Abstract
Supercritical carbon dioxide (SCCO2) extraction and fractionation of Spirulina platensis was carried out to obtain functional compounds with antioxidant, antimicrobial and enzyme inhibitory activities. Extraction of SCCO2 was carried out using 200 g of Spirulina powder at 40 ºC under 120 bar pressure with CO2 flow rate of 1.2 kg h−1. SCCO2 fraction obtained was further treated with hexane and ethyl acetate to identify its components. Individual components were identified by comparing mass spectra of samples with standard data and retention indices (RI) of C5–C20 n-alkanes mixture using the kovat index formula. The phenolic and flavonoid content of the SCCO2 extract was found to be 0.34 ± 0.01 g/100 g and 0.12 ± 0.01 g/100 g respectively. The SCCO2 extract had antioxidant activity with IC50 value of 109.6 ± 3.0 μg mL−1 for DPPH (2,2-Diphenyl-1-picryl hydrazyl radical), IC50 value of 81.66 ± 2.5 μg mL−1 for reducing power and IC50 value of 112.70 ± 0.8 μg mL−1 for hydroxyl radical scavenging activity. Further, antioxidant activity study on oxidative induced DNA damage was analysed to elucidate the positive role of SCCO2 extract. SCCO2 extracts showed high antimicrobial activity against Gram-positive bacteria (Staphylococcus aureus FRI 722 and Bacillus cereus F 4810) compared to that of Gram negative bacteria (Escherichia coli MTCC 108 and Yersinia enterocolitica MTCC 859). The SCCO2 extract exhibited inhibitory activity on both Angiotensin-1 converting enzyme and α-glucosidase with IC50 values of 274 ± 1.0 μg mL−1 and 307 ± 2.0 μg mL−1 respectively.
Keywords: SCCO2; Spirulina; Angiotensin −1 converting enzyme; DPPH, Phenolics
Introduction
Spirulina platensis
a blue green microalga, is used since ancient times as a source of food because of its high protein and micronutrient content (Dillon et al. 1994). Spirulina is widely studied, not only for its nutritional value but also for its reported medicinal properties. Several studies have shown that Spirulina and its extracts could prevent or inhibit cancer in humans and animals and have immuno-promoting effects (Hirahashi et al. 2002; Subhashini et al. 2004), antimicrobial activity (Demule et al. 1996; Ozdemir et al. 2004), antioxidant activity (Madhyastha et al. 2009), antihypertensive activity (Suetsuna and Chen 2001; Lu et al. 2010).
There is an increasing interest in natural antioxidants from microalgae. Among phytochemicals, phenolics and flavonoids are receiving attention mainly because of their wide range of potential applications and serve as natural antioxidants. These bioactive compounds retard or inhibit autoxidation by acting on radical scavengers and consequently are essential antioxidants that protect against the propagation of the oxidative chain. Phenolics and flavonoids with various beneficial pharmacological effects include antioxidant capacity (Lopez et al. 2011), antihypertensive (Loizzo et al. 2007), α-glucosidase inhibitory activity (Shobana et al. 2009), defensive mechanism against pathogenic microorganisms (Cowan 1999).
In recent years, SCCO2 extraction has received greater attention as an important alternative to traditional solvent extraction methods for several reasons. This technique provides an efficient extraction by eliminating concentration steps and use of organic solvents which are harmful environmentally. SCCO2 extraction method extracts soluble components from a raw material exploiting the unique properties of gases above their critical points. Carbon dioxide is an ideal solvent for extracting some classes of natural substances like phenolics and flavonoids which are used for food as it is nontoxic, non-explosive, readily available and easy to remove. Consequently, the quality of SCCO2 extracts is higher than those obtained by liquid-liquid extraction with organic solvents or by steam distillation, which can either induce thermal degradation or leave toxic residual solvent in the products. SCCO2 extraction has been used to separate and purify active components such as carotenoids and γ-linolenic acid from Spirulina and is compared with solvent extraction method (Careri et al. 2001; Mendes et al. 2006). Data provided in the present work demonstrates the advantages of SCCO2 extraction for compounds with pharmaceutical value from Spirulina.
Materials and methods
Reagents
Food grade carbon dioxide (99.9 % purity) was obtained from M/s Kiran Corporation (Mysore, India). Hippuryl histidyl-leucine (HHL), Hippuric acid, 2,2-Diphenyl-1-picryl hydrazyl (DPPH), p-nitrophenyl α-D-glucopyronoside (PNPG), α-glucosidase, triflouroacetic acid (TFA) were obtained from Sigma–Aldrich Co (St. Louis, USA). Sterile paper discs and ampicillin are obtained from Himedia Labs (Mumbai, India). All other reagents and chemicals are of analytical grade.
Spirulina culture
Spirulina platensis
(CFTRI) is a local isolate, maintained under standard conditions and grown in modified Zarrouk’ s medium as described by Sarada et al. (1999). The culture was harvested using a nylobolt cloth and oven-dried at 50 °C. The algal cells thus obtained were stored at −20 °C for further use.
Bacterial strains and culture medium
The bacterial strains used were Bacillus cereus F 4810, Public Health Laboratory (London, UK), Staphylococcus aureus FRI 722, Public Health Laboratory (The Netherlands), Escherichia coli MTCC 108 and Yersinia enterocolitica MTCC 859, Microbial Type Culture Collection, Institute of Microbial Technology (Chandigarh, India). Nutrient agar and brain heart infusion agar media and their respective broths were used.
Nutrient agar media composition: Heart infusion agar media Composition
| Nutrient agar media composition | Heart infusion agar media Composition | ||
| Ingredients | g/L | Ingredients | g/L |
| Peptic digest of animal tissue | 5.0 | Calf brain, infusion form | 200.0 |
| Sodium chloride | 5. 0 | Beef heart, infusion form | 250.0 |
| Beef extract | 1.5 | Proteose peptone | 10.0 |
| Yeast extract | 1.5 | Dextrose | 2.0 |
| Agar | 15.0 | Sodium chloride | 5.0 |
| Final pH (at 25°C) | 7.4 ± 0.2 | Disodium phosphate | 2.5 |
| Agar | 15.0 | ||
| Final pH (at 25°C) | 7.4 ± 0.2 | ||
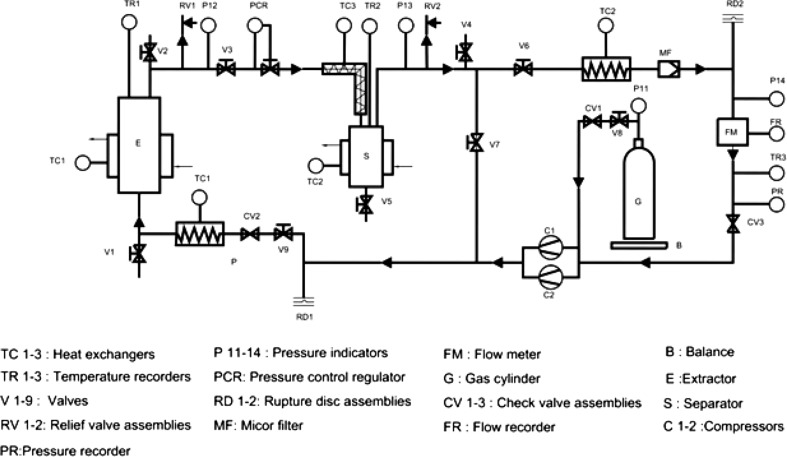
The SCCO2 extraction system used in the present work was NOVA Swiss WERKE AG; EX 1000–1.4–1.2 V type (Switzerland) designed for a working pressure of up to 100 Mpa, temperature up to 100 °C with extractor volume of 4 L. It operates at set pressure of ± 10 % and a temperature of ± 0.5 °C. The dried and powdered Spirulina biomass (200 g) was loaded into the extraction vessel. A flexible electrical heating tape with a regulator was wound around the pipe connecting the separator to the expansion valve to prevent the blocking of the separator pipe with the extracted material. CO2 supplied from a gas cylinder was compressed by a diaphragm compressor to the desired pressure by adjusting the pressure controller and heated to the specified temperature by means of a heat exchanger to reach the supercritical state. At the extractor exit, CO2 along with the extracted material was depressurized to separate the material and was recycled by recompression. The flow rate was maintained at 1.8–2.0 kg h−1 and an average yield of three experiments was taken. The schematic flow diagram of the SCCO2 extraction is given in Fig. 1 as described earlier by Udaya Sankar (1989).
Fig 1.
Schematic flow diagram of the SCCO2 extraction Unit
Supercritical CO2 extraction for Spirulina biomass was carried out at two sets of conditions - temperature of 40 and 50 °C and pressure of 120 and 280 bar with CO2 flow rates at 1.2 and 2.9 kg h−1. Yield was measured by weight and extract was stored at 4 ºC in dark for further analysis. From the SCCO2 extraction volatile compounds were prepared by hydrodistillation and analysed using GCMS. More than hundred compounds were identified containing mainly hydrocarbons, terpenes, phenols, acids and aldehydes (Table 1.)
Table 1.
List of important identified compounds in SCCO2 extract of Spirulinausing Kovat index formula
| Compound | Nature of the Compound | Kovat Index | Reference |
|---|---|---|---|
| Linalool | Terepene alcohol | 1082 | J. Agric. Food Chem, 1991, 39, 1494-1497 |
| Benzoic acid | Aromatic carboxylic acid | 1259 | J. Agric. Food Chem, 1991, 39, 1494-1497 |
| 3-methoxy-4-hydroxy cinnamic acid | Phenolic acid | 1481 | J. Agric. Food Chem, 1991, 39, 1494-1497 |
| n-Undecane | Alkane hydrocarbon | 1096 | Flavour Fragr. J, 2003, 18, 376-79 |
| β-Curcumene | Diphenylheptanoids | 1503 | Flavour Fragr. J, 2003, 18, 376-79 |
| n-Heptadecane | Alkane hydrocarbon | 1692 | Flavour Fragr. J, 2003, 18, 376-79 |
| λ -Teripinene | Terepene | 1742 | Flavour Fragr. J, 2006, 21, 410-15 |
| Hexadecane | hydrocarbon | 1600 | J. Agric. Food Chem, 1988, 36, 553-560 |
| Trans-Sabiene hydrate | Monoterepenes | 1053 | Flavour Fragr. J, 2004, 19, 424-433 |
| Estragole | phenylpropene | 1175 | Flavour Fragr. J, 2004, 19, 424-433 |
| α-Phellandrene | Cyclic monoterepenes | 1005 | Chemistry of Natural components vol 41, No 4,2005 |
| β-Caryophyllene | Sesquiterpene | 1429 | Chemistry of Natural components vol 41, No 4,2005 |
| β-Selinene | Sesquiterpene | 1496 | Chemistry of Natural components vol 41, No 4,2005 |
| Benzaldehyde | Aromatic aldehyde | 1005 | Flavour Fragr. J, 2006, 21, 859-863 |
| Phytol | acyclic diterpene alcohol | 1496 | J. Agric. Food Chem, 2000, 48, 1140-11149 |
Identification of the individual components was done by comparing mass spectra of samples and comparing individual components retention indices on the HP-1 column with data available in literature using Kovat index formula.
Preparation of extracts
Known quantities of SCCO2 extracts were dissolved in DMSO for evaluation of bioactivity. Similarly known quantity of SCCO2 extract was extracted with hexane for volatile compounds analysis by GC and GCMS. For extraction of phenols and flavonoids ethyl acetate was used.
Gas chromatography–mass spectrometry (GC-MS) analysis
Volatile components in the extract were analysed by GC and GC/MS using a HP gas chromatograph equipped with FID detector and a 30 m × 0.25 mM HP-1 capillary column (0.33 μm coating). Temperature gradient was from 70 °C to 280 °C, increasing by 10 °C/min. The injector temperature was 150 °C (1 μL injection size), with a detector temperature of 250 °C. Helium was used as carrier gas at 2 mL/min flow rate.
Determination of total phenolics
Total phenolics content from SCCO2 extract was estimated by the modified Folin-Ciocalteu method of Singleton and Rossi (1965). 200 μL of diluted sample was added to 1 mL of 1:10 diluted Folin-Ciocalteu reagent. After 5 min, 800 μL of saturated sodium carbonate (75 g/L) was added and incubated for 30 min at 37 °C. The absorbance at 750 nm was measured for determining total phenolics. Gallic acid was used for standard calibration curve.
Determination of flavonoid content
The flavonoids content in the SCCO2 extract from S. Platensis was measured by the AlCl3 method as described by Djeridane et al. (2006). The extracts were diluted to an appropriate concentration using absolute ethanol. Then 1 mL of diluted sample was mixed with 1 mL of 2 % (w/v) methanolic solution of aluminium chloride. After incubating the mixture for 15 min at room temperature, the absorbance of the reaction mixture was read at 430 nm with a spectrophotometer. Rutin was used as standard.
DPPH free radical-scavenging assay
Neutralization of DPPH (1, 1-diphenyl-2-picrylhydrazyl) free radical by the SCCO2 extracts was done by modified method of Brand-Williams et al. (1995). For each antioxidant activity, six different concentrations of SCCO2 extract in the concentration range of 10 μg mL−1 to 300 μg mL−1 was used for the assay. DPPH solution (0.14 mL) in methanol was placed in an eppendorf tube and 0.1 mL of sample extract was added. Methanol was used as blank with DPPH methanol solution as a reference sample and ascorbic acid, BHT as standards. Absorbance of this reaction mixture was measured at 517 nm.
Reducing power activity
The reducing power activity of the SCCO2 extract was evaluated according to the method of Yen and Chen (1995) with a modification. To 0.5 mL of extract 1.25 mL of phosphate buffer (pH 6.6) and 1.25 mL of 1 % K3Fe (CN) 6 were added and the mixture was incubated at 50 °C in a water bath for 20 min. After the mixture was cooled to room temperature, 1.25 mL of 10 % TCA, 1.25 mL of H2O and 0.25 mL of 0.1 % FeCl3.6 H2O were added and after 10 min at room temperature the absorbance was read at 700 nm.
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity of extract was measured according to the method of Halliwell et al. (1987). One ml of the reaction solution was prepared by mixing aliquots of SCCO2 extract, 1 mM FeCl3, 1 mM EDTA, 20 mM H2O2, 1 mM L-ascorbic acid and 30 mM deoxyribose in potassium phosphate buffer (pH 7.4). The reaction mixture was incubated for 1 h at 37 °C and further heated in a boiling water bath for 15 min after adding 1 mL of 2.8 % (w/v) trichloroacetic acid and 1 mL of 1 % (w/v) 2-thiobarbituric acid. The colour developed was measured at 532 nm against a blank containing phosphate buffer.
Protective effect of SCCO2 extract on oxidation- induced DNA damage
The hydroxyl radical was generated by Fenton reaction according to the modified method of Huang et al. (2006). 15 μL of reaction mixture containing the SCCO2 extract (100 μg), 5 μL of calf thymus DNA (mg/mL−1), 18 mM FeSO4 and 60 mM hydrogen peroxide were incubated at room temperature for 15 min. 2 μL of 1 mM EDTA was added to stop the reaction. Blank test contained only thymus DNA and control test contained all the reaction components except SCCO2 extract. The treated DNA reaction mixture was subjected to agarose electrophoresis and stained with ethidium bromide to examine under UV light.
In vitro assay of ACE inhibitory activity
The Angiotensin-1-converting enzyme (ACE) inhibitory activity was assayed using reverse phase high performance liquid chromatography (RP-HPLC) by Wu and Ding (2002) as modified by (Mallikarjun Gouda et al. 2006). ACE was extracted from kidney acetone powder in the laboratory at 4 ˚C for 16–18 h using 10 mL of sodium borate buffer pH 8.3 containing 300 mM NaCl. The extract was centrifuged at 15, 000 x g for 60 min at 4 °C. The supernatant was dialyzed using the same buffer for 24 h (500 ml × 3).. The assay mixture contained 0.1 mL of 0.1 M borate buffer pH 8.3 containing 1 % NaCl, 0.05 mL of 5 mM HHL and 0.05 mL of ACE enzyme extract in a total volume of 0.45 mL. The reaction was arrested after incubation at 37 °C for 30 min by adding 0.25 mL of 1 N HCl. The Hippuric acid was separated from HHL by RP-HPLC using Phenomenex Luna C18 column (250 x 4.6 mm, 5 μ) and isocratic elution with 50 % methanol containing 0.1 % TFA at a flow rate of 0.8 ml/min. ACE activity was assayed by monitoring the release of hippuric acid from the substrate, Hippuryl histidyl-leucine (HHL) at 228 nm. One unit of ACE is defined as that amount of enzyme, which releases 1 μmole of hippuric acid per hour at 37 °C and at pH 8.3. To determine the inhibition of the enzyme activity, the enzyme was pre-incubated with the SCCO2 extract before adding the substrate. IC50 value is calculated as the concentration of extract required to decrease ACE activity by 50 %. The % inhibition curves were plotted using a minimum of three determinations for each concentration.
Assay for α-glucosidase inhibitory activity
The inhibitory activity of α-glucosidase was determined according to the modified method of Kurihara et al. (1995). To start the reaction 4 mM p-nitrophenyl α-D-glucopyranoside (0.05 mL) and 2 U/mL α-glucosidase (0.025 mL) in 0.05 M phosphate buffer (pH 7) were added to the sample solution (1.0 mL). Each reaction was carried out at 37 °C for 15 min and stopped by adding cold 0.2 M Na2CO3 (750 μL). Enzymatic activity was quantified by measuring absorbance at 405 nm. One unit of α-glucosidase activity was defined as the amount of enzyme that liberates 1.0 μmole p-nitrophenol per min. The IC50 value was defined as the concentration of α-glucosidase inhibitor that inhibited 50 % of α-glucosidase activity.
Antibacterial activity
The agar plates inoculated with the test organisms were Bacillus cereus, Staphylococcus aureus, Escherichia coli and Yersinia enterocolitica. Nutrient agar and brain heart infusion agar media and their respective broths used were incubated for 1 h before introducing the sterile discs impregnated with SCCO2 extracts to be tested for antibacterial activity. These plates were incubated at 37 °C for 24 h. After incubation, all the plates were observed for zones of bacterial growth inhibition, and the diameters of these zones were measured in millimeters. All tests were performed under sterile conditions in duplicate and repeated three times. Ampicillin discs (10 μg −1 disc) were used as positive control.
Results and Discussion
S. platensis
biomass yielded maximum SCCO2 extract of 2.86 ± 0.15 g/100 g dry biomass at 40 °C with a pressure of 120 bar and CO2 flow rate of 1.2 kg−1 h. Volatile compounds were prepared by hydrodistillation and analysed using GCMS. More than hundred compounds were identified and some important compounds like hydrocarbons, terpenes, phenols and aldehydes are listed in Table 1.
Effect on phenolic and flavonoids on biological activity
Results indicated the presence of phenolic compounds (0.34 ± 0.01 g/100 g) and flavonoids (0.12 ± 0.01 g/100 g) in SCCO2 extract of Spirulina. A direct correlation between antioxidant capacity and reducing power of certain algal extracts were reported (Peksel et al. 2013; Lopez et al. 2011). The reducing properties are generally associated with the presence of reductones, such as phenolics and flavonoids which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom. The observed antibacterial activity of SCCO2 extracts of Spirulina is in accordance with the opinion of Cowan (1999) who showed that several classes of phenolic acids and flavonoids in plants involve in the plant defence mechanism against pathogenic microorganisms. Earlier studies indicates that flavonoids isolated from leaves of Ailanthus excels (Roxb) are involved in ACE inhibitory activity (Loizzo et al. 2007). Phenolics purified from seed coat of finger millet was shown to inhibit α-glucosidase (Shobana et al.2009), a carbohydrate hydrolysing enzyme.
Antibacterial activity
Antimicrobial activity of SCCO2 extracts was tested against four different bacteria, including two Gram-positive bacteria (Staphylococcus aureus FRI 722 and Bacillus cereus F 4810) and two Gram negative bacteria (Escherichia coli MTCC 108 and Yersinia enterocolitica MTCC 859) and the results are presented in Table 2. All plates have zones of growth inhibition and the diameters of zones were measured in millimetres.
Table 2.
Antimicrobial activity of SCCO2 extract of Spirulina
| Microorganisms | Diameter of zone of inhibition (mm) | ||
|---|---|---|---|
| Control | SCCO2 extract | Ampicillin | |
| Escherichia coli MTCC 108 | 6 | 10 | 21 |
| Staphylococcus aureus FRI 722 | 6 | 14 | 22 |
| Bacillus cereus F 4810 | 6 | 15 | 15 |
| Yersinia enterocolitica MTCC 859 | 6 | 8 | 14 |
Extracts were more effective against Gram-positive bacteria with zone of inhibition of ‘14–15 mm’ than with Gram negative bacteria where zone of inhibition is ‘8–10 mm’.
The higher resistance of Gram-negative bacteria to external agents was documented earlier and is attributed to the presence of lipopolysaccharides in their outer membranes, which make them inherently resistant to antibiotics, detergents and hydrophilic dyes. Antibacterial activity of volatile compounds obtained through solvent extraction of Spirulina, red and brown algae was reported earlier (Ozdemir et al. 2004; Karabay-Yavasoglu et al. 2007; Demirel et al. 2009), Linalool, terepenols, β-caryophyllene and other terepenoid compounds present in the SCCO2 extract (Table 1) might also be contributing for the antimicrobial activity along with phenolics and flavonoid compounds.
Antioxidant activity
The antioxidant activity of SCCO2 extract was studied using DPPH, hydroxyl radical scavenging activity, reducing power and oxidative induced DNA damage and the results are shown in Table 3 and Fig. 2 The antioxidant activity against DPPH in SCCO2 extract is contributed by these phenolic and flavonoid compounds. The IC50 value for antioxidant activity of Spirulina extract was found to be 109.6 ± 3.0 μg mL−1 compared with the BHT (23.3 ± 2.0 μg mL−1) and ascorbic acid (7.33 ± 0.9 μg mL−1). Hydroxyl radical scavenging activity of the SCCO2 extract exhibited IC50 value of 112.70 ± 0.89 μg mL−1 as against standard BHT IC50 value of 28.97 ± 0.25 μg mL−1. In determining reducing power of SCCO2 extract of Spirulina, ascorbic acid and BHT were used as positive controls (Table 3). The IC50 value of SCCO2 extract for reducing power was found to be 81.66 ± 2.5 μg mL−1 compared to that of standard ascorbic acid (9.76 ± 0.25 μg mL−1) and BHA (17.36 ± 1.85 μg mL−1).
Table 3.
Antioxidant activity of SCCO2 extract of Spirulina
| Sample | *IC50 (μg mL−1) | ||
|---|---|---|---|
| DPPH | Reducing Power | Hydroxyl radical scavenging assay | |
| SCCO2 extract | 109.6 ± 3.0 | 81.66 ± 2.5 | 112.70 ± 0.89 |
| Ascorbic acid | 7.3 ± 0.3 | 9.76 ± 0.2 | - |
| BHT | 23.3 ± 2.0 | 1 7.36 ± 1.8 | 28.97 ± 0.25 |
*IC50 value: concentration at which the DPPH and Hydroxyl radicals were scavenged by 50%: Absorbance was 0.5 for reducing power respectively. Each value is expressed as mean ± SD
Fig 2.
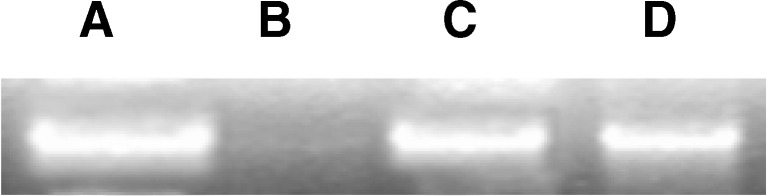
Protection capacity of the SCCO2 extracts of Spirulina against hydroxyl radical induced calf thymus DNA damage. Lane A: Reaction mixture without hydroxyl radical Lane B: Reaction mixture with hydroxyl radical and without sample Lane C: Reaction mixture with hydroxyl radical and with standard antioxidant Lane D: Reaction mixture with hydroxyl radical and SCCO2 extract
Further, antioxidant activity study on oxidative induced DNA damage was analysed to elucidate the positive role of SCCO2 extract. In this study the hydroxyl radical generating system was based on the Fenton reaction (Fe2+ + H2O2). Free radicals could damage macromolecules in cells, such as DNA, protein, and membrane lipids. Results indicated that SCCO2 extract protected against hydroxyl radical-induced calf thymus DNA damage (Fig. 2). The protective capacity of SCCO2 extract may possibly be due to presence of phenolic and flavonoid components present in the extract.
Angiotensin-1 converting enzyme and α-Glucosidase inhibitory activity
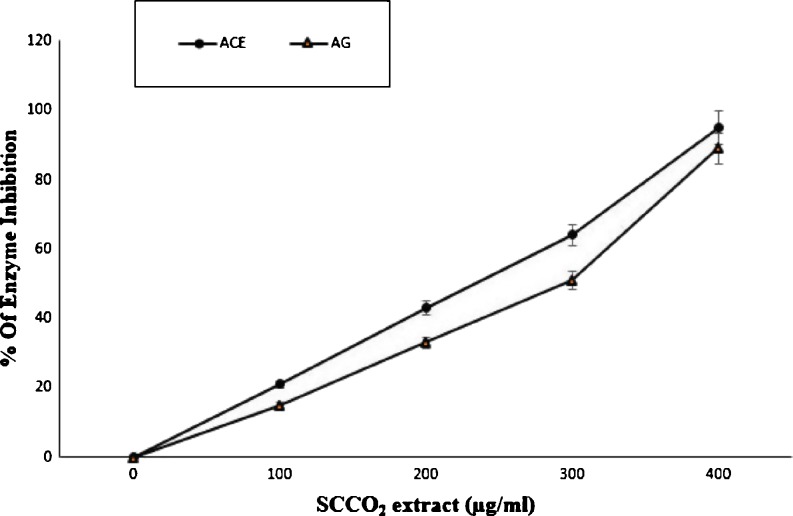
Effect of SCCO2 extract of Spirulina on Angiotensin-1converting enzyme (ACE) and α-Glucosidase (AG) inhibition is shown in Fig. 3.0. ACE is a key component in the renin angiotensin aldosterone system (RAAS) which regulates blood pressure. As the over expression of RAAS is associated with vascular hypertension, ACE inhibition has become a major target control for hypertension. The research on potential ACE inhibitors is expanding broadly and are focused on natural product derivatives such as peptides, phenolics, flavonoids and terpenes. Earlier studies indicates that flavonoids isolated from leaves of Ailanthus excels (Roxb) involved in ACE inhibitory activity (Loizzo et al. 2007). The current study is focused on investigating the ACE inhibitory property of SCCO2 extract and found to be at IC50 of 274 ± 1.0 μg mL−1
Fig 3.
Effect of SCCO2 extract of Spirulina on Angiotensin-1 Converting enzyme (ACE) and α-Glucosidase (AG) inhibition
Diabetes mellitus is an endocrine disorder characterized by hyperglycemia and is associated with disturbances of carbohydrate, fat and protein metabolism resulting in defects in insulin secretion, or action, or both. A therapeutic approach for treating diabetes is to decrease postprandial hyperglycemia.. The SCCO2Spirulina extract showed α-glucosidase inhibition with an IC50 value of 307 ± 2.0 μg mL−1 as shown in Fig. 3 and was found to be concentration dependent. Xiancui et al. (2005) earlier reported microalgae as a source of α- glucosidase inhibitor.
Conclusion
In conclusion, it is revealed that the SCCO2 extract of Spirulina platensis possess significant antihypertensive, antidiabetic, antioxidant as well as antimicrobial activities. The phytoconstituents like, phenolics, tannins, flavonoids, terpenoids etc. might be contributing for these activities. The previous studies of both solvent and supercritical extracts of microalgal and plant extracts (Herrero et al.2006) mainly focused on antioxidant and antimicrobial activities. The present study showed α-glucosidase and angiotensin 1-converting enzyme inhibitory activities in the supercritical extract of Spirulina which is used as health food in several parts of the world. The present results therefore substantiates that the whole Spirulina biomass can be used as food supplement and further studies are envisaged to elucidate these bioactivities in vivo models.
Acknowledgement
Authors thank Dr. Varadaraj M C, Chief Scientist, Food Microbiology Department and Dr. Pradeep Singh Negi, Principal Scientist, Fruit and Vegetable Technology Department, Central Food Technological Research Institute, Mysore for their help in microbiological studies. The authors thank Director, CSIR-CFTRI, for his encouragement and support. MGKG acknowledges the SRF fellowship provided by CSIR, Govt. of India.
Conflict of Interest
The authors have declared that there is no conflict of interest
References
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Careri M, Furlattini L, Mangia A, Musci M, Anklam E, Theobald A, Von Holst C. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina pacifica algae: a chemometric approach. J Chromatogr A. 2001;912:61–71. doi: 10.1016/S0021-9673(01)00545-3. [DOI] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirel Z, Yilmaz-Koz FF, Karabay-YavasogluUN OG, Sukatar A. Antimicrobial and antioxidant activity of brown algae from the Aegean Sea. J Serb Chem Soc. 2009;74:619–628. doi: 10.2298/JSC0906619D. [DOI] [Google Scholar]
- Demule MCZ, Decaire GZ, Decano MS. Bioactive substances from Spirulina platensis (Cyanobacteria) Phyton-Int J Exp Bot. 1996;58:93–96. [Google Scholar]
- Dillon JC, Phuc AP, Dubacq JP. Nutritional value of the alga Spirulina. World Rev Nutr Diet. 1994;77:32–46. doi: 10.1159/000424464. [DOI] [PubMed] [Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method: a simple “test tube”assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Herrero M, Cifuentes A, Ibanez E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006;98:136–148. doi: 10.1016/j.foodchem.2005.05.058. [DOI] [Google Scholar]
- Hirahashi T, Matsumoto M, Hazeki K, Saeki Y, Ui M, Seya T. Activation of the human innate immune system by Spirulina: augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int Immuno pharmacol. 2002;2:423–434. doi: 10.1016/S1567-5769(01)00166-7. [DOI] [PubMed] [Google Scholar]
- Huang DJ, Chen HJ, Hou WC, Lin CD, Lin YH. Sweet potato Ipomoea batatas [L.] Lam“Tainong 57’) storage root mucilage with antioxidant activities in vitro. Food Chem. 2006;98:774–781. doi: 10.1016/j.foodchem.2005.07.018. [DOI] [Google Scholar]
- Karabay-Yavasoglu NU, Sukatar A, Ozdemir G, Horzum Z. Antimicrobial activity of volatile components and various extracts of the red alga Janiarubens. Phytother Res. 2007;21:153–156. doi: 10.1002/ptr.2045. [DOI] [PubMed] [Google Scholar]
- Kurihara H, Ando J, Hatano M, Kawabata J. Sulfoquinovo syldiacylglycerol as an α-glucosidase inhibitor. Bioorgan Med Chem Lett. 1995;5:1241–1244. doi: 10.1016/0960-894X(95)00196-Z. [DOI] [Google Scholar]
- Loizzo MR, Said A, Tundis R, Rashed SG, Hufner A, Menichini F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae) Phytother Res. 2007;21:32–36. doi: 10.1002/ptr.2008. [DOI] [PubMed] [Google Scholar]
- Lopez A, Rico M, Rivero A, Suárez de Tangil M. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011;125:1104–1109. doi: 10.1016/j.foodchem.2010.09.101. [DOI] [Google Scholar]
- Lu J, Ren DF, Xue YL, Sawano Y, Miyakawa T, Tanokura M. Isolation of an antihypertensive peptide from alcalase digest of Spirulina platensis. J Agric Food Chem. 2010;58:7166–7171. doi: 10.1021/jf100193f. [DOI] [PubMed] [Google Scholar]
- Madhyastha HK, Sivashankari S, Vatsala TM. C-phycocyanin from Spirulina fussiformis exposed to blue light demonstrates higher efficacy of in vitro antioxidant activity. Biochem Eng J. 2009;43:221–224. doi: 10.1016/j.bej.2008.11.001. [DOI] [Google Scholar]
- Mallikarjun Gouda KG, Gowda LR, Rao AA, Prakash V. Angiotensin I-converting enzyme inhibitory peptide derived from glycinin, the 11S globulin of soybean (Glycine max) J Agric Food Chem. 2006;54:4568–4573. doi: 10.1021/jf060264q. [DOI] [PubMed] [Google Scholar]
- Mendes RL, Reis AD, Palavra AF. Supercritical CO2 extraction of γ-linolenic acid and other lipids from Arthrospira Spirulina maxima: Comparison with organic solvent extraction. Food Chem. 2006;99:57–63. doi: 10.1016/j.foodchem.2005.07.019. [DOI] [Google Scholar]
- Ozdemir G, Ulku Karabay N, Dalay MC, Pazarbasi B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother Res. 2004;18:754–757. doi: 10.1002/ptr.1541. [DOI] [PubMed] [Google Scholar]
- Peksel A, Imamoglu S, AltasKiymaz N, Orhan N. Asphodelus Aestivus Brot.; Antioxidant activity; Free radical; Scavenging activity; Superoxide anion radical. Int J Food Prop. 2013;16:1339–1350. doi: 10.1080/10942912.2011.587622. [DOI] [Google Scholar]
- Sarada R, Pillai MG, Ravishankar GA (1999) Phycocyanin from Spirulina sp. Influence of processing of biomass on phycocyanin yield, analysis of efficacy of various extraction methods and stability studies on phycocyanin. Process Biochem 34:795–801
- Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusinecoracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- Subhashini J, Mahipal SV, Reddy MC, Mallikarjuna Reddy M, Rachamallu A, Reddanna P. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem Pharmacol. 2004;68:453–462. doi: 10.1016/j.bcp.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Suetsuna K, Chen JR. Identification of antihypertensive peptides from peptic digests of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar Biotechnol. 2001;3:305–309. doi: 10.1007/s10126-001-0012-7. [DOI] [PubMed] [Google Scholar]
- Udaya Sankar K. Studies on physico-chemical characteristic of essential oil of pepper (Pipernigrum L.) obtained by supercritical carbon dioxide. J Sci Food Agric. 1989;48:105–112. [Google Scholar]
- Wu J, Ding X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int. 2002;35:367–375. doi: 10.1016/S0963-9969(01)00131-4. [DOI] [Google Scholar]
- Xiancui L, Rongli N, Xiao F, Lijun H, Lixin Z. Macroalage as a source of alpha-glucosidase inhibitors. Chin J Oceanol Limn. 2005;23:354–356. doi: 10.1007/BF02847160. [DOI] [Google Scholar]
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. doi: 10.1021/jf00049a007. [DOI] [Google Scholar]