Abstract
Cold Pre-fermentative Maceration (CPM) is an interesting winemaking technique to enhance the extraction of pigments, aroma and flavour compounds from grape skins to wine. This work aimed to evaluate the effect of Cold Pre-fermentative Maceration (CPM) on the composition of Tannat red wines produced in Uruguay in several vintages. For this purpose, wines elaborated by CPM were compared with control wines produced by Traditional Maceration (TM) in 4 years. Control wines (TM) were made with classical fermentation on skins. The CPM was carried out with additions of dry ice for 5 days to keep the must at 10 ºC. Wines were analysed at devatting. The impact of CPM on composition and color of wines was different in each year. Color intensity was significantly higher in CPM than control wines in 2007 and 2009 whereas the anthocyanins levels were higher in control wines every year. However, CPM wines had the highest polyphenols contents in 2007, 2009 and 2010 principally due to their catechins and proanthocyanidins contents. Anthocyanin profile was similar in the wines of each vintage, according to the varietal fingerprint. The highest contents of higher alcohols were verified in CPM wines. The Cold Pre-fermentative Maceration could have an important effect on the characteristics of Tannat red wines, although it depends strongly on the composition of the grapes of origin.
Keywords: Winemaking, Maceration, Cold pre-fermentative maceration, Anthocyanin, Wine
Introduction
Winemaking procedures employed during skin contact influence the extraction of phenolic compounds from grape skins. Consequently, the composition of wines and their sensorial attributes could be modified, with particular impact on their color features (Sacchi et al. 2005; Gambacorta et al. 2011; González-Neves et al. 2013). Cold Pre-fermentative Maceration (CPM) is an interesting technique for several grape varieties to enhance the extraction of pigments, aroma and flavour compounds from grape skins to must (Gómez-Míguez et al. 2007; Heredia et al. 2010). Nevertheless, several studies provided contradictory results of this practice on the color and composition of wines. Many works showed an increase in color and anthocyanin content of wines made by CPM (Reynolds et al. 2001; Parenti et al. 2004; De Beer et al. 2006; Gómez-Míguez et al. 2007; Busse-Valverde et al. 2011; González-Neves et al. 2013) although other papers showed little impact or negative effects of this technique (Girard et al. 2001; Pérez-Lamela et al. 2007; Gambacorta et al. 2011). These results are related to the effects of many factors which conditioned the cold soak such as grape variety, ripeness of berries, temperature, skin contact time and refrigeration technique of must (Parenti et al. 2004; Álvarez et al. 2006; Gambacorta et al. 2011; Ortega-Heras et al. 2012).
During cold maceration treatment, the must is held at a low temperature, usually 10–15 ºC, for several days. The skin contact takes place in the absence of ethanol because these low maceration temperatures prevent yeasts from starting the fermentation process (Gómez-Míguez et al. 2007). This situation determines preferential solubility of the water-soluble compounds, thus enhancing the extraction of anthocyanin and tannins of low molecular weight (Sacchi et al. 2005; Álvarez et al. 2006; Ortega-Heras et al. 2012). In addition, the pigment release could be favored since as the cap has not been formed, the contact between the solid parts of grapes and the must is better (Ortega-Heras et al. 2012). Moreover, the absence of ethanol allows the formation of higher molecular weight pigments that enhance color stability (Canals et al. 2005).
Tannat (Vitis vinifera L.) is the main red variety cultivated in Uruguay, since the middle of the 19th century. This variety, very rich in pigments and tannins, is very well adapted to the ecological conditions of the country and produces wines of outstanding qualities. Application of CPM in this variety could be interesting because this practice may contribute to obtain highly colored wines with moderate astringency (González-Neves et al. 2010b, 2012, 2013).
For that reason, the aim of this work was to evaluate the effect of Cold Pre-fermentative Maceration on the colour and composition of young red wines produced from Tannat grapes in several vintages.
Materials and methods
Grapes
The essays were carried out in the vintages 2006, 2007, 2009 and 2010. Tannat grapes were grown in the south of Uruguay and were harvested at optimum maturity, regarding the relationship between sugars contents, total acidity and pH. Samples of 250 berries were taken periodically using the method outlined by Carbonneau et al. (1991), extracting fractions of bunches in the middle zone of the spurs. Each fraction of the bunch had from 3 to 5 berries, randomly selected alternatively from the upper and lower parts of the clusters.
Basic analyses of grapes (soluble solids contents, total acidity and pH) were carried out according to (OIV 2007), using a refractometer Atago N1 (Atago, Japan) and a pH meter Hanna HI8521 (Hanna instruments, Italy). These analyses were realised in the juice obtained from the berries manually crushed and the crushing of the pulp with a spinning juice extractor Phillips HR2290 (Phillips, The Netherlands).
At harvest, sample grapes were analysed also according to Glories and Augustin (1993), in order to determine the potential in total anthocyanins (ApH1), the potential in extractable anthocyanins (ApH3.2) and the phenolic richness of the grapes (A280). After grinding the grapes with a blender Phillips HR2855 (Phillips, The Netherlands), model solutions at two different pH values (3.2 and 1.0) were added; them were homogenised and macerated for 4 hours. Before analysis, the extracts were filtered for separating the grinded fragments of grapes and then were centrifuged for 3 min at 3,500 rpm, with a MSE Mistral 2000 centrifuge (Sanyo-Gallenkamp, Great Britain). The anthocyanin contents of the two extracts (pH 3.2 and pH 1.0) were measured according to the spectrophotometric method proposed by Ribéreau-Gayon and Stonestreet (1965). The phenolic richness was estimated by measuring the absorbance at 280 nm of the pH 3.2 extract. All the measurements were carried out by duplicate with a Shimadzu UV-1240 Mini (Shimadzu, Japan) spectrophotometer, using glass (for the anthocyanin analyses) and quartz (for the absorbance at 280 nm analyses) cells with 1 cm path length. The indexes were calculated considering the respective dilution of the grape extracts, according to González-Neves et al. (2004).
Winemaking
Wines elaborated in each year by classical fermentation on skins (Control wines) were compared with wines made by Cold Pre-fermentative Maceration (CPM).
The clusters were transported in plastic boxes (20 kg each one) to the winery. Two batches of 70 Kg of grapes in each one were employed for each winemaking technique. Grapes were destemmed and crushed with an Alfa 60 R crusher (Italcom, Italy), and the barrelling was in stainless-steel tanks (100 L capacity each).
Potassium metabisulfite (50 mg SO2/100 kg of grapes) was added and dry active yeast (Saccharomyces cerevisiae WE372; Anchor, South African) was inoculated in all the musts. The yeasts inoculation were realised immediately in the Control musts while the yeasts were added after pre-fermentative process in CPM.
Control wines were made with classical fermentation on skins (maceration in simultaneous with alcoholic fermentation) for 7 (in 2007) or 8 days (in 2006, 2009 and 2010), according to the polyphenolic potential of grapes at harvest (González-Neves et al. 2004, 2010b). Two pumping over followed by punching the cap were carried out daily along the skin contact. The temperature of fermentation was controlled between 26 and 28 °C.
The CPM wines were vinified with an initial skin contact at low temperature before fermentation, freezing the must with solid carbon dioxide (dry ice). The musts were maintained at low maceration temperatures (near to 10 ºC) for 5 days. After that, classical fermentation on skins was carried out for 7 (2007) or 8 days (2006, 2009 and 2010) at 26 - 28 ºC.
At devatting, the fermentation was finished in all the cases. The pressing of the marc was carried out with a stainless steel manual press. Free-run juices and press juices were mixed and wines were kept in glass recipients of 10-l capacity.
Wine analyses
The wines were analysed at devatting. Basic composition (alcohol contents, dry extract, total and volatile acidity, pH, sugars, total and free sulphur dioxide contents) was determined according to the classical oenological methods (OIV 2007).
Total polyphenols, catechins and proanthocyanidins contents were determined by spectrophotometric methods. Total polyphenols were analysed with Folin-Ciocalteu reagent, according to Singleton and Rossi (1965). Catechins were analysed according to the method proposed by Swain and Hillis (1959). Proanthocyanidins contents were determined according to Ribéreau-Gayon and Stonestreet (1966). The colour of the wines was evaluated with the indexes proposed by Glories (1984).
The wines were centrifuged for 3 min at 3,000 rpm before analysis. All the measurements were carried out in a Shimadzu UV-1240 Mini (Shimadzu, Japan) spectrophotometer, using glass cells with 1 mm path length for the colour analyses and glass cells with 1 cm path length for the polyphenolic analyses.
The anthocyanin contents of the wines were determined by HPLC-DAD, according to Revilla et al. (1999). After filtration through Sartorius filters (Sartorius, USA) (0.45 μm diameter) the samples were injected directly into a a chromatographic system equipped with two pumps Waters 510 and 515, a Rheodyne 7725i injector (20 μm loop) and a photodiode detector Waters 2996 (Waters Corp., USA). The system was controlled with Millenium 32 Software (Waters Corp., USA). A Phenomenex Luna C18 reverse phase column, 5 μm, 150 × 4.6 mm (Waters Corp., USA) was used as stationary phase, with a mobile phase flow rate of 0.8 mL/min. The solvent A was an aqueous solution (10 %) of formic acid, and solvent B was an aqueous solution of methanol (45 %) and formic acid (10 %). The gradient established was: from 35 to 95 % B for 20 min, from 95 to 100 % B for 5 min, isocratic 100 % B for 5 min (Revilla et al. 1999). Two replications of the analyses were performed in all the cases. The identification of the compounds was carried out taking into account the spectrum of each one and the retention time of each peak. Previously, the identification was confirmed (González-Neves et al. 2007) using as a reference a chromatographic system with a mass spectrophotometer (Hewlett Packard 1100 Series LC-MS). The concentration of each pigment was calculated considering a calibration curve with malvidin glucoside chloride and the results are expressed in malvidin-3-O-glucoside mg/L.
The major volatile compounds were analysed following the gas chromatography (GC) method based on direct injection of the sample (Bertrand 1993) into a Agilent 6890 N gas chromatograph with a flame ionisation detector (Agilent Tech., USA). The analyses were carried out using a HP–INNOWAX HP–19091 N–133 bonded fused silica capillary column (30 m × 0.25 mm i.d.) coated with polyethylene glycol (0.25 μm phase thickness); the column temperature varied from 35 °C (3 min) to 165 °C at 8 °C/min. The following additional parameters were used: injector temperature, 220 °C; injection mode, splitless; volume injected, 1 μL; carrier gas was Hydrogen. Identification was carried out by comparison of the retention times with those of corresponding pure standards, and quantification was based on the calibration curves of respective standards.
Statistical analyses
The data were analysed with Statgraphics Plus package, 4.1 version (Statgraphics Corp., USA). Analyses of Variance and media separation by Tukey at 5 % were performed.
Results and discussion
Composition of grapes
Table 1 presents the composition of grapes. An important vintage effect was found. Thus, the grapes analysed in 2006 had the highest sugars contents, phenolic richness and anthocyanin potential while the berries collected in 2010 had the lowest levels of sugars, phenolic richness and pH. The values of the extractability index of anthocyanins (EA%) were high in every year, which is typical for this variety (González-Neves et al. 2004, 2010b). This result indicate a low extractability of these compounds, related to difficulties for their passage from the grapes to the must during maceration, and justifies the realization of different procedures to maximize their extraction from the beginning of the skin contact (Cagnasso et al. 2008; González-Neves et al. 2010b).
Table 1.
Basic composition and phenolic potential of grapes
| Year | S (g/L) | TA (meq/L) | pH | A280 (AU) | ApH1 (mg/L) | ApH3.2 (mg/L) | EA% | Mp% |
|---|---|---|---|---|---|---|---|---|
| 2006 | 230.0 a ± 1.1 | 106.1 a ± 1.7 | 3.36 b ± 0.04 | 66.2 a ± 1.1 | 2571.0 a ± 40.8 | 1283.0 a ± 19.5 | 50.1 c ± 0.8 | 22.5 c ± 0.5 |
| 2007 | 205.2 b ± 1.5 | 97.9 b ± 1.5 | 3.42 b ± 0.05 | 50.6 c ± 2.0 | 1810.3 b ± 85.2 | 920.5 b ± 42.0 | 49.2 c ± 0.6 | 27.3 b ± 0.8 |
| 2009 | 224.2 a ± 1.0 | 77.5 c ± 1.2 | 3.72 c ± 0.03 | 55.1 b ± 2.3 | 1972.8 b ± 127.5 | 856.3 b ± 59.8 | 56.6 a ± 0.6 | 37.9 a ± 1.7 |
| 2010 | 201.0 b ± 1.3 | 98.7 b ± 3.3 | 3.26 a ± 0.04 | 47.2 d ± 3.3 | 1887.2 b ± 146.8 | 861.3 b ± 81.5 | 54.4 b ± 1.2 | 27.1 b ± 1.9 |
Mean values ± standard deviations. Values with the same letter in the same column have no statistical significant differences according to a Tukey test (p < 0.05). S: sugars contents, TA: total acidity, A280: phenolic richness, ApH1: total anthocyanin potential, ApH3.2: extractable anthocyanin potential, EA%: extractability of anthocyanins; Mp%: proportion of seed tannins. AU: absorbance units. Anthocyanins expressed in mg/L of malvidin glucoside
General and polyphenolic composition of wines
Chemical basic characteristics of wines were in agreement with grape ripeness (Table 2). Winemaking technologies exerted little influence on the basic characteristics of wines. Important differences were observed in the alcohol content of wines produced in 2006 (Table 2), indicating some heterogeneity of the grapes employed in this year. Some authors (Girard et al. 2001; Parenti et al. 2004) reported increases in ethanol concentrations of wines produced by cold maceration, but only small differences in this sense were verified in 2007 and 2009.
Table 2.
Basic composition of wines
| Year | Treatment | Alcohol (% v/v) | Total acidity (meq/L) | Volatile acidity (meq/L) | pH | Residual sugars (g/L) | Total dry extract (g/L) | Total sulphur dioxide (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 2006 | Control | 12.8 a ± 0.1 | 123.9 a ± 1.5 | 4.6 ns ± 0.8 | 3.47 ns ± 0.02 | 1.9 ns ± 0.1 | 34.6 a ± 0.5 | 41.2 ns ± 8.2 |
| CPM | 12.1 b ± 0.1 | 112.6 b ± 3.4 | 4.7 ns ± 0.4 | 3.49 ns ± 0.03 | 2.0 ns ± 0.1 | 33.3 b ± 0.2 | 45.1 ns ± 4.5 | |
| 2007 | Control | 11.4 b ± 0.1 | 76.5 ns ± 1.4 | 7.7 a ± 0.6 | 3.79 ns ± 0.02 | 2.0 ns ± 0.1 | 32.4 ns ± 0.2 | 54.8 ns ± 6.7 |
| CPM | 11.8 a ± 0.1 | 85.2 ns ± 3.6 | 4.0 b ± 0.3 | 3.70 ns ± 0.05 | 1.9 ns ± 0.1 | 32.5 ns ± 0.1 | 58.2 ns ± 8.9 | |
| 2009 | Control | 12.2 b ± 0.1 | 91.3 ns ± 1.3 | 1.9 ns ± 0.9 | 3.69 ns ± 0.02 | 1.8 ns ± 0.1 | 30.6 ns ± 0.3 | 50.0 b ± 5.7 |
| CPM | 12.5 a ± 0.1 | 93.5 ns ± 2.4 | 2.1 ns ± 0.3 | 3.67 ns ± 0.03 | 1.8 ns ± 0.1 | 31.7 ns ± 0.7 | 67.5 a ± 6.9 | |
| 2010 | Control | 10.8 a ± 0.1 | 106.1 ns ± 2.0 | 2.0 b ± 0.0 | 3.44 ns ± 0.01 | 1.6 ns ± 0.1 | 29.6 ns ± 0.3 | 56.0 ns ± 6.5 |
| CPM | 10.5 b ± 0.1 | 105.1 ns ± 2.2 | 2.2 a ± 0.0 | 3.46 ns ± 0.01 | 1.5 ns ± 0.1 | 29.9 ns ± 0.3 | 53.1 ns ± 4.5 |
Mean values ± standard deviations. Values with the same letter in the same year mean that there are no statistically significant differences between the wines, according to a Tukey test (p < 0.05)
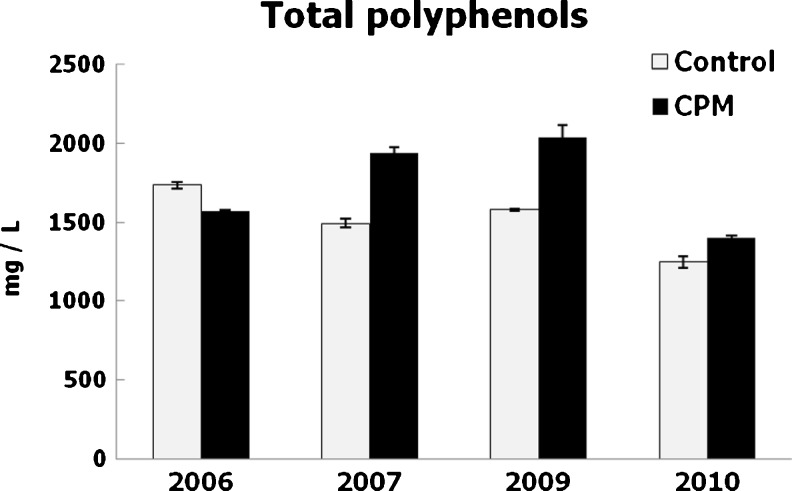
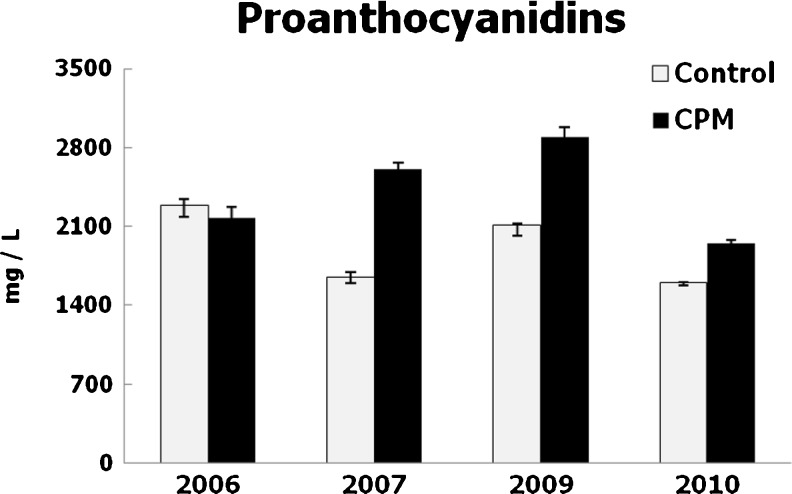
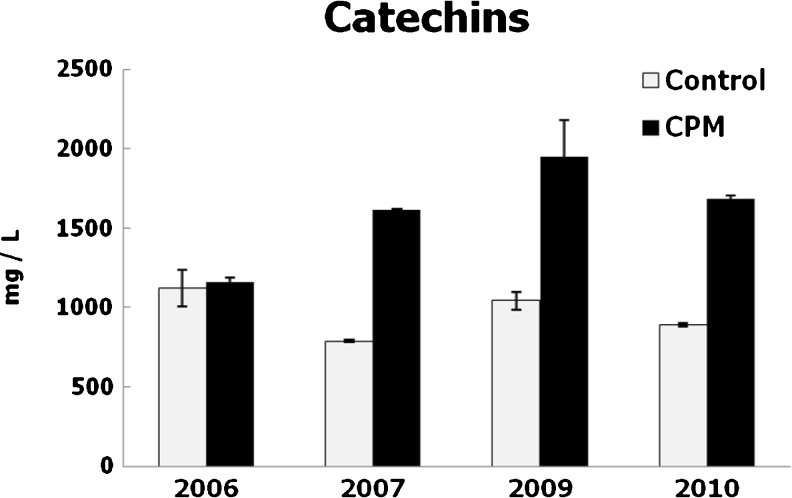
The impact of CPM on the polyphenol composition of wines was very different in the different vintages. Polyphenol contents were modified significantly in most years by CPM, with very important increases of monomeric (catechins) and polymeric tannins (proanthocyanidins) (Figs. 1 to 3). The wines produced by CPM had the highest levels of total polyphenols and proanthocyanidins in 2007, 2009 and 2010, and they were the richest in catechins every year. The increase of tannins contents could be atributed to the total lenght of skin contact because the pre-fermentative maceration was continued by a classical fermentation on skins. Nevertheless, Busse-Valverde et al. (2010) indicated that the increases in tannin contents by cold soak treatments were mainly due to an increase in seed proanthocyanidins, which also were extracted in the absence of ethanol. Moreover, the increase in catechin contents could be due to the differential extraction of these compounds in aqueous medium at pre-fermentation (Álvarez et al. 2006). Increases of hydrocinnamic acid derivatives by CPM (Gil-Muñoz et al. 1999) may contribute to enhace total polyphenol levels.
Fig. 1.
Total polyphenol contents of wines. Mean values and standard deviations, expressed in mg/L of gallic acid
Fig. 3.
Proanthocyanidin contents of wines. Mean values and standard deviations, expressed in mg/L of cyanidin chloride
Anthocyanin composition of wines
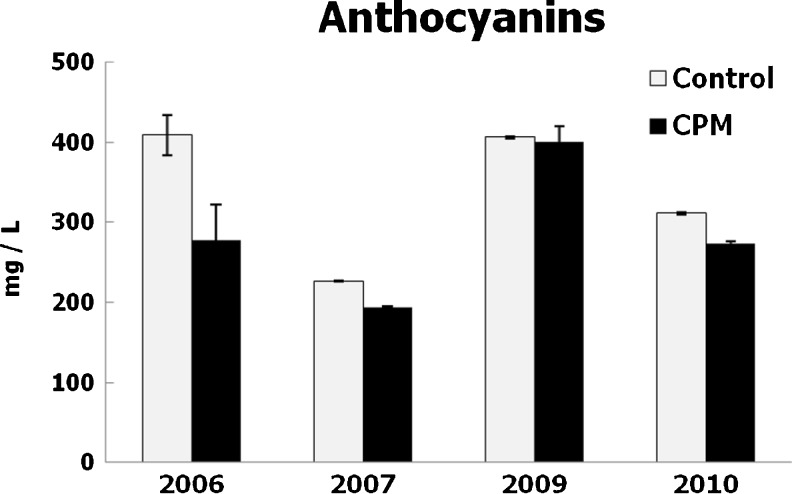
The effect of CPM on the anthocyanin composition of the wines produced in 2006 to 2009 was reported in a previous paper (González-Neves et al. 2012). The cold pre-fermentative process did not increase the anthocyanin levels; control wines had higher contents of these pigments in every vintage (Fig. 4) that agrees to the results of many papers (Girard et al. 2001; Pérez-Lamela et al. 2007; Puertas et al. 2008; Gil-Muñoz et al. 2009; Gambacorta et al. 2011). However, several works showed an increase in the anthocyanin contents of wines produced by pre-fermentative maceration at low temperature (Gómez-Plaza et al. 2000; Reynolds et al. 2001; Parenti et al. 2004; De Beer et al. 2006; Gómez-Míguez et al. 2007; Alvarez et al. 2009; Gil-Muñoz et al. 2009; Heredia et al. 2010; Busse-Valverde et al. 2011; González-Neves et al. 2013). Presumably, the characteristics of Tannat grapes, and principally the low extractability of anthocyanins in spite of their richness (Table 1), affect strongly the effect of the pre-fermentative skin contact on the release of these compounds. Dry ice could have an extractive effect by contact with skins, coupled with a protective effect due to the displacement of oxygen after sublimation (Parenti et al. 2004; Heredia et al. 2010).
Fig. 4.
Anthocyanin contents of wines. Mean values and standard deviations, expressed in mg/L of malvidin glucoside
Control wines produced in 2006 and 2009 had significantly higher contents of anthocyanins than those produced in 2007 and 2010 (Fig. 4). This result is based on the differences verified in the grape composition (Table 1) and on the size of the berries harvested each year. The water deficit in early ripeness determined a decrease in the size of the berries produced in 2009 (data not shown). The small size of berries enhances the extraction of anthocyanins (Ortega-Regules et al. 2008); therefore, differences in the composition of grapes were minimized by the effect of winemaking and are not directly reflected in the composition of wines (Table 1 and Figs. 2 to 5).
Fig. 2.
Catechin contents of wines. Mean values and standard deviations, expressed in mg/L of D-catechin
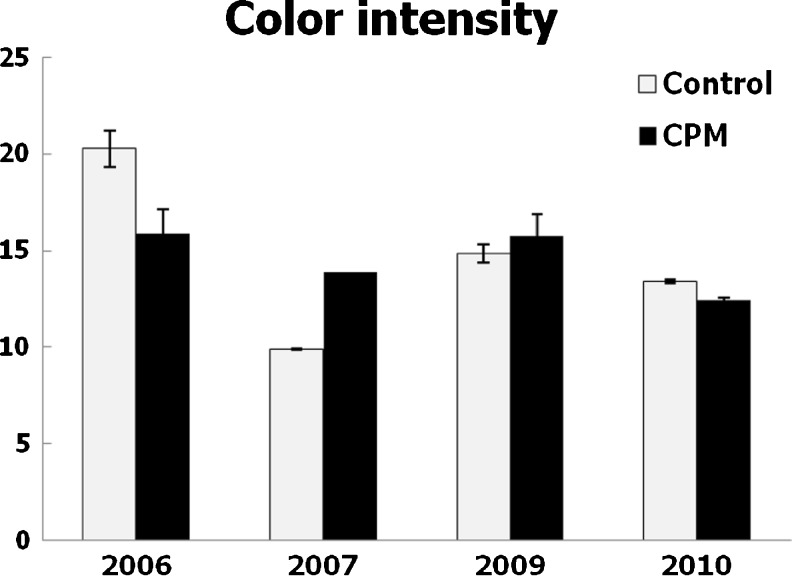
Fig. 5.
Color intensity of wines. Mean values and standard deviations, expressed in absorbance units
Anthocyanin profile of wines
The levels of the different types of molecules were quite different in the wines of each year and were modified by the winemaking technique (Table 3). The solubility and stability of each type of anthocyanin has incidence on its extraction and its reactions during vinification, which may establish a differential effect of each technique of winemaking on the anthocyanin profile. The vintage modifies the levels of all anthocyanins and principally malvidin, acetylated and non-acylated glucosides. In turn, winemaking technique had different impact on the levels of the different types of anthocyanin in each year.
Table 3.
Anthocyanin composition of wines. Concentrations of the five anthocyanidins and the three types of glucosides
| Year | Treatment | Anthocyanidins | Glucosides | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | ||||||||
| Dp | Cy | Pt | Pn | Mv | NA | Ac | Cm | ||
| 2006 | Control | 34.0 a ± 3.6 | 2.5 a ± 0.1 | 62.8 a ± 4.9 | 17.4 a ± 1.0 | 292.1 a ± 15.1 | 294.9 a ± 17.3 | 68.0 a ± 3.5 | 43.4 a ± 3.8 |
| CPM | 16.2 b ± 4.2 | 1.9 b ± 0.1 | 35.1 b ± 7.2 | 13.0 b ± 1.1 | 212.9 b ± 31.6 | 202.5 b ± 30.2 | 48.2 b ± 7.4 | 27.1 b ± 5.2 | |
| 2007 | Control | 11.1 a ± 0.1 | 1.4 ns ± 0.1 | 22.4 ns ± 0.1 | 10.3 a ± 0.1 | 181.9 a ± 0.6 | 166.4 a ± 0.5 | 40.8 a ± 0.1 | 18.1 a ± 0.2 |
| CPM | 9.7 b ± 0.1 | 1.5 ns ± 0.1 | 22.3 ns ± 0.1 | 8.4 b ± 0.1 | 152.4 b ± 0.7 | 141.0 b ± 0.6 | 36.2 b ± 0.1 | 17.0 b ± 0.1 | |
| 2009 | Control | 29.0 a ± 0.1 | 1.3 b ± 0.1 | 56.7 ns ± 0.6 | 13.0 ns ± 0.1 | 306.4 ns ± 1.9 | 290.1 ns ± 3.0 | 78.9 a ± 0.5 | 34.7 ns ± 2.1 |
| CPM | 28.2 b ± 0.2 | 2.7 a ± 0.2 | 55.3 ns ± 5.3 | 13.3 ns ± 0.6 | 300.7 ns ± 10.9 | 284.6 ns ± 14.9 | 75.6 b ± 1.9 | 36.9 ns ± 2.2 | |
| 2010 | Control | 21.8 a ± 0.1 | 0.0 ns ± 0 | 47.1 a ± 0.1 | 9.1 a ± 0.1 | 233.2 a ± 0.9 | 229.2 a ± 0.6 | 55.9 a ± 0.1 | 25.4 a ± 0.1 |
| CPM | 18.6 b ± 0.1 | 0.0 ns ± 0 | 39.8 b ± 0.1 | 7.1 b ± 0.1 | 208.4 b ± 2.2 | 200.6 b ± 2.2 | 49.8 b ± 0.1 | 22.7 b ± 0.1 | |
Mean values ± standard deviations. Values with the same letter in the same year mean that there are no statistically significant differences between the wines, according to a Tukey test (p < 0.05). Dp: delphinidin; Cy: cyanidin; Pt: petunidin; Pn: peonidin; Mv: malvidin; NA: non-acylated glucosides; Ac: acetyl glucosides; Cm: coumaroyl glucosides
The anthocyanic profile of wines, determined by the relationship between the different types of molecules, was slightly modified by CPM. Thus, the proportion of each anthocyanin was more affected by the vintage factors than by the winemaking techniques. These results confirm the large influence of climate on the anthocyanin biosynthesis and on the extractability of these compounds from the grapes, which modifies the effect of the winemaking techniques in each year (Cagnasso et al. 2011; González-Neves et al. 2012). However, these changes do not modify significantly the characteristic fingerprint of the variety. Tannat grapes and wines are characterized by higher contents of delphinidin, petunidin and non-acylated glucosides than Merlot and Cabernet Sauvignon grapes and wines produced in Uruguay (González-Neves et al. 2007, 2010b).
The proportion of delphinidin in the total of anthocyanins was comprised between 4.9 and 8.3 %; cyanidin was ranging from 0.0 at 0.8 %; petunidin from 9.9 at 15.4 %; peonidin from 2.6 at 4.7 %; malvidin from 72.1 at 80.1 %. Concerning the acylation, 71.7 to 73.8 % of the glucosides were non acylated, 16.6 to 19.5 % were acetyl glucosides, 8.3 to 10.4 % were coumaryl glucosides.
The results obtained in this study support the conclusion that anthocyanin profile of wines is mostly dependent on the grape variety rather than the winemaking technology (Puertas et al. 2008; González-Neves et al. 2010a). Nevertheless, the effect of technology on the anthocyanin composition of wines needs to be further investigated, since it seems to be linked to the intrinsic characteristics of grapes in terms of ripening and richness in phenols (Gambacorta et al. 2011).
Color of wines
The impact of CPM on the color of wines was very different in each year (Fig. 5). Color intensity was increased by CPM in 2007 and 2009. The best effect of this practice on wine color was verified in 2007, when the grapes were less ripe although they had the best extractability of anthocyanin (Table 1). The color of red wines was enhanced by cold pre-fermentative maceration in numerous experiences (Gómez-Plaza et al. 2000; Reynolds et al. 2001; De Beer et al. 2006; Gómez-Míguez et al. 2007; Gil-Muñoz et al. 2009; Heredia et al. 2010; Busse-Valverde et al. 2011; González-Neves et al. 2013) but it was not significantly in others (Alvarez et al. 2009). Anyway, in these cases it is hoped that it increases colour stability (Álvarez et al. 2009).
The worst results of CPM were verified in the vintage 2006, when control wines had been the richest in total polyphenols and anthocyanin (Figs. 1 and 4). The grapes produced in this year had the best ripeness (Table 1), but also important differences were observed in the alcohol content of wines (Table 2), indicating some heterogeneity of the raw material used in each winemaking. Consequently, the results can not be attributed accurately to the effect of winemaking technique.
The results of our work are in agreement with several authors (Reynolds et al. 2001; Llaudy et al. 2005; Álvarez et al. 2006) which suggest that the effectiveness of CPM are strongly related to the ripeness of the grapes. These authors reported that the best results are related to grapes with low richness in anthocyanins. However, Ortega-Heras et al. (2012) signaled that the cold pre-fermentative maceration with dry ice enhances the release of anthocyanin and other polyphenols and the formation of new pigments, increasing colour stability and intensity, particularly when the grapes reached a higher maturity degree. Anyway, the results of this study together with earlier data (González-Neves et al. 2012, 2013) confirm that different characteristics of the grape, such as the size of berry or the extractability of anthocyanins, could modify the effectiveness of CPM on the enhancement of the wine color.
In a previous work, the wines made in 2006 were analysed along 1 year (González-Neves et al. 2010a). In this case, the results suggest that CPM would promote the formation of derivative pigments of anthocyanins which contribute to maintain better the color along the time. Other studies through time should be made to confirm these preliminary conclusions.
The small amount of grapes employed in the vinifications could modify the results favoring the anthocyanin extraction in the traditional maceration. Moreover, the little volume of juice corresponding to the winemaking scale employed may increase the polyphenolic fixation onto the grape skins and seeds, particularly in longer macerations. These considerations justify the precaution in the adoption of alternative winemaking techniques in an industrial scale.
Major volatile composition of wines
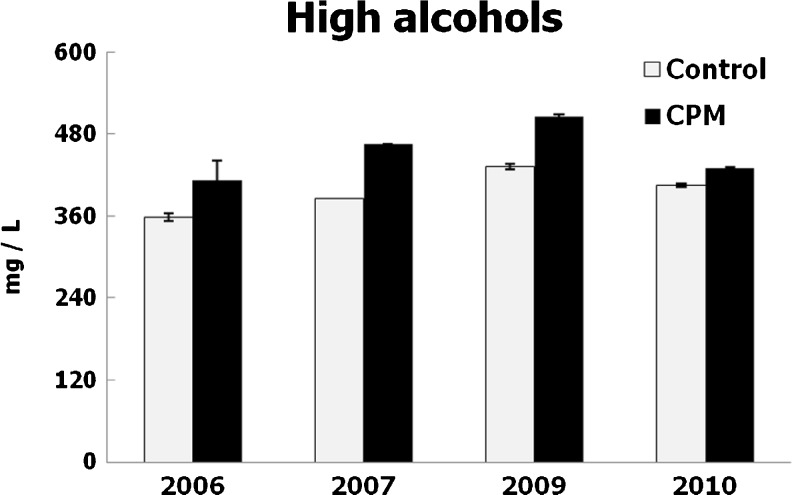
The volatile composition of wines was modified by winemaking. Ethyl acetate contents were increased by cold soak, mainly in 2006 and 2009, in agree with Salinas et al. (2003). The contents of higher alcohols were increased by CPM every year (Fig. 6). The main differences were verified in the levels of 2-methyl-1-propanol and 2 and 3-methyl-1butanol (Table 4). These results could be related to the development of non-Saccharomyces yeast strains along the cold maceration (Romano et al. 1992). Aroma modifications induced by these yeasts may contribute to the quality enhancement and improved complexity of wines elaborated by using CPM (Bosso et al. 2006).
Fig. 6.
Higher alcohol contents of wines. Mean values and standard deviations, expressed in mg/L of higher alcohols
Table 4.
Major volatile composition of wines
| Year | Treatment | Acetaldehide (mg/L) | Ethyl acetate (mg/L) | Methanol (mg/L) | 1-propanol (mg/L) | 2-methyl-1- propanol (mg/L) | 2 and 3-methyl-1-butanol (mg/L) |
|---|---|---|---|---|---|---|---|
| 2006 | Control | Nd | 23.4 b ± 0.6 | 111.5 b ± 5.0 | 30.6 ns ± 1.3 | 43.6 b ± 07 | 283.8 b ± 4.1 |
| CPM | Nd | 35.4 a ± 12.1 | 128.1 a ± 0.7 | 31.0 ns ± 1.9 | 75.6 a ± 0.5 | 305.7 a ± 29.5 | |
| 2007 | Control | Nd | 32.1 b ± 0.1 | 138.2 ns ± 0.1 | 40.4 a ± 0.1 | 44.3 b ± 0.1 | 300.3 b ± 0.2 |
| CPM | Nd | 33.8 a ± 0.3 | 139.2 ns ± 1.6 | 32.5 b ± 0.1 | 64.6 a ± 0.3 | 368.6 a ± 0.7 | |
| 2009 | Control | 14,0 a ± 2.0 | 22.6 b ± 0.4 | 103.9 b ± 1.5 | 22.4 b ± 0.3 | 72.6 b ± 0.4 | 337.4 b ± 3.4 |
| CPM | 7,7 b ± 0.6 | 39.9 a ± 1.1 | 115.5 a ± 4.4 | 24.7 a ± 0.2 | 88.5 a ± 5.1 | 392.0 a ± 4.6 | |
| 2010 | Control | 7,3 b ± 0.2 | 30.4 a ± 0.4 | 88.6 a ± 0.7 | 52.6 b ± 0.3 | 80.3 b ± 0.6 | 272.5 b ± 1.4 |
| CPM | 8,3 a ± 0.1 | 27.3 b ± 0.3 | 79.7 b ± 0.7 | 53.3 a ± 0.1 | 91.2 a ± 0.5 | 285.1 a ± 0.8 |
Mean values ± standard deviations. Values with the same letter in the same year mean that there are no statistically significant differences between the wines, according to a Tukey test (p < 0.05)
Higher alcohols were among the aromas released as secondary products of yeast metabolism. These compounds could be synthesized by yeast through either the anabolic pathway from glucose, or the catabolic pathway from their corresponding amino acids (Tao et al. 2008).
Couasnon (1999) signaled that the saturation of the musts with carbonic dioxide, produced by the ice dry, could modify the production of higher alcohols during the alcoholic fermentation and explain the increases of these compounds in the CPM wines. In addition, Baumes (1998) indicates that an increase in carbonic gas pressure was reported among the factors enhancing higher alcohol production along fermentation.
Conclusions
The impact of CPM on the wines color and composition was very different in each year. The anthocyanin contents of Tannat wines were not increased by the cold pre-fermentative maceration. In turn, CPM process contributes to increase tannin contents and particularly catechin contents of wines. Moreover, this technique may enhance color intensity and stability, because it could promote anthocyanin derivative pigments formation.
Our results confirm that the effect of Cold Pre-fermentative Maceration depends strongly on the composition of the grapes, but others features like the size of berries and the extractability of anthocyanins modify the results of this technique. Further studies on industrial scale and with longer ageing time of wines will allow to confirm these conclusions.
Anthocyanin composition of wines is mostly dependent on the grape variety rather than winemaking technique. Climate along grape ripeness has a large influence on the anthocyanin biosynthesis, which modifies the effect of the winemaking in each year.
Acknowledgments
The authors are grateful to L. Barreiro, J. Balado, V. Berriel, R. Bochicchio, G. Gatto, and A. Tessore for their participation in this work.
References
- Álvarez I, Aleixandre J, García M, Lizama V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal Chim Acta. 2006;563:109–115. doi: 10.1016/j.aca.2005.10.068. [DOI] [Google Scholar]
- Álvarez I, Aleixandre J, García M, Lizama V, Aleixandre J. Effect of the prefermentative addition of copigments on the polyphenolic composition of Tempranillo wines after malolactic fermentation. Eur Food Res Technol. 2009;228:501–510. doi: 10.1007/s00217-008-0957-0. [DOI] [Google Scholar]
- Baumes R. Les constituants volatils du stade fermentaire. Paris: Lavoisier; 1998. pp. 193–201. [Google Scholar]
- Bertrand A. Les principales substances chimiques actuellement dosées par chromatographie. Paris: Lavoisier; 1993. pp. 1–16. [Google Scholar]
- Bosso A, Panero L, Di Stefano R (2006) La criomacerazione con neve carbonica abbinata alla tecnica dell’estrazione differita degli antociani. Ind Bevande XXXV:449–461.
- Busse-Valverde N, Gómez-Plaza E, López-Roca J, Gil-Muñoz R, Fernández-Fernández J, Bautista-Ortín A. Effect of different enological practices on skin and seed proanthocyanidins in three varietal wines. J Agric Food Chem. 2010;58:11333–11339. doi: 10.1021/jf102265c. [DOI] [PubMed] [Google Scholar]
- Busse-Valverde N, Gómez-Plaza E, López-Roca J, Gil-Muñoz R, Bautista-Ortín A. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J Agric Food Chem. 2011;59:5450–5455. doi: 10.1021/jf2002188. [DOI] [PubMed] [Google Scholar]
- Cagnasso E, Rolle L, Caudana A, Gerbi V. Relationship between grape phenolic maturity and red wine phenolic composition. Ital J Food Sci. 2008;3:365–380. [Google Scholar]
- Cagnasso E, Torchio F, Gerbi V, Río Segade S, Giacosa S, Rolle L. Evolution of the phenolic content and extractability indices during ripening of nebbiolo grapes from the piedmont growing areas over six consecutive years. S Afr J Enol Vitic. 2011;31(2):229–241. [Google Scholar]
- Canals R, Llaudy M, Valls J, Canals J, Zamora F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J Agric Food Chem. 2005;53:4019–4025. doi: 10.1021/jf047872v. [DOI] [PubMed] [Google Scholar]
- Carbonneau A, Moueix A, Leclair N, Renoux J. Proposition d’une méthode de prélèvement de raisins à partir de l’analyse de l’hétérogéneité de maturation sur un cep. Bull OIV. 1991;727(728):679–690. [Google Scholar]
- Couasnon M. Une nouvelle technique, la maceration prefermentaire a froid. extraction a la neige carbonique. 2e. partie : la technologie de la neige carbonique. Rev Œnol. 1999;93:28–32. [Google Scholar]
- De Beer D, Joubert E, Marais J, Manley M. Maceration before and during fermentation: effect on pinotage wine phenolic composition, total antioxidant capacity and objective colour parameters. S Afr J Enol Vitic. 2006;27:137–150. [Google Scholar]
- Gambacorta G, Antonacci D, Pati S, La Gatta M, Faccia M, Coletta A, La Notte E. Influence of winemaking technologies on phenolic composition of Italian red wines. Eur Food Res Technol. 2011;233:1057–1066. doi: 10.1007/s00217-011-1613-7. [DOI] [Google Scholar]
- Gil-Muñoz R, Gómez-Plaza E, Martínez A, López-Roca J. Evolution of phenolic compounds during wine fermentation and post-fermentation: influence of grape temperature. J Food Comp Anal. 1999;12:259–272. doi: 10.1006/jfca.1999.0834. [DOI] [Google Scholar]
- Gil-Muñoz R, Moreno-Pérez A, Vila-López R, Fernández-Fernández J, Martínez-Cutillas A, Gómez-Plaza E. Influence of low temperature prefermentative techniques on chromatic and phenolic characteristics of Syrah and Cabernet Sauvignon wines. Eur Food Res Technol. 2009;228:777–788. doi: 10.1007/s00217-008-0989-5. [DOI] [Google Scholar]
- Girard B, Yuksel D, Cliff M, Delaquis P, Reynolds A. Vinification effects on the sensory, colour and GC profiles of pinot noir wines from British Columbia. Food Res Int. 2001;34:483–499. doi: 10.1016/S0963-9969(00)00177-0. [DOI] [Google Scholar]
- Glories Y. La couleur des vins rouges. 2e. Partie: Mesure, origine et interpretation. Conn Vigne Vin. 1984;18(4):253–271. [Google Scholar]
- Glories Y, Augustin M. Maturité phénolique du raisin, conséquences technologiques: application aux millésimes 1991 et 1992. Bordeaux: CIVB; 1993. pp. 56–61. [Google Scholar]
- Gómez-Míguez M, González-Miret G, Heredia F. Evolution of colour and anthocyanin composition of Syrah wines elaborated with pre-fermentative cold maceration. J Food Eng. 2007;9:271–278. doi: 10.1016/j.jfoodeng.2006.01.054. [DOI] [Google Scholar]
- Gómez-Plaza E, Gil-Muñoz R, López-Roca J, Martínez A. Color and phenolic compounds of a young red wine. Influence of wine-making techniques, storage temperature, and length of storage time. J Agric Food Chem. 2000;48:736–741. doi: 10.1021/jf9902548. [DOI] [PubMed] [Google Scholar]
- González-Neves G, Charamelo D, Balado J, Barreiro L, Bochicchio R, Gatto G, Gil G, Tessore A, Carbonneau A, Moutounet M. Phenolic potential of tannat, cabernet-sauvignon and merlot grapes and their correspondence with wine composition. Anal Chim Acta. 2004;513:191–196. doi: 10.1016/j.aca.2003.11.042. [DOI] [Google Scholar]
- González-Neves G, Franco J, Barreiro L, Gil G, Moutounet M, Carbonneau A. Varietal differentiation of tannat, cabernet-sauvignon and merlot grapes and wines according to their anthocyanic composition. Eur Food Res Technol. 2007;225:111–117. doi: 10.1007/s00217-006-0388-8. [DOI] [Google Scholar]
- González-Neves G, Gil G, Barreiro L, Bochicchio R, Gatto G, Tessore A, Favre G. Pigment profile of red wines cv Tannat made with alternative winemaking. J Food Comp Ana. 2010;23(5):447–454. doi: 10.1016/j.jfca.2009.08.021. [DOI] [Google Scholar]
- González-Neves G, Gil G, Ferrer M, Charamelo D, Balado J, Bochicchio R, Gatto G, Tessore A. Prediction of the color and polyphenolic composition of the young red wines from the phenolic potential of the grapes. Int J Food Sci Technol. 2010;45(9):1843–1851. doi: 10.1111/j.1365-2621.2010.02343.x. [DOI] [Google Scholar]
- González-Neves G, Gil G, Favre G, Ferrer M. Influence of grape composition and winemaking on the anthocyanin composition of red wines of Tannat. Int J Food Sci Technol. 2012;47:900–909. doi: 10.1111/j.1365-2621.2011.02920.x. [DOI] [Google Scholar]
- González-Neves G, Gil G, Favre G, Baldi C, Hernández N, Traverso S. Influence of winemaking procedure and grape variety in the colour and composition of young red wines. S Afr J Enol Vitic. 2013;34(1):138–146. [Google Scholar]
- Heredia F, Escudero M, Hernandez D, Gordillo B, Meléndez A, Vicario I, González M. Influence of the refrigeration technique on the colour and phenolic composition of Syrah red wines obtained by pre-fermentative cold maceration. Food Chem. 2010;118(2):377–383. doi: 10.1016/j.foodchem.2009.04.132. [DOI] [Google Scholar]
- Llaudy M, Canals R, Cabanillas P, Canals J, Zamora F (2005) La maceración prefermentativa en frío. ACE Revista de Enología. http://www.acenologia.com. Accessed 18 Sept 2008.
- OIV . Récueil des méthodes internationales d’analyse des moûts et des vins. Paris: OIV; 2007. [Google Scholar]
- Ortega-Heras M, Pérez-Magariño S, González-Sanjosé M. Comparative study of the use of maceration enzymes and cold pre-fermentative maceration on phenolic and anthocyanic composition and colour of a Mencía red wine. LWT – Food. Sci Technol. 2012;48:1–8. [Google Scholar]
- Ortega-Regules A, Romero-Cascales J, Ros García J, Bautista-Ortín A, López-Roca M, Fernández-Fernández J, Gómez-Plaza E. Anthocyanins and tannins in four grape varieties (Vitis vinifera L.). evolution of their content and extractability. J Int Sci Vigne Vin. 2008;42:147–156. [Google Scholar]
- Parenti A, Spugnoli P, Calamai L, Ferrari S, Gori C. Effects of cold maceration on red wine quality from Tuscan Sangiovese grape. Eur Food Res Technol. 2004;218:360–366. doi: 10.1007/s00217-003-0866-1. [DOI] [Google Scholar]
- Pérez-Lamela C, García-Falcón M, Simal-Gándara J, Orriols-Fernández I. Influence of grape variety, vine system and enological treatments on the colour stability of young red wines. Food Chem. 2007;101:601–606. doi: 10.1016/j.foodchem.2006.02.020. [DOI] [Google Scholar]
- Puertas B, Guerrero R, Jurado M, Jiménez M, Cantos V. Evaluation of alternative winemaking processes for red wine color enhancement. Food Sci Tech Int. 2008;14:21–27. doi: 10.1177/1082013208095686. [DOI] [Google Scholar]
- Revilla I, Pérez-Magariño S, González-Sanjosé M, Beltrán S. Identification of anthocyanin derivatives in grape skin extracts and red wines by liquid chromatography with diode array and mass spectrometric detection. J Chrom A. 1999;847:83–90. doi: 10.1016/S0021-9673(99)00256-3. [DOI] [Google Scholar]
- Reynolds A, Cliff M, Girard B, Kopp T. Influence of fermentation temperature on composition and sensory properties of Semillon and Shiraz wines. Am J Enol Vitic. 2001;52(3):235–240. [Google Scholar]
- Ribéreau-Gayon P, Stonestreet E. Le dosage des anthocyanes dans le vins rouge. Bull Soc Chimie. 1965;9:2649–2653. [PubMed] [Google Scholar]
- Ribéreau-Gayon P, Stonestreet E. Dosage des tanins dans du vin rouge et détermination de leur structure. Chimie Anal. 1966;48:188–196. [Google Scholar]
- Romano P, Suzzi G, Comi G, Zironi R. Higher alcohol and acetic acid production by apiculate wine yeasts. J Applied Bacteriol. 1992;73(2):126–130. doi: 10.1111/j.1365-2672.1992.tb01698.x. [DOI] [Google Scholar]
- Sacchi K, Bisson L, Adams D. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am J Enol Vitic. 2005;56(3):197–206. [Google Scholar]
- Salinas R, Garijo J, Pardo F, Zalacain A, Alonso G. Color, polyphenol, and aroma compounds in rosé wines after prefermentative maceration and enzymatic treatments. Am J Enol Vitic. 2003;54(3):195–202. [Google Scholar]
- Singleton V, Rossi J. Colorimetry of total phenolics with phosphomolybdic and phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Swain T, Hillis W. The phenolic constituents of Prunus domestica I The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tao Y, Li H, Wang H, Zhang L. Volatile compounds of young Cabernet Sauvignon red wine from Changli County (China) J Food Comp Anal. 2008;21:689–694. doi: 10.1016/j.jfca.2008.05.007. [DOI] [Google Scholar]