent RNA polymerases isolated from Saccharomyces strain 1016 were also sensitive to the antibiotic. Lomofungin does not appear to react generally with proteins, as bovine serum albumin did not prevent the inhibitory effect and numerous other enzymes were not affected by lomofungin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman R., Schultz L. D., Hall B. D. Transcription in yeast: separation and properties of multiple FNA polymerases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1702–1706. doi: 10.1073/pnas.69.7.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogt T. M., Planta R. J. Characteristics of DNA-dependent RNA polymerase activity from isolated yeast nuclei. FEBS Lett. 1972 Jan 15;20(1):47–52. doi: 10.1016/0014-5793(72)80014-0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- COOPER J., SRERE P. A., TABACHNICK M., RACKER E. The oxidative pentose phosphate cycle. II. Quantitative determination of intermediates and enzymes. Arch Biochem Biophys. 1958 Apr;74(2):306–314. doi: 10.1016/0003-9861(58)90002-x. [DOI] [PubMed] [Google Scholar]
- Gascón S., Lampen J. O. Purification of the internal invertase of yeast. J Biol Chem. 1968 Apr 10;243(7):1567–1572. [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Gottlieb D., Nicolas G. Mode of action of lomofungin. Appl Microbiol. 1969 Jul;18(1):35–40. doi: 10.1128/am.18.1.35-40.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Johnson L. E., Dietz A. Lomofungin, a new antibiotic produced by Streptomyces lomondensis sp. n. Appl Microbiol. 1969 May;17(5):755–759. doi: 10.1128/am.17.5.755-759.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klo S. C., Cano F. R., Lampen J. O. Lomofungin, an inhibitor of ribonucleic acid synthesis in yeast protoplasts: its effect on enzyme formation. Antimicrob Agents Chemother. 1973 Jun;3(6):716–722. doi: 10.1128/aac.3.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Osmotic regulation of invertase formation and secretion by protoplasts of Saccharomyces. J Bacteriol. 1971 Apr;106(1):183–191. doi: 10.1128/jb.106.1.183-191.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O., McLellan W. L., Jr, el-Nakeeb M. A. Antibiotics and fungal physiology. Antimicrob Agents Chemother (Bethesda) 1965;5:1006–1015. [PubMed] [Google Scholar]
- Menon I. A. Differential effects of -amanitin on RNA polymerase activity in nuclei and mitochondria. Can J Biochem. 1971 Dec;49(12):1395–1398. doi: 10.1139/o71-201. [DOI] [PubMed] [Google Scholar]
- Mondal H., Ganguly A., Das A., Mandal R. K., Biswas B. B. Ribonucleic acid polymerase from eukaryotic cells. Effects of factors and rifampicin on the activity of RNA polymerase from chromatin of coconut nuclei. Eur J Biochem. 1972 Jun 23;28(1):143–150. doi: 10.1111/j.1432-1033.1972.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Ponta H., Ponta U., Wintersberger E. DNA-dependent RNA polymerases from yeast. Partial characterization of three nuclear enzyme activities. FEBS Lett. 1971 Nov 1;18(2):204–208. doi: 10.1016/0014-5793(71)80445-3. [DOI] [PubMed] [Google Scholar]
- REICH E. ACTINOMYCIN: CORRELATION OF STRUCTURE AND FUNCTION OF ITS COMPLEXES WITH PURINES AND DNA. Science. 1964 Feb 14;143(3607):684–689. doi: 10.1126/science.143.3607.684. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Ward D. C., Reich E., Goldberg I. H. Base specificity in the interaction of polynucleotides with antibiotic drugs. Science. 1965 Sep 10;149(3689):1259–1263. doi: 10.1126/science.149.3689.1259. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T. Poisonous principles of mushrooms of the genus Amanita. Four-carbon amines acting on the central nervous system and cell-destroying cyclic peptides are produced. Science. 1968 Mar 1;159(3818):946–952. doi: 10.1126/science.159.3818.946. [DOI] [PubMed] [Google Scholar]



