Abstract
In order to enhance folate levels in fermented foods, a folate producing probiotic lactic acid bacterium isolated from cow’s milk and identified as Lactococcus lactis CM28 by 16S rRNA sequencing was used to fortify skim milk. Optimization of medium additives such as folate precursors, prebiotics and reducing agents along with suitable culture conditions enhanced folate levels in skim milk. Optimization resulted in a four fold increase in the extracellular folate (61.02 ± 1.3 μg/L) and after deconjugation the total folate detected was 129.53 ± 1.2 μg/L. The effect of refrigerated storage on the viability of L. lactis, pH, titratable acidity (TA) in terms of percentage lactic acid and finally on the stability of folate was determined. Only a slight variation in pH (4.74 ± 0.02 to 4.415 ± 0.007) and acidity (0.28 ± 0.028 to 0.48 ± 0.014 %) was noted during folate fermentation. During storage, only less than a log unit reduction was noted in the viable count of the probiotic after 15 days and about 90 % of the produced folate was retained in an active state.
Keywords: Folate, Fortification, Lactococcus lactis, Probiotics, Skim milk
Introduction
Folate is a generic term for a number of folic acid derivatives that differ in their state of oxidation. It is an essential B group vitamin with a daily recommended intake of 200–400 μg. For pregnant women, a double dose is recommended. They have a major role as a cofactor in normal cellular functions and also in growth and development (Blount et al. 1997; Sybesma et al. 2003b). Rapidly proliferating cells requires higher amount of folate for DNA replication, repair and methylation (Jacob 2000). The protective role of folate against neural tube defects in newborn is well established (Hibbard 1964; Lucock 2000; Roy 2007). Folate deficiency is also been linked to cardiovascular diseases (Boushey et al. 1996; Brattstrom 1996), anaemia, poor cognitive performance and certain forms of cancer (Ames 1999).
Vegetables and dairy products are good sources of dietary folate. Even though widely available in various foods, folate deficiency still prevails. Natural inhibitors present in the food along with the physiological conditions in the gut could contribute to the decreased bioavailability of folate. Many developed countries including US and Canada have made mandatory folate fortification programmes. Folic acid is composed of para amino benzoic acid (PABA) linked to a pteridine ring at one end and L-glutamic acid at the other end. In natural folates, the extent of the reduction state of the pteroyl group, the number of glutamate residues and the nature of the substituent on the pteridine ring are different (LeBlanc et al. 2007). Higher levels of synthetic folic acid in the diet are linked to the masking of vitamin B12 deficiency while natural folates have a low tendency to mask B12 deficiency. Due to the potential risks of using synthetic folic acid, fortification by natural folate would be a better alternative.
Fermented milk can contribute significantly to the daily recommended folate level (Alm 1980) and the folate producing probiotics could aid in this fortification. Probiotics are live microbes which when administered are beneficial to the host in many ways. However, in milk fermentation, majority of the bacteria are folate utilizers thereby decreasing the amount of folate (Lin and Young 2000). Hence, only by the judicial selection of a suitable starter culture or a consortium of folate producers it is possible to increase the dietary folate content. A number of lactic acid bacteria (LAB) such as Streptococcus thermophilus, Lactococcus lactis, Lactobacillus plantarum have been reported to produce folate (LeBlanc et al. 2007; Sybesma et al. 2003b).
LAB have a GRAS (Generally Recognized as Safe) status and a long history of safe use in various traditional fermented foods. The aim of this work is to study the efficacy of a probiotic L. lactis strain in folate fortification of skim milk, optimization of fermentation conditions to increase the total and bioavailable folate and to see the stability of the produced folate on refrigerated storage.
Materials and methods
Microorganism, media and cultivation conditions
The lactic acid bacterium isolated from raw cow’s milk using MRS (de Man, Rogosa, Sharpe medium)—CaCO3 plate and identified as Lactococcus lactis by 16S rRNA sequencing (99 % similarity) was used for the present study. The NCBI Genbank accession number of the strain is KJ676682 with the strain name Lactococcus lactis CM28. The culture displayed many of the probiotic properties and was reported earlier by us (Gangadharan et al. 2010). It was subcultured in M17 medium (Himedia, India) supplemented with 0.5 % glucose and incubated at 37 °C under static condition. Lactobacillus casei NCIM 2364 (obtained from North Maharashtra University, Jalgaon on the basis of material transfer agreement) was used for microbiological assay for the detection of folate and was maintained in MRS medium.
Folic acid standards were procured from Sigma (MO, USA). All other media and reagents were purchased from Himedia, India unless otherwise stated.
Inoculum for folate fortification of skim milk
L. lactis CM28 was inoculated in folic acid assay medium supplemented with a nutrient solution and incubated at 37 °C for 18 h. The particular solution was composed of (mg/ml) L-Glutamate-30, L-Alanine-20, L-Glycine-20, L-Histidine- 20, L-Serine-10, L-Threonine-10, L-Cysteine-10, L-Arginine-20, L-Asparagine-10, L-Isoleucine- 20, L-Methionine-10, L-Valine- 10, Ascorbic acid- 50, Nicotinamide- 20, Calcium pantothenate -20, Pyridoxal HCl -20, Riboflavin-20, Biotin -10, p-aminobenzoate (PABA) −1. 1 % v/v of this culture was used as inoculum for the fortification studies.
Effect of culture conditions and additives on fermentative production of folate
Fermentation studies were carried out in 4%w/v skim milk medium. Optimum incubation time and temperature was determined by checking the levels of extracellular folate at various incubation times (6, 7, 8, 9, 10, 11 and 12 h) and temperature (30, 37 and 42 °C). The effect of various additives on folate production was studied individually and the optimized levels of each were incorporated in the subsequent experiments. The efficacy of three prebiotics namely sorbitol, mannitol and fructooligosaccharides (FOS) were analyzed by their addition at varying concentrations of 0.2, 0.4, 0.6, 0.8 and 1 % w/v into the skim milk medium. The effect of the two folate precursors (PABA and glutamate) was evaluated by supplementation at different concentrations (25, 50, 75 and 100 μmol/L). Fermentation was also done in presence of reducing agents like sodium ascorbate, sodium thioglycholate and cysteine hydrochloride (0.2 % w/v) at both static and agitating (100 rpm) conditions.
Folate analysis
Folate was quantified by microbiological assay using Lactobacillus casei NCIM2364, a folate auxotroph as the indicator strain. For the assay, the culture was grown in folic acid casei medium supplemented with sterile folic acid (30 pg/ml) and stored as glycerol stock at −80 °C until use. 20 μl of this was used as the inoculum for the microbiological assay (Horne 1997; Horne and Patterson 1988; Wilson and Horne 1982).
For the extraction of intracellular free folate, the cells were resuspended in 0.1 mol/L phosphate buffer, pH 6.1 containing 0.5 % sodium ascorbate. The cells were then sonicated (Sonics, USA, amplitude 47 % and 30 S ON and OFF cycle for 5 min on ice), boiled at 100 °C for 5 min and centrifuged at 12,000×g for 5 min at 4 °C. The supernatant was filtered and used to estimate the intracellular free folate. The extracellular folate was extracted from the fermented milk by adding 10 ml of extraction buffer (0.1 mol/L phosphate buffer with 0.5 % sodium ascorbate) to the fermented medium followed by boiling for 15 min and centrifugation at 12,000 ×g for 10 min. The supernatant was then filtered through a 0.45-μm filter and used directly or stored at −20 °C until use (Lin and Young 2000).
The microbiological assay was performed in a 96 well plate. For the assay 150 μl of folic acid assay medium (2×), 8 μl working buffer, 20 μL inoculum, varying concentration of folic acid standards (calcium salt of folinic acid, Sigma-Aldrich) ranging from 50 to 300 pM or samples were added to the wells and the final volume was made up to 300 μL using sterile water. Working buffer contained 0.5 ml 1 M potassium phosphate buffer pH 6.1, 1.6 g sodium ascorbate and 9.5 ml milli Q water, filter sterilized through 0.22 μ filter. Controls were also kept without inoculum to ensure the sterility of the assay. The plates were then incubated at 37 °C and the growth was measured at 600 nm by using a microtiter plate reader (Teccan infinite M200). Since the microbiological assay is less sensitive to longer chain polyglutamyl folate, the total folate was quantified after enzymatic deconjuagation of the samples by human plasma (Sigma-Aldrich), as a source of γ-glutamyl hydrolase, at 37 °C and pH 4.8 for 4 h (Sybesma et al. 2003b). To 1 g human plasma, 5 ml 0.1 M 2-mercaptoethanol (Sigma-Aldrich) with 0.5 % sodium ascorbate (Sigma-Aldrich) was added and clarified the supernatant by centrifugation. To 20 μl sample, 1 μl of the above enzyme preparation was added and incubated at 37 °C for 4 h in dark. Samples were diluted and 10 μl of it was used for the assay.
Storage stability of the fermented milk
Fermentative production of folate was performed using the optimized medium and culture conditions. After incubation at 37 °C for 8 h, the fermented skim milk was stored at 5 °C for 15 days. Samples were collected at 5 days interval and analyzed for folate, pH, lactic acid % in terms of titratable acidity (TA) and viable count of bacteria. TA is determined by titration of the fermented milk with 0.1 N NaOH using phenolphthalein as the indicator of endpoint. For the viable count of bacteria, the samples were serially diluted in sterile saline and appropriate dilutions were plated onto M17 agar plates supplemented with 0.5 % glucose. The plates were then incubated at 37 °C for 1–2 days. Colony forming units (CFU) were enumerated and the viable count was expressed in log CFU/ml.
All experiments were done in triplicates. Values are expressed as mean ± standard deviation of three independent determinations.
Results and discussion
Effect of culture conditions and additives on fermentative production of folate
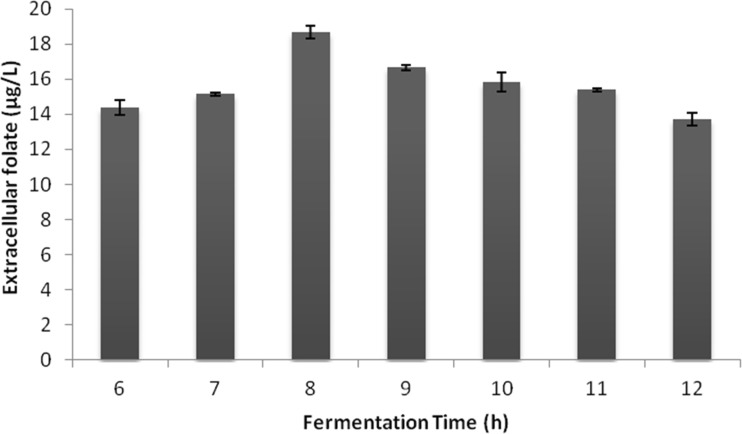
The folate content in milk is low when compared to other folate rich foods (Konings et al. 2001) and pasteurization will result in further reduction of folate level. Fermentation of milk by folate producing probiotic bacteria could be an alternative to develop a functional fermented food with enriched levels of folate. The folate binding proteins present in milk can increase its stability and bioavailability and thus it is an ideal matrix for folate fortification (LeBlanc et al. 2007). The levels of folate in fermented milk are influenced by the cultivation conditions, presence of folate precursors, prebiotic supplementation etc. In the present study, the extracellular folate production by the LAB isolate L.lactis CM28 was highest (18.66 ± 0.36 μg/L) at 8 h of fermentation (Fig. 1) by using 4 % skim milk medium supplemented with 6 μM glycine and 6 μM methionine. The gradual decrease in folate level after 8 h of fermentation could be due to the increased utilization of folate for growth and cell division. There were previous reports where the folate production was highest between 6 and 8 h of fermentation (Lin and Young 2000; Padalino et al. 2012). For fermentation, the incubation temperature of 37 °C was found to be optimum resulting in 18.59 ± 0.28 μg/L extracellular folate compared to 16.72 ± 0.56 μg/L and 8.06 ± 0.6 μg/L at 30 °C and 42 °C respectively. The optimum growth and higher biomass attained at 37 °C could be the reason for increased folate production (Gangadharan and Nampoothiri 2011; Iyer et al. 2010). But, it was reported by Sybesma et al (2003b) that there was a ten fold increase in folate production when there was a 90 % decrease in specific growth rate.
Fig. 1.
Optimization of fermentation time for folate production by Lactococcus lactis CM28 in skim milk medium
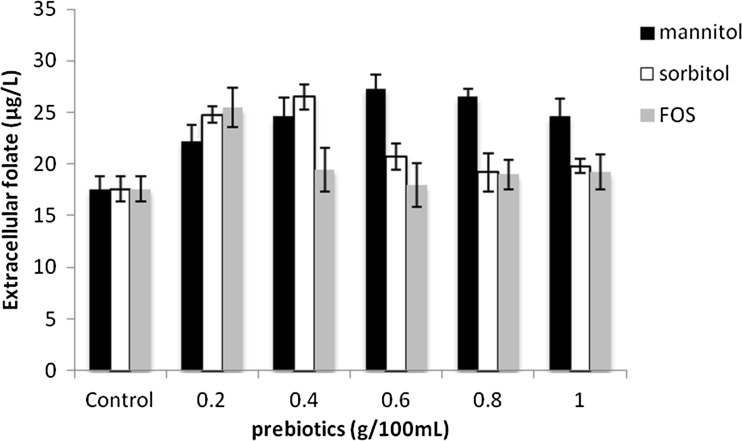
Prebiotics are non digestible food ingredients that promote the growth and survival of the probiotics. In this study the effect of sorbitol, mannitol and FOS at varying concentrations (0.2, 0.4, 0.6, 0.8 and 1 % v/w) were checked. An increase in extracellular folate production was observed in presence of these three prebiotics (Fig. 2). When compared to different concentrations of sorbitol and FOS, 0.6 % mannitol had a superior effect on extracellular folate levels which increased upto 27.31 ± 1.4 μg/L. In contrary, Padalino et al (2012) have reported that the use of prebiotics like FOS and galactooligosaccharides (GOS) in the medium had a little effect on the folate production level even though it stimulated the growth of the probiotic bacteria. In their study among the five LAB strains studied, an increase in folate production was observed in Lactobacillus plantarum with FOS supplementation and with the addition of GOS only Streptococcus thermophilus showed elevated folate production. In another study the simulative effect of four prebiotics FOS, mannitol, maltodextrin and pectin on the growth of Lactobacillus strains was reported (Yeo and Liong 2010). In their study, Pricope et al (2010) showed the effect of sorbitol in reducing the lag period of Bifidobacteria. The synergistic effect of Lactobacillus paracasei and FOS combination on faecal microflora of weaned pigs was reported by Bomba et al (2002). Hence, in general it can be concluded that supplementation of prebiotics can stimulate the growth but the functional properties like enhanced folate production can be strain dependent.
Fig. 2.
Effect of prebiotics on the fermentative production of folate by Lactococcus lactis CM28 in skim milk Black square—Mannitol (0.2, 0.4, 0.6, 0.8 g/100 ml), white square—Sorbitol (0.2, 0.4, 0.6, 0.8 g/100 ml) Grey square—Fructooligosaccharide (FOS) (0.2, 0.4, 0.6, 0.8 g/100 ml)
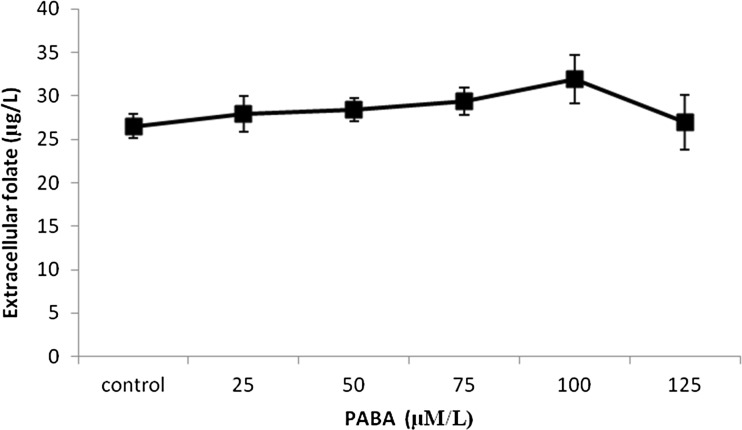
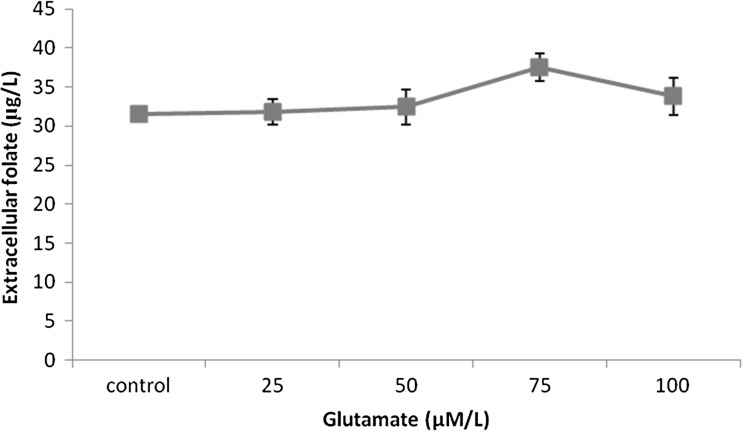
Folate is synthesized from its precursors PABA, glutamate and GTP. PABA is obtained from the shikimate pathway and GTP is produced through the purine biosynthetic pathway. The folate production by Lactococcus lactis was found to be dependent on the concentration of PABA in the culture medium (Sybesma et al. 2003b). Our results were also in accordance to this. The addition of varying concentration of PABA into the skim milk medium increased the folate production. Maximum of 31.93 ± 2.8 μg/L extracellular folate was obtained when 100 μmol/L PABA was supplemented (Fig. 3). The elevated PABA levels in the medium could result in the inactivation of folylpolyglutamate synthetase, the enzyme responsible for the elongation of the polyglutamate tail of the folate molecule thereby increasing the amount of secreted folate (Wegkamp et al. 2007). The intracellular retention of polyglutamyl folates is higher than the monoglutamyl folates. Similarly, the addition of glutamate also resulted in an increase in the released folate. The cumulative effect of PABA and glutamate resulted in 37.56 ± 1.8 μg/L extracellular folate (Fig. 4).
Fig. 3.
Effect of PABA on folate production by Lactococcus lactis CM28 in skim milk
Fig. 4.
Effect of glutamate on folate production by Lactococcus lactis CM28 in skim milk
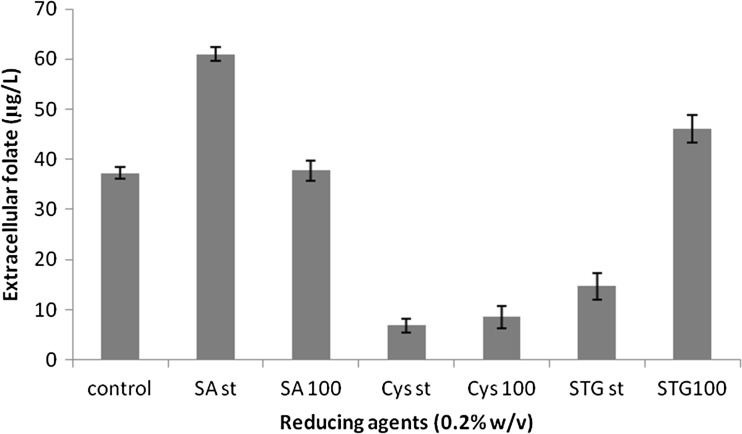
Folate bioavailability is highly dependent on the physico-chemical properties of the folate forms, food ingredients present and stability of the food folate (Seyoum and Selhub 1998). Folates are highly susceptible to oxidation by air, heat and the acid-peptic milieu of the stomach. Significant loss in activity occurs due to oxidation resulting in conversion to inactive forms such as p-aminobenzoyl glutamate (Forssén et al. 2000). Several studies have shown that reducing agents like sodium ascorbate can prevent the oxidative damage of folate and also result in the reversion of inactive forms to active folate. When the medium was supplemented with 0.2 % sodium ascorbate, 61.02 ± 1.3 μg/L extracellular folate was detected (Fig. 5). This increase could be due to the increased stability of the folate in presence of sodium ascorbate or the higher biomass achieved in microaerophilic environment provided by the reducing agent (Gangadharan and Nampoothiri 2011).
Fig. 5.
Effect of reducing agents on fermentative production of folate by Lactococcus lactis CM28 in skim milk. SA st—Sodium ascorbate (0.2 %) at static condition, SA 100—Sodium ascorbate (0.2 %) with agitation (100 rpm), Cys st—Cysteine (0.2 %) at static condition, Cys 100—Cysteine (0.2 %) with agitation (100 rpm), STG st—Sodium thioglycholate (0.2 %) at static condition, STG 100—Sodium thioglycholate (0.2 %) with agitation (100 rpm)
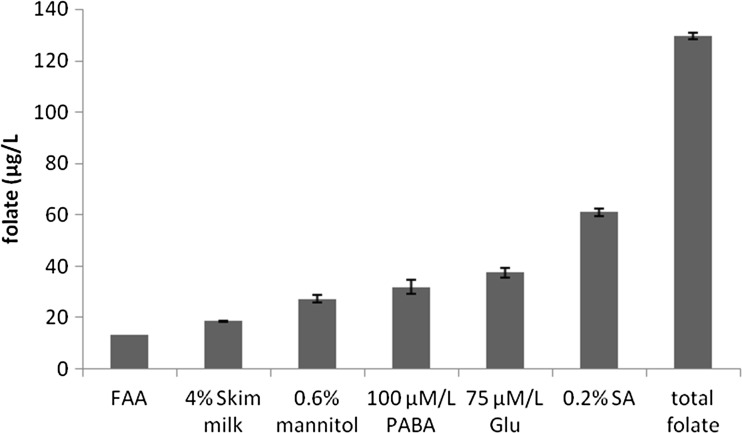
The total folate (extra and intracellular) production was also determined under optimized conditions after deconjugation with human plasma. The optimized medium was skim milk (4 %) supplemented with glycine (6 μmol/L), methionine (6 μmol/L), mannitol (0.6 %), PABA (100 μmol/L), glutamate (75 μmol/L) and sodium ascorbate (0.2 %) and fermented for 8 h at 37 °C at static conditions. A fourfold increase in the extracellular folate (61.02 ± 1.3 μg/L) was obtained after optimization which can be correlated to the bioaccessible folate. Extracellular folates will contain shorter polyglutamyl tails which make them more bioavailable than polyglutamyl folates. Monoglutamyl folates can be directly absorbed in the human gut whereas polyglutamyl folates require enzymatic deconjugation before intestinal absorption and metabolic utilization. The effective intake of folates is highly dependent on the degree of polyglutamylation. (Gregory 2001; Sybesma et al. 2003a). The major portion of folate produced by Lactococcus lactis is retained intracellularly (Sybesma et al. 2003b). In this study, after deconjugation the total folate detected was 129.53 ± 1.2 μg/L (Fig. 6). This indicated that approximately 53 % of folate produced by the LAB strain Lactococcus lactis CM28 is in the polyglutamyl form and is retained intracellularly. Pompei et al (2007) demonstrated that intracellular accumulation of folate is highly strain dependent in Bifidobacteria.
Fig. 6.
Folate levels quantified at optimized condition. FAA Folic acid assay medium, Glu glutamate, SA Sodium ascorbate. Total folate corresponds to extra and intracellular folate
Storage stability of fermented milk
Probiotics, upon ingestion should survive the acid milieu of the stomach and reach the intestine in high numbers, adhere to the intestinal walls and multiply to exert their health benefits on the host. According to the recommendation of the International Dairy Federation the minimum number of probiotic cells at the time of consumption should be ≥107 CFU/g (Ouwehand and Salminen 1998). It is also important that the probiotic product retains its functionality throughout the storage period (Daneshi et al. 2013). The shelf life of yoghurt is usually about 2 weeks under refrigerated conditions. The folate levels, pH, TA and viable counts of the fermented milk were measured initially and then every 5 days for 15 days (Table 1). About 90 % of the folate was retained in an active form after 15 days. The viable count of bacteria was 6 × 1010 CFU/ml (10.78 log CFU/ml) after 15 days which were sufficient to exert health benefits on the host. Less than one log unit reduction was there in the viable count of bacteria during the storage period. Over the storage time, it was noticed that the TA slightly increased and pH decreased. The post acidification of fermented milk on cold storage was due to β galactosidase activity (Kailasapathy 2006). This in turn could be the reason for the slight decrease in the viability of the probiotic bacteria.
Table 1.
Storage stability studies of the fermented milk
| Days | 1 | 5 | 10 | 15 |
|---|---|---|---|---|
| pH | 4.74 | 4.53 | 4.42 | 4.42 |
| TA (% lactic acid) | 0.28 ± 0.028 | 0.315 ± 0.007 | 0.42 ± 0.028 | 0.48 ± 0.014 |
| Viable count (log CFU/ml) | 11.6 ± 0.28 | 11.48 ± 0.1 | 10.9 ± 0.11 | 10.78 ± 0.07 |
| Folate stability (%) | 100 | 99.24 ± 0.11 | 95.11 ± 1.32 | 90.78 ± 0.71 |
Mean ± SD; n = 3
Conclusion
Folate is an important cofactor involved in the biosynthesis of nucleic acids and is required in high amounts during growth phase. The results from the present study show that the folate producing lactic acid bacterium isolated from cow’s milk can be employed to combat the suboptimal intake of this vitamin. Optimization of medium and culture conditions resulted in a fourfold increase in the bioavailable extracellular folate. Since the strain L. lactis CM28 exhibited significant probiotic properties along with folate production it could be used to develop functional foods that could provide various health benefits. Also, the strain showed an acceptable viability and retained about 90 % of the produced folate in an active form over the storage period of 15 days at 5 °C. Thus, it can be concluded that this strain can be used as a monostrain probiotic or can be used along with other folate producing strains for the enhancement of the vitamin in various dairy as well as other fermented foods.
Acknowledgments
First author JBD acknowledge the Research fellowships provided by the Department of Science and Technology (DST), India, and Kerala State Council for Science, Technology and Environment (KSCSTE). Authors also thank Department of Biotechnology (DBT), New Delhi and Council for Scientific and Industrial research (CSIR), New Delhi (under Net work Project FUNHEALTH (CSC 0133)) for the grant for probiotic research.
References
- Alm L. Effect of fermentation on B-vitamin content of milk in Sweden. J Dairy Sci. 1980;65:353–359. doi: 10.3168/jds.S0022-0302(82)82199-1. [DOI] [PubMed] [Google Scholar]
- Ames BN. Micronutrient deficiencies cause DNA damage and cancer. Food Sci Agric Chem. 1999;1:1–15. [Google Scholar]
- Blount BC, Marck MM, Wehr CM, MacGregor T, Hiatt RA, Wang G, Wickramasingle SN, Everon RB, Ames BN (1997) Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc Natl Acad Sci U S A 94:3290–3295 [DOI] [PMC free article] [PubMed]
- Bomba A, Nemcová R, Gancarcíková S, Herich R, Guba P, Mudronová D. Improvement of the probiotic effect of the micro-organisms by their combination with maltodextrins, fructooligosaccharides and poly unsaturated fatty acids. Br J Nutr. 2002;1:S95–S99. doi: 10.1079/BJN2002634. [DOI] [PubMed] [Google Scholar]
- Boushey CJ, Beresford AA, Omenn GS, Moltulsky AG (1996) A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA 274:1049–1057 [DOI] [PubMed]
- Brattstrom L. Vitamins as homocysteine-lowering agents. J Nutr. 1996;126:1276S–1280S. doi: 10.1093/jn/126.suppl_4.1276S. [DOI] [PubMed] [Google Scholar]
- Daneshi M, Ehsani MR, Razavi SH, Labbafi M. Effect of refrigerated storage on the probiotic survival and sensory properties of milk/carrot juice mix drink. Electron J Biotechnol. 2013;16:5. doi: 10.2225/vol16-issue5-fulltext-2. [DOI] [Google Scholar]
- Forssén KM, Jägerstad MI, Wigertz K, Witthöft CM. Folates and dairy products: a critical update. J Am Coll Nutr. 2000;19:100S–110S. doi: 10.1080/07315724.2000.10718071. [DOI] [PubMed] [Google Scholar]
- Gangadharan D, Nampoothiri KM. Folate production using Lactococcus lactis ssp cremoris with implications for fortification of skim milk and fruit juices. LWT Food Sci Technol. 2011;9:1859–1864. doi: 10.1016/j.lwt.2011.05.002. [DOI] [Google Scholar]
- Gangadharan D, Sivaramakrishnan S, Pandey A, Nampoothiri KM. Folate-producing lactic acid bacteria from cow’s milk with probiotic characteristics. Int J Dairy Technol. 2010;63:339–348. doi: 10.1111/j.1471-0307.2010.00590.x. [DOI] [Google Scholar]
- Gregory JF. Case study: folate bioavailability. J Nutr. 2001;131:1376S–1382S. doi: 10.1093/jn/131.4.1376S. [DOI] [PubMed] [Google Scholar]
- Hibbard BM. The role of folic acid in pregnancy. J Obstet Gynaecol Br Commonw. 1964;7:529–542. doi: 10.1111/j.1471-0528.1964.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Horne DW. Microbiological assay of folates in 96-well microtiter plates. In: McCormick DB, Suttie JW, Wayne C, editors. Methods in enzymology, vol 281. London: Academic; 1997. pp. 38–43. [DOI] [PubMed] [Google Scholar]
- Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin Chem. 1988;34:2357–2359. [PubMed] [Google Scholar]
- Iyer R, Tomar SK, Singh AK. Response surface optimization of the cultivation conditions and medium components for the production of folate by Streptococcus thermophilus. J Dairy Res. 2010;77:350–356. doi: 10.1017/S0022029910000166. [DOI] [PubMed] [Google Scholar]
- Jacob RA. Folate, DNA methylation, and gene expression: factors of nature and nurture. Am J Clin Nutr. 2000;72:903–904. doi: 10.1093/ajcn/72.4.903. [DOI] [PubMed] [Google Scholar]
- Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT Food Sci Technol. 2006;39:1221–1227. doi: 10.1016/j.lwt.2005.07.013. [DOI] [Google Scholar]
- Konings EJM, Roomans HHS, Dorant E, Goldbohm RA, Saris WHM, van den Brandt PA. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am J Clin Nutr. 2001;73:765–776. doi: 10.1093/ajcn/73.4.765. [DOI] [PubMed] [Google Scholar]
- LeBlanc JG, de Giori GS, Smid EJ, Hugenholtz J, Sesma F (2007) Folate production by lactic acid bacteria and other food-grade microorganisms. Commun Curr Res Educ Top Trends Appl Microbiol 329–339
- Lin MY, Young CM. Folate levels in cultures of lactic acid bacteria. Int Diary J. 2000;10:409–413. doi: 10.1016/S0958-6946(00)00056-X. [DOI] [Google Scholar]
- Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- Ouwehand AC, Salminen SJ. The health effects of cultured milk products with viable and nonviable bacteria. Int Dairy J. 1998;8:749–758. doi: 10.1016/S0958-6946(98)00114-9. [DOI] [Google Scholar]
- Padalino M, Conesa DP, Nicolás RL, Saseta CF, Berruezo GR. Effect of fructooligosaccharides and galactooligosaccharides on the folate production of some folate producing bacteria in media cultures or milk. Int Dairy J. 2012;27:27–33. doi: 10.1016/j.idairyj.2012.06.006. [DOI] [Google Scholar]
- Pompei A, Cordisco L, Amaretti A, Zanoni S, Mattezzi D, Rossi M. Folate production by Bifidobacteria as a potential probiotic property. Appl Environ Microbiol. 2007;73:179–185. doi: 10.1128/AEM.01763-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricope L, Dumitrascu L, Nicolau A, Borda D, Georgescu L. The influence of saccharin and sorbitol upon the BB-12® activity in milk and the rheological characteristics of fermented products. AUDJG Food Technol. 2010;34:74–81. [Google Scholar]
- Roy MP. Folate and neural tube defects. Am J Clin Nutr. 2007;85:285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- Seyoum E, Selhub J. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J Nutr. 1998;128:1956–1960. doi: 10.1093/jn/128.11.1956. [DOI] [PubMed] [Google Scholar]
- Sybesma W, Starrenburg M, Kleerebezem M, Mierau I, de Vos WM, Hugenholtz J. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl Environ Microbiol. 2003;69:3069–3076. doi: 10.1128/AEM.69.6.3069-3076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybesma W, Starrenburg M, Tijsseling L, Hoefnagel MHN, Hugenholtz J. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl Environ Microbiol. 2003;69:4542–4548. doi: 10.1128/AEM.69.8.4542-4548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegkamp A, van Oorschot W, de Vos WM, Smid EJ. Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl Environ Microbiol. 2007;73:2673–2681. doi: 10.1128/AEM.02174-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Horne DW. Use of glycerol-cryoprotected Lactobacillus casei for microbiological assay of folic acid. Clin Chem. 1982;28:1198–1200. [PubMed] [Google Scholar]
- Yeo SK, Liong MT. Effect of prebiotics on viability and growth characteristics of probiotics in soymilk. J Sci Food Agric. 2010;90:267–275. doi: 10.1002/jsfa.3808. [DOI] [PubMed] [Google Scholar]