Abstract
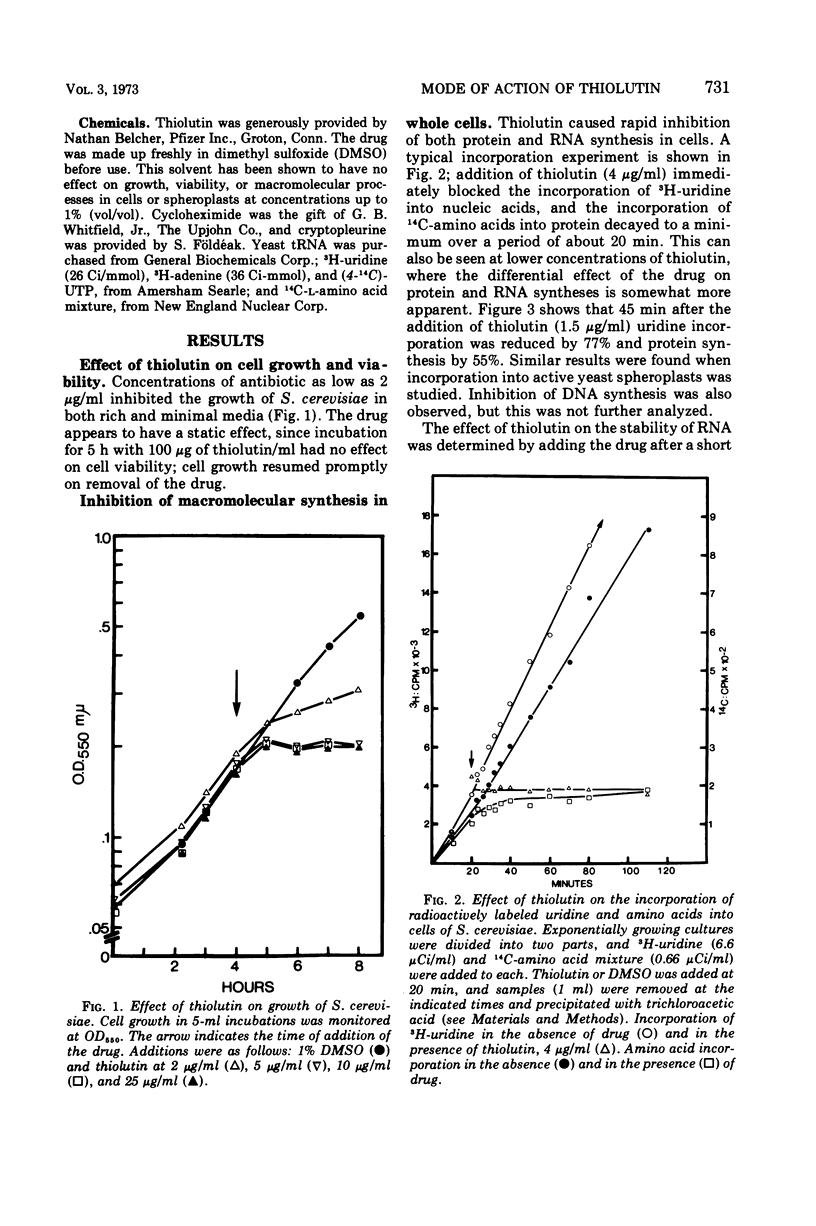
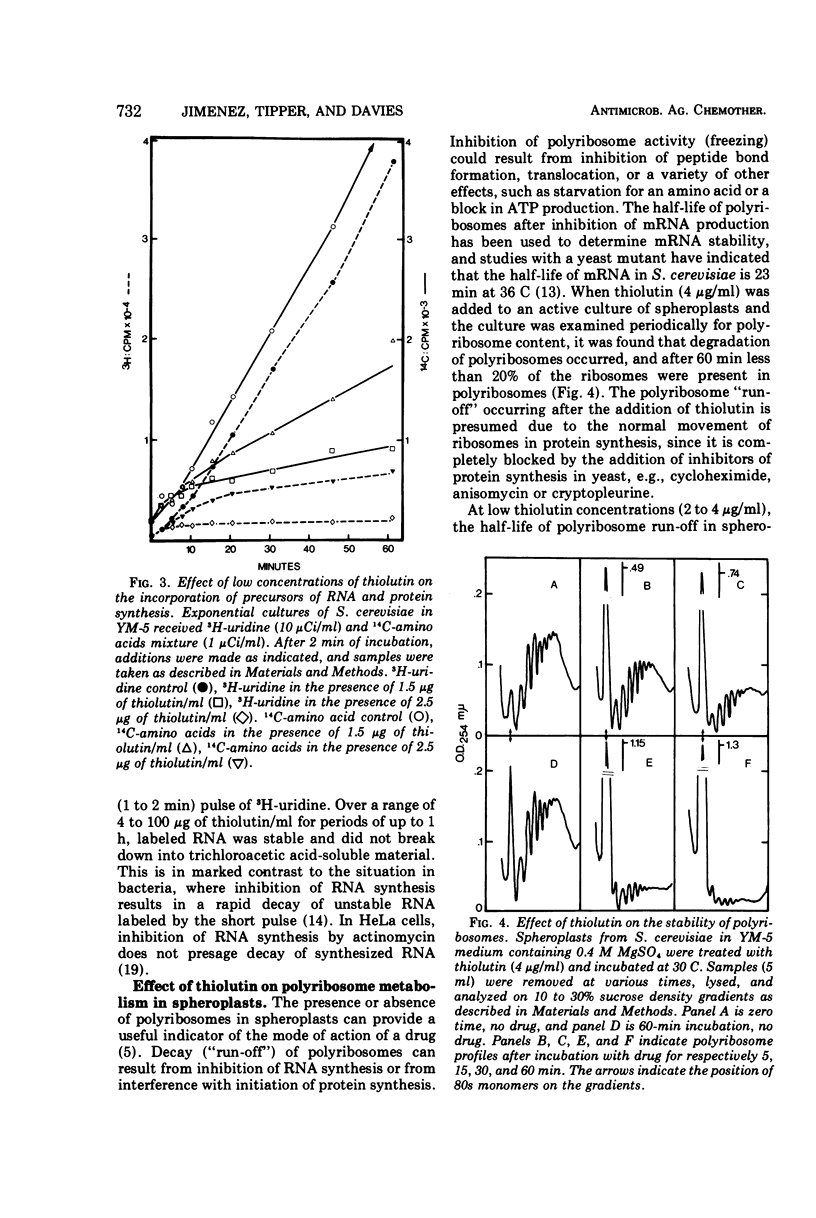
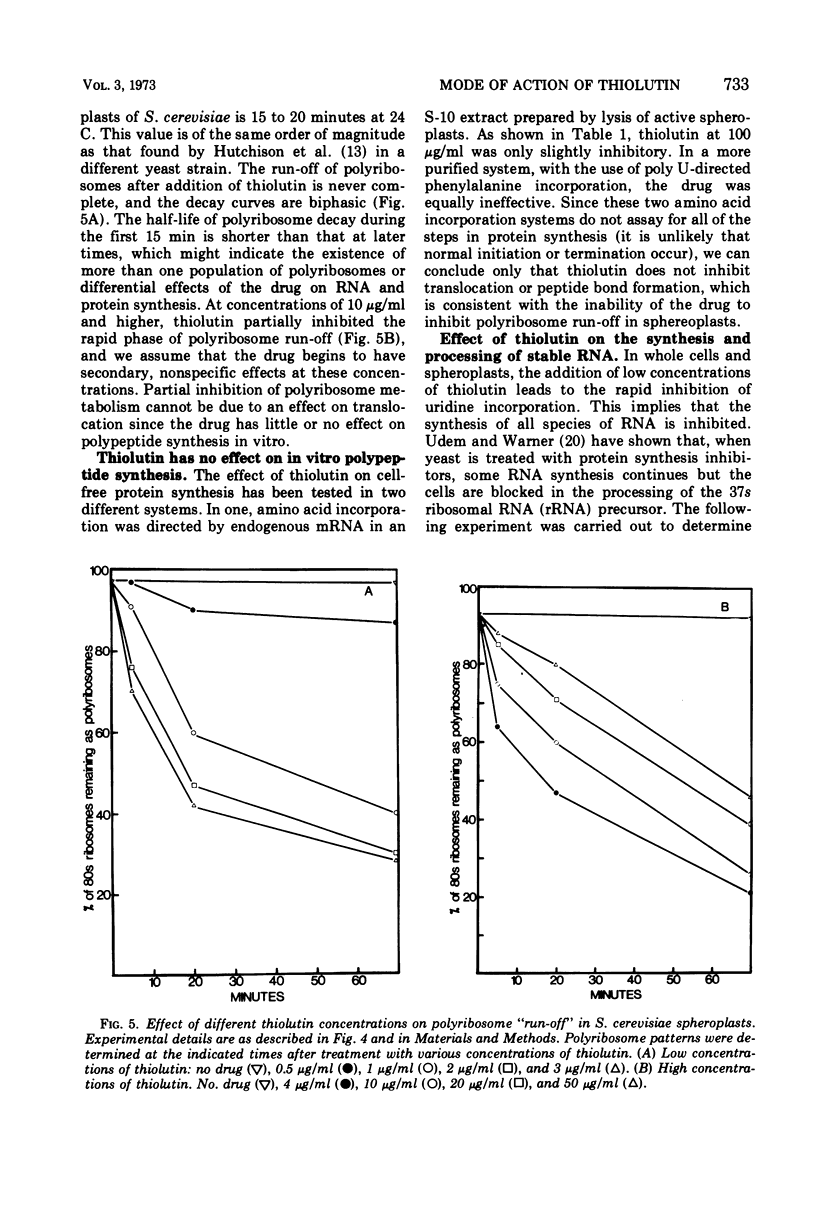
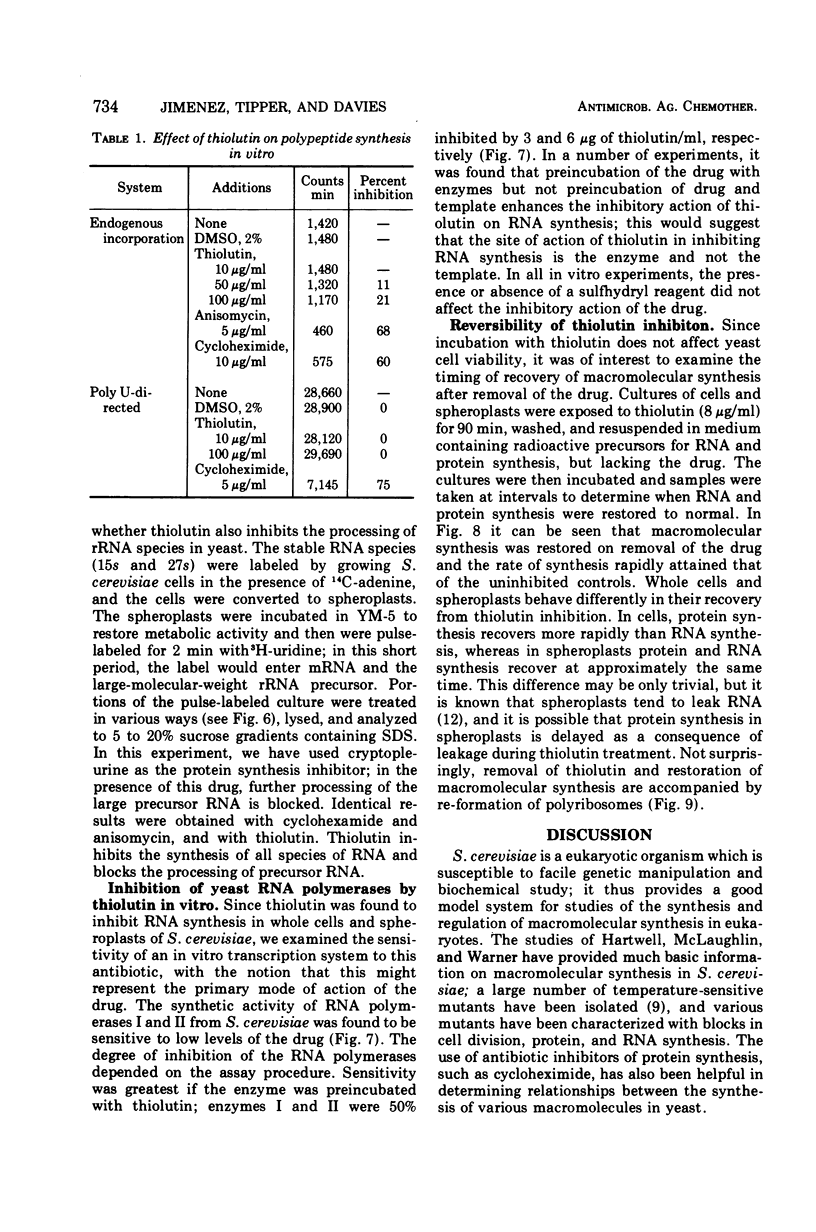
The sulfur-containing antibiotic thiolutin has been shown to be a potent, reversible inhibitor of the growth of Saccharomyces cerevisiae. Viability was unaffected over the concentration range of 4 to 100 μg/ml. At concentrations as low as 2 μg/ml, the drug inhibited ribonucleic acid (RNA) and protein synthesis in whole cells and spheroplasts. At these low concentrations, protein synthesis continued for a short period of time after RNA synthesis was completely stopped. With higher drug concentrations (greater than 20 μg/ml) protein synthesis was inhibited; concentrations of thiolutin up to 100 μg/ml did not affect translocation or peptide bond formation in cell-free protein-synthesizing systems from yeast. The effect of thiolutin on the activity of partially purified deoxyribonucleic acid-dependent RNA polymerases was examined, and the drug was found to be a potent inhibitor of RNA synthesis in vitro. Inhibition was greatest when the polymerase was preincubated with thiolutin. Several mechanisms are discussed to explain the multiple effects of thiolutin on S. cerevisiae. Since the action of the drug is easily reversed, thiolutin may prove to be of use in studies of various stages of yeast growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman R., Schultz L. D., Hall B. D. Transcription in yeast: separation and properties of multiple FNA polymerases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1702–1706. doi: 10.1073/pnas.69.7.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogt T. M., Planta R. J. Characteristics of DNA-dependent RNA polymerase activity from isolated yeast nuclei. FEBS Lett. 1972 Jan 15;20(1):47–52. doi: 10.1016/0014-5793(72)80014-0. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- Dezelee S., Sentenac A., Fromageot P. Role of DNA-RNA hybrids in eukaryots 1. Purification of yeast RNA polymerase B. FEBS Lett. 1972 Mar;21(1):1–6. doi: 10.1016/0014-5793(72)80148-0. [DOI] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick E. W., Maitra U., Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. XVI. The purification and properties of ribonucleic acid polymerase from yeast: preferential utilization of denatured deoxyribonucleic acid as template. J Biol Chem. 1969 Jan 25;244(2):413–424. [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. A mutant of yeast apparently defective in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1969 Feb;62(2):468–474. doi: 10.1073/pnas.62.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S. Temperature-sensitive mutants of yeast exhibiting a rapid inhibition of protein synthesis. J Bacteriol. 1968 Nov;96(5):1664–1671. doi: 10.1128/jb.96.5.1664-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta H., Ponta U., Wintersberger E. DNA-dependent RNA polymerases from yeast. Partial characterization of three nuclear enzyme activities. FEBS Lett. 1971 Nov 1;18(2):204–208. doi: 10.1016/0014-5793(71)80445-3. [DOI] [PubMed] [Google Scholar]
- Ponta H., Ponta U., Wintersberger E. Purification and properties of DNA-dependent RNA polymerases from yeast. Eur J Biochem. 1972 Aug 18;29(1):110–118. doi: 10.1111/j.1432-1033.1972.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- Roth R. M., Dampier C. Dependence of ribonucleic acid synthesis on continuous protein synthesis in yeast. J Bacteriol. 1972 Feb;109(2):773–779. doi: 10.1128/jb.109.2.773-779.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]



