Abstract
The effects of pectin and sucrose addition on the rheological parameters and freezing kinetic of passion fruit pulp were studied. The effect of the air–blast freezing of passion fruit at -20 °C on the rheological parameters before and after freezing was evaluated. The rheological analyses were carried out using a viscometer at 25 °C, and the readings were converted into rheological measurements using the Mitschka method and fitted to the Ostwald–de Waele model. The freezing kinetics was analyzed using a controlled–temperature cold–stage instrument, which was coupled to a microscope integrated with a video capture system. The concentrations of sucrose and pectin were established using a Composite Rotational Design. The pulps combined with the additives exhibited a pseudoplastic behavior, and the values for the index flow (n) and consistency index (k) before and after freezing differed and were dependent on the additive concentrations. The rates of increase for the frozen areas were evaluated in a microfreezer and were significantly influenced by the additive concentrations. These concentrations were those that presented a lower index flow and a higher consistency index. The results are discussed in terms of the solubility and interaction of the pectin added to the fruit pulp with low pH, the presence of sucrose and low temperature.
Keywords: Rheological behavior, Frozen fruit pulps, Additives, Kinetic Freezing
Introduction
Preservation of fruit in the form of juice, pulp and other products boosts product availability and permits the use of production surplus. Frozen fruit pulp is derived from the edible portion of the fruit and involves crushing and/or pulping the fruit and then freezing the processed paste. Air–blast freezing is the process most often applied in small agroindustries. When fruit is frozen in the form of pulp, the effect of the freezing process is verified by alterations in pulp consistency and composition, which are caused by chemical reactions during this subsequent storage (Fernandes et al. 2010). The consistency and global appearance of the pulp are best maintained when the pulp is frozen quickly (Wang and Chang, 1994).
Several reports in the literature show that fruit pulps behave as non–Newtonian fluids, with shear–thinning behavior. Pelegrine et al. (2002) determined that pineapple and mango pulps are shear–thinning fluids, as their apparent viscosities decrease with increases in shear rate, and the flow behavior index (n) was less than 1. A study comparing the available data in the literature for fruit pulps, including guava, raspberry, pineapple, apricot, apple, mango, tamarind and black currant found that all these fruit pulps showed shear–thinning behavior (Krokida et al. 2001).
Fruit pulps with added pectin and sucrose have been used as raw material in various processes by the food industry, including the production of fruit preserves, candy, ice cream, ice pops and lollypops. The use of these additives affects the rheological behavior of the fruit pulps, altering the concentration of the constituents compared with the original pulp and consequently altering the physicochemical and microstructural characteristics of the products in the subsequent stages of processing, such as freezing and thawing (Fernandes et al. 2010, Conceição et al. 2012).
Food processing critically affects the pectin structure–function relationship and thus the textural and rheological quality attributes of fruit and vegetable products depending on the technique applied and the intensity of the process. The rates at which desired and undesired quality–related reactions take place are functions of both intrinsic and extrinsic factors. The intrinsic factors refer to the product–specific properties, including pH and water activity. Extrinsic factors that affect the quality of processed foods are process–specific factors, including temperature, residence time and pressure (Van Buggenhout et al. 2009).
As an ingredient in food applications, pectin is known to be a principal component for the formation of gels. The rheological characteristics, such as the viscosity or viscoelasticity of systems, change in subtle ways depending on the pectin source and the environmental conditions. The rheological properties of pectin gels from apples and citrus fruits have been studied extensively (Sila et al. 2009). However, most of this research has focused on the thermal processing domain, while pectin engineering studies in the freezing technology domain are currently rather limited (Van Buggenhout et al. 2009). In low–temperature applications, the effects of pectin on the rheological parameters can be related to its solubility properties. The structural characteristics of pectin and the distribution of hydrophilic and hydrophobic groups within the molecule determine the solubility properties of the polymer. Partial demethoxylation of pectin decreases its solubility in water. Generally, pectin solubility in water is increased by decreasing the polymer size and increasing the methoxy–ester groups; however, the pH, temperature, and the solute concentrations in the environment are also important (Sila et al. 2009)
The pH values observed for the yellow passion fruit pulp after freezing and thawing ranged from 2.67 to 3.77 and were higher than those for the fresh samples previously reported, from 2.54 to 3.77 (Raimundo et al. 2009) or 2.79 to 3.02 (De Marchi et al. 2000). Pectin is very stable near pH 3.5, at its pKa value. The pectin polymer contains a negative charge in the acidic side chain. The addition of sugar to the fruit pulp–pectin mixture also influences the equilibrium between the pectin and the water, destabilizing the pectin and forming a mesh similar to a network structure that is capable of retaining liquid. This mesh then agglutinates the sugar in the form of a gel. The rigidity of the network structure is also influenced by the concentration of sugar and by the acidity of the medium.
Rheological behavior is the mechanical behavior of materials subjected to a deformation process due to a tension field and can be important to mass transfer–related phenomena that occur during the freezing processes. There is an ongoing discussion regarding the influence of the presence of hydrocolloids in the form of ice crystals and the roles of the ice crystal sizes. The ice crystals can be smaller, but this depends on the capacity of the hydrocolloids to bond with the water and increase the viscosity of the mixture (Blanshard and Franks 1987). The freezing process is extremely dependent on product–related properties. Knowledge of the factors that may affect the change of the fruit pulp state is valuable for the monitoring and control of ice crystals (George 1993). The incorporation of various concentrations of additives into the pulp can modify the freezing kinetics and the arrangement of the water molecules in the crystalline state (Fernandes et al. 2010).
The objectives of this work are thus as follows: i) to evaluate the effect of air–blast freezing and the addition of sucrose and pectin on the rheological behavior of passion fruit pulps and ii) to establish a correlation between the concentrations of the additives and the rheological behavior and freezing kinetics of the fruit pulp mixtures.
Material and Methods
2.1 Preparation of samples
The yellow variety of passion fruit used in this work was harvested from various areas within the Lavras region, Minas Gerais state (Brazil). To extract the pulp, passion fruits were sanitized and crushed using a food processor (Braun AG 4243, São Paulo–Brazil). The resulting mixture was then passed through a Granutest (Telastem, São Paulo–Brazil) sieve with 1.2 mm openings (corresponding to Tyler–14, 14 mesh per inch and ABNT–16). Sucrose (P.A., 99.8 %–Isofar, Brazil) and low methoxy pectin (citrus pectin) (PROQUÍMIOS, São Paulo–Brazil) at the concentrations listed in the experimental design section (2.6) were added to the pulps, and the mixtures were then homogenized using a Turratec grinder (Tecnal, model–TE–102, Brazil).
2.2 Air–blast freezing
To measure the effects of freezing on the rheological behavior, the passion fruit pulps were stored in cylindrical polyethylene containers (1 L) and frozen at–20°C using an air–blast (Kit Frigor, Brazil) tunnel. The cooling time and cooling rate were obtained using type T thermocouples (copper–constantan) inserted into the geometric center of the cylindrical containers and connected to a signal conditioning system (National Instruments–Model SCXI–Hungary). The temperature measurements were collected at intervals of 1 min using software LabVIEW 8.5 (National Instruments–Ireland).
2.3. Rheological behavior
The characterization of the rheological behavior of systems composed of passion fruit pulps with added sucrose and pectin before and after freezing/thawing was performed using torque unit readings from a viscometer (model RVT–Brookfield Engineering Laboratories, MA, USA) with a reading precision of ±1.0 % and a measurement range of 100 mPa s to 8,000 Pa s at 25°C. The velocities used were 0.5, 1, 2.5, 5, 10, 20, 50 and 100 rpm, and the sample volume was 500 mL, contained within a 600 mL beaker. Spindles (numbers 2, 3 and 4–Brookfield Engineering Laboratories, MA, USA) were used for the passion fruit pulps according to the reading limit at maximum speed. We used the Mitschka method (Briggs and Steffe 1997) to convert the readings into rheological measurements (shear stress, shear rate and apparent viscosity).
Microfreezer analyses
In this study, passion fruit pulp samples were subjected to freezing conditions to obtain constant heat–flow removal and a freezing rate of 0.4 °C/min. To study the microstructure of the freezing front in the pulps, we visualized the samples by light microscopy during freezing using a microscope (Meiji, Series ML 5000–Japan) equipped with a Peltier temperature–controlled cold–stage system and a polarized light filter, which was coupled to a video capture system. Photomicrographs were obtained at 1–s intervals from the start of the freezing front movement. The increase of frozen area in the passion fruit pulps as a function of time was analyzed using Sigma Scan–Pro 5.0 image analysis software.
2.6 Experimental design
We used the rotational composite design (Table 1) to study the effect of freezing on the rheological parameters and the increase of frozen area in the passion fruit pulps as a function of time. Triplicate samples were analyzed at 25 °C before freezing and after thawing. Thawing occurred at room temperature, controlled to 20 ± 1 °C.
Table 1.
Rotational composite experimental design
| Samples | Codified | Real (g/100 g of pulp) | ||
|---|---|---|---|---|
| Sucrose | Pectin | Sucrose | Pectin | |
| 1 | -1 | -1 | 2.91 | 0.15 |
| 2 | +1 | -1 | 17.09 | 0.15 |
| 3 | -1 | +1 | 2.91 | 0.85 |
| 4 | +1 | +1 | 17.09 | 0.85 |
| 5 | 0 | 0 | 10.00 | 0.50 |
| 6 | 0 | 0 | 10.00 | 0.50 |
| 7 | 0 | 0 | 10.00 | 0.50 |
| 8 | - 1.41 | 0 | 0.00 | 0.50 |
| 9 | + 1.41 | 0 | 20.00 | 0.50 |
| 10 | 0 | - 1.41 | 10.00 | 0.00 |
| 11 | 0 | + 1.41 | 10.00 | 1.00 |
A nonlinear regression was fit to the rheological behavior data of treatments according to the Ostwald–de Waele model (power law) and assessed with regard to the statistical parameter coefficient of determination (R2). These statistical analyses were performed using R® version 2.4.1 software (R Development Core Team, Vienna, Austria, 2006) and Statistica version 6.0 software (Statsoft®). The data obtained by the response surface methodology were analyzed using Statistica version 6.0 software (Statsoft®) for the response variables index of flow (n) and consistency index (K), while Minitab 14 was used for the variable ‘area occupied by ice crystals.’
Results and Discussion
3.1 Rheological behavior
Although the Ostwald–de Waele model is often described as a power law, it is in fact an empirical relationship. This widely used equation takes the form
| 1) |
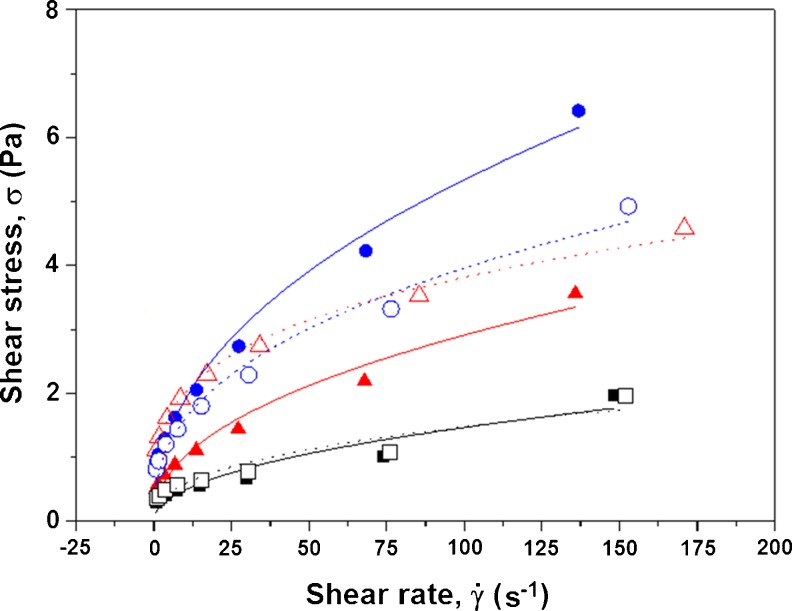
where σ is the shear stress, K is a consistency index, is the shear rate, and n is a dimensionless number that indicates the closeness to Newtonian flow (Bourne 2002). The experimental points (means of triplicates) and the curve fit to the Ostwald-de Waele model (power law) of the treatments before freezing (BF) and after thawing (AF) from the air-blast freezing are presented in Fig. 1 for the systems with sucrose at a concentration of 10 g/100 mL and different concentrations of pectin. The coefficients of determination (R2) are also shown for these treatments (legend).
Figure 1.
Relationship between shear stress (Pa) and deformation rate (s-1) before freezing (BF) and after thawing (AF) in passion fruit pulps with the addition of (-■-) 10 g of sucrose/100 mL of pulp and 0 g of pectin/100 mL of pulp, BF, R2 = 0.92; (-□-) 0 g of pectin/100 mL of pulp, AF, R2 = 0.92; (-▲-) 0.5 g of pectin/100 mL of pulp, BF, R2 = 0.96; (-∆-) 0.5 g of pectin/100 mL of pulp, AF, R2 = 0.99; (-●-) 1.0 g of pectin/100 mL of pulp, BF, R2 = 0.98; (-○-) 1.0 g of pectin/100 mL of pulp, AF, R2 = 0.98
According to Steffe (1996), the curve fit models for all treatments showed that the pulps behave like pseudoplastic fluids (n < 1). In these fluids, the shear stress increases with the shear rate, with greater increases corresponding to higher concentrations of pectin in the systems. However, after thawing of the samples, these behaviors are different. Figure 1 shows that there is a reversal of this trend of pseudoplasticity for concentrations of 0.5 and 1.0 g of pectin/100 mL of pulp. In the systems with 10 g of sucrose/100 mL of pulp in the absence of added pectin, the results for the unfrozen and thawed samples were similar. For the systems with 10 g of sucrose/100 mL of pulp and 0.5 g of pectin/100 mL of pulp, higher variations in the shear stress as a function of the shear rate were observed for the thawed samples compared to the unfrozen samples. For the systems with 10 g of sucrose/100 mL of pulp and 1.0 g of pectin/100 mL of pulp, the unfrozen samples had higher variations in the shear stress as a function of the shear rate compared to those samples frozen and thawed for the same additives.
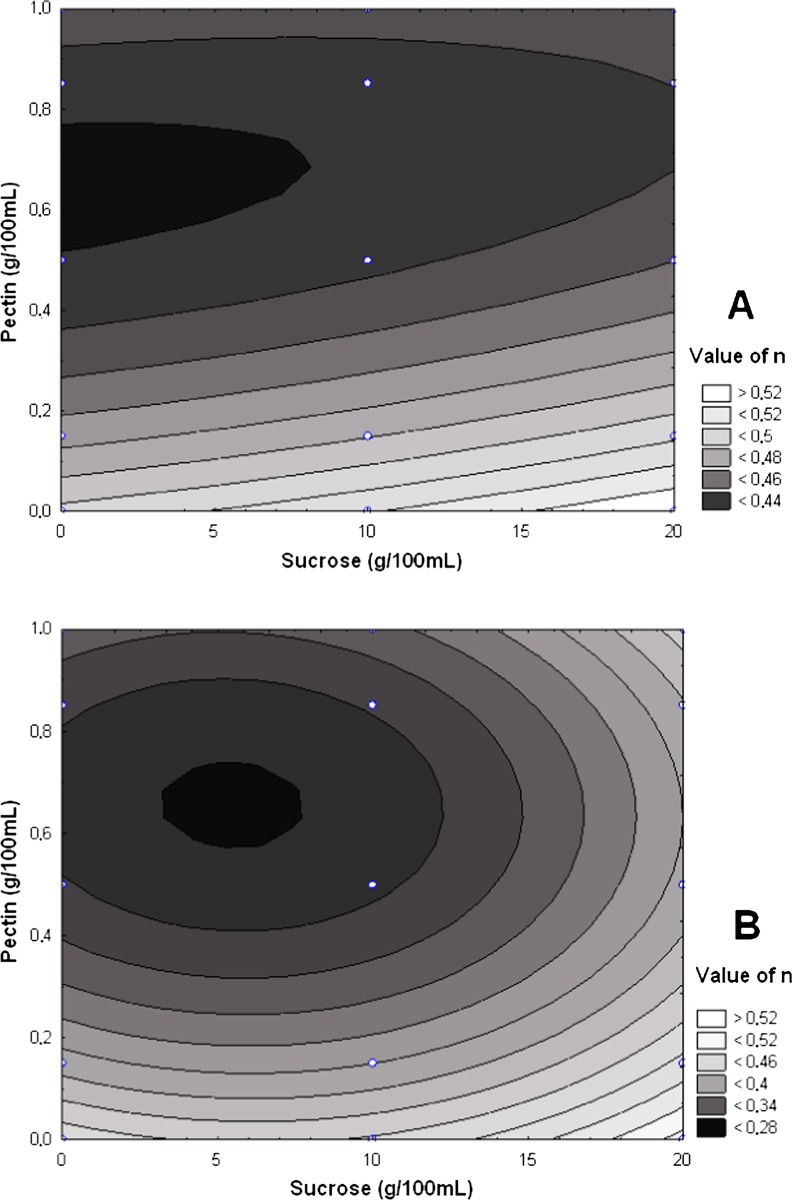
The model parameters to fit the experimental data of K (consistency coefficient) and the respective coefficients of determination (R2) are illustrated for the unfrozen samples in Fig. 2A and for frozen and thawed samples in Fig. 2B. For n (flow behavior index), the results are presented for the unfrozen samples in Fig. 3A and for the frozen and thawed samples in Fig. 3B. The n values were found to be less than unity (Figure A and 3B), thus demonstrating the pseudoplastic character of the pulps (Steffe 1996; Ferreira et al. 2002; Cabral et al. 2002; Silva et al. 2005; Cabral et al. 2005; Sato and Cunha 2007).
Figure 2.
Consistency index (k) as a function of sucrose and pectin concentrations: (A) before freezing R2 = 0.92; (B) after thawing R2 = 0.94
Figure 3.
Flow index (n) as a function of sucrose and pectin concentrations: (A) before freezing, R2 = 0.88; (B) after thawing, R2 = 0.93.
Figures 2B and 3B show that the highest consistency index and the lowest flow index are demonstrated by the samples that had been frozen with concentrations of pectin above 0.5 g/100 mL of pulp. The mechanism can be related to the separation of the structural water as ice during the freeze-concentration of the systems. A comparison of the differences between the viscosities of the unfrozen and frozen/thawed pulps shows that the higher values for the viscosity after thawing can be attributed to the size of the particles suspended in the pulp (Pelegrine et al. 2002). During the cooling and separation of the structural water as ice, the reduction in the temperature leads to a reduction in the molecular distances due to increases in the intermolecular forces. Additionally, with the temperature reduction, the shear stress is reduced, causing a rearrangement of the particles and aggregation into bigger particles. These particles flow with more difficulty, resulting in a viscosity increase (Conceição et al. 2012).
With less water, acidic pH and in the presence of sucrose, the conditions boost the formation of the pectin-sucrose network. However, the increase of sucrose concentration (20 g/100 mL of pulp) is not favorable for the separation of water as ice, and it remain in the unfrozen phase. After thawing, the change in the consistency index and flow index as functions of the additive concentrations can be explained by the size of the suspended particles, which influence its alignment when subjected to the shear stress.
Because shear stress is directly proportional to viscosity, an increase in viscosity is associated with the increase in pectin concentration for unfrozen pulps. The behavior of unfrozen and thawed samples treated with 20 g of sucrose/100 mL of pulp in the absence of pectin (data not shown) was similar to that of the samples treated with 10 g of sucrose/100 mL of pulp, as shown in Figs. 2 and 3.
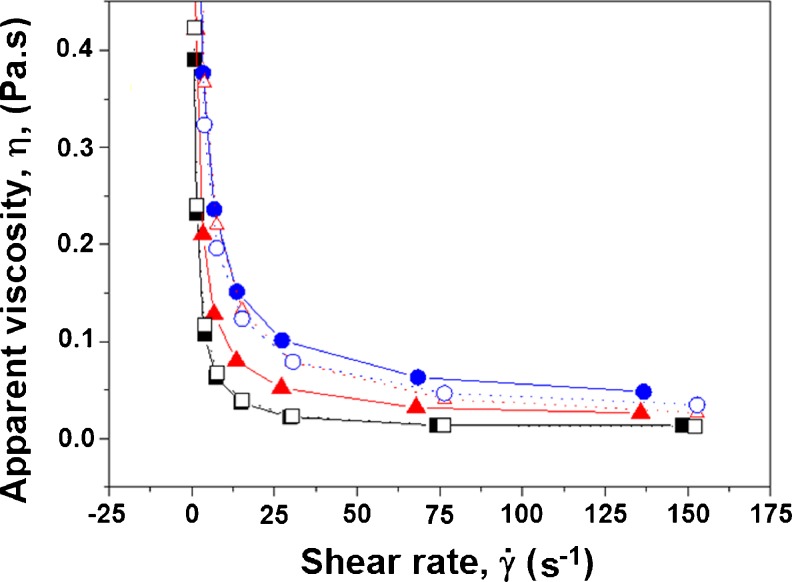
Figure 4 shows the curves of the apparent viscosity as a function of the shear rate. These curves for the frozen and thawed treatments describe a pseudoplastic behavior, thus confirming the previous analyses. In this non-Newtonian type of fluid, the apparent viscosity decreases with increases in the shear rate. According to Haminiuck et al (2007), the decrease in apparent viscosity with increasing shear rate can be explained by the structural breakdown of the pulp caused by the hydrodynamic forces generated and the increased alignment of the constituent molecules.
Figure 4.
Relationship between apparent viscosity (Pas) and deformation rate (s-1) of passion fruit pulps before freezing (BF) and after thawing (AF) with the following additions: (-■-) 10 g of sucrose/100 mL of pulp and 0 g of pectin/100 mL of pulp, BF; (-□-) 0 g of pectin/100 mL of pulp, AF; (-▲-) 0.5 g of pectin/100 mL of pulp, BF; (-∆-) 0.5 g of pectin/100 mL of pulp, AF; (-●-) 1.0 g of pectin/100 mL of pulp, BF; (-○-) 1.0 g of pectin/100 mL of pulp pectin, AF.
3.2. Analyses of the concentration of the systems on the freezing kinetics
To verify and correlate the rheological behavior effects with the kinetics of ice crystal formation during freezing for the passion fruit pulp systems with added sucrose and pectin, we obtained microfreezer photomicrographs and analyzed the development of the freezing front in these systems.
The cooling rate of the Peltier cold–stage system was set up to be similar to the cooling rate for air–blast freezing of the samples packed in polyethylene containers (1 L) at an air–cooling temperature of–20 ± 2 ºC. The results for the cooling rates measured in the air–blast freezer were 0.38 °C/min (10 g of sucrose/100 mL of pulp, 0.5 g of pectin/100 mL of pulp), 0.36 °C/min (10 g of sucrose/100 mL of pulp, 0 g of pectin/100 mL of pulp) and 0.32 °C/min (10 g of sucrose/100 mL of pulp, 1.0 g of pectin/100 mL of pulp). This difference observed in the heat removal rate can be a consequence of the increase in the pectin concentration. Air–blast freezing was chosen because it is the most frequently applied method in small agroindustries. This study aimed to determine whether the changes in the rheological behavior caused by the additives before freezing modify the rate of ice crystal growth at the microscopic level and the degree to which the treatments can influence this growth rate.
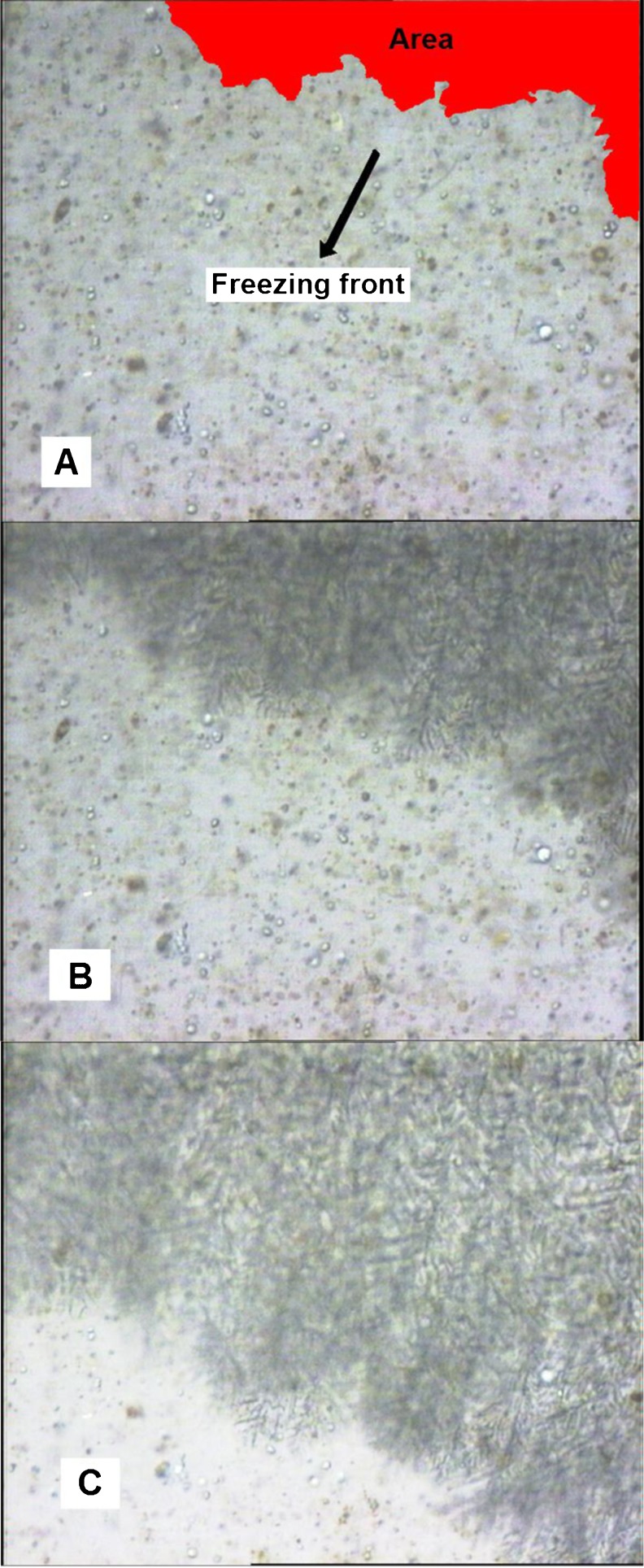
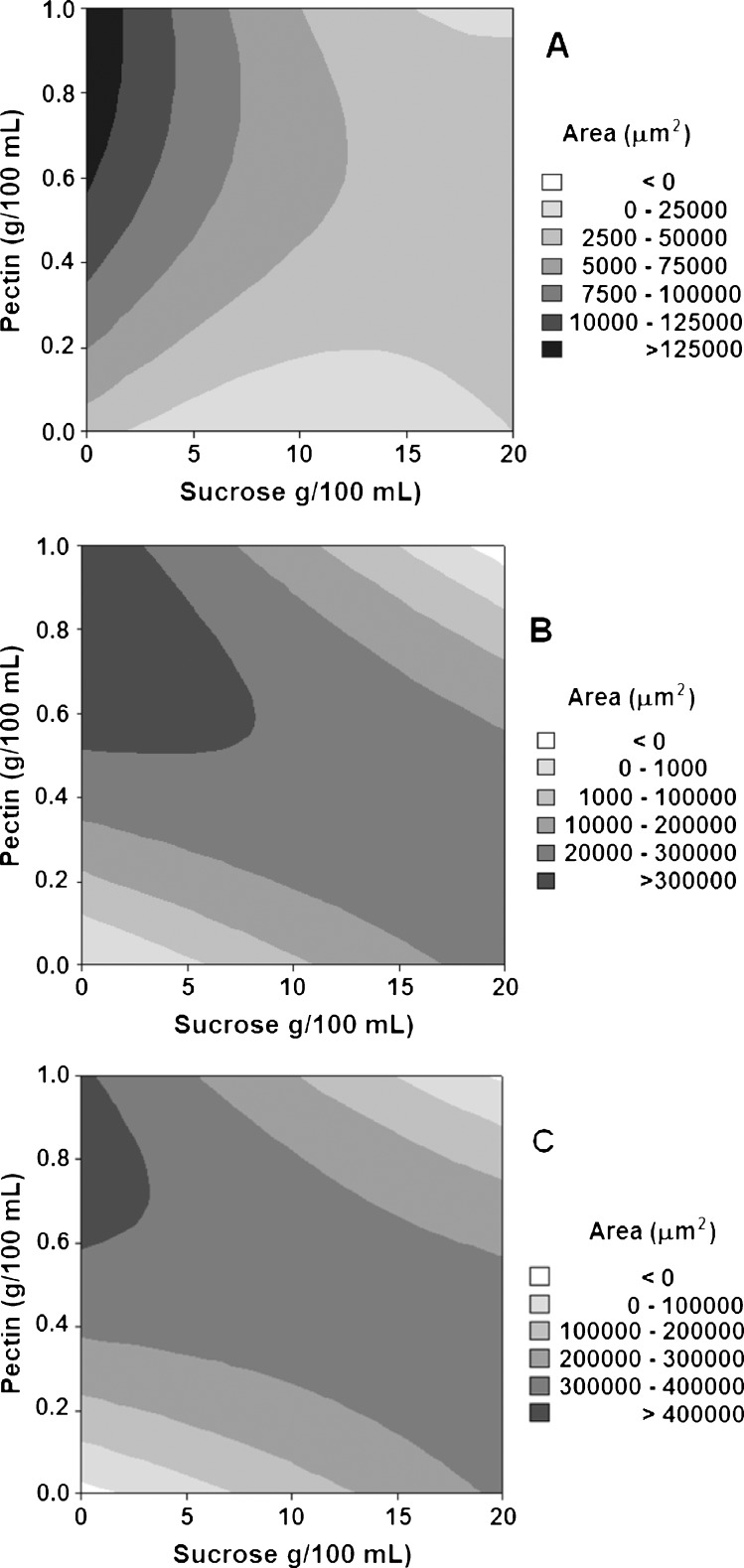
Figure 5 shows photomicrographs selected at time intervals of 2, 6 and 10 s after the freezing of pulp mixtures containing 17.09 g of sucrose/100 g of pulp and 0.85 g of pectin/100 g of pulp (sample 4) began. Figure 5 shows the variation of the area of the ice crystals growing in the field of the microscope. The variations in the areas occupied by the ice crystals as a function of pectin and sucrose concentrations at 2, 6 and 10 s intervals for all the treatments were quantified using image analysis software, as shown in Fig. 6.
Figure 5.
Photomicrographs of passion fruit pulp with the addition of 17.1 g of sucrose/100 mL of pulp and 0.85 g of pectin/100 mL of pulp at time intervals 2 (A), 6 (B) and 10 (C) seconds during the freezing process.
Figure 6.
Variation of area occupied by the ice crystals during the freezing of passion fruit pulps as a function of the pectin and sucrose concentrations at time intervals (A) 2, (B) 6 and (C) 10 s after freezing began.
Figure 6 shows that the freezing front movement occurs with greater velocity following an inverse relationship for treatments in which high sucrose concentrations are associated with low pectin concentrations, there are intermediate sucrose and pectin concentrations and high pectin concentrations are associated with low sucrose concentrations.
With respect to the rheological behavior and results, Fig. 6 reveals an interactive effect between the sucrose and pectin factors on the apparent viscosity of the systems, which may influence the freezing front velocity. This result suggests the existence of an optimum combination of sucrose and pectin concentrations in which the synergistic interaction between the pulp components and the additives may increase the amount of free water available in the medium and therefore favor the freezing front mobility.
The results shown in Figs. 5 and 6 reveal that the addition of pectin significantly (P < 0.05) influenced the velocity of the increase in the frozen area. This increase was more pronounced with pectin concentrations of 0.6 g/100 mL to 1.0 g/100 mL and sucrose concentrations lower than 8 g/100 mL of pulp.
One of the properties of pectin is an ability to produce strong interactions in acidic pH and in the presence of sucrose because the formation of the pectin–sucrose chain is dependent on hydrogen bonds and hydrophobic interactions, which in turn are functions of temperature (Sato and Cunha 2007).
The interactions in the pectin–sucrose system are complex and depend strongly on temperature. Hydrogen bond interactions predominate at low temperatures, while hydrophobic interactions predominate at higher temperatures. Thus, depending on the concentration and temperature, the bonding zones are formed and stabilized by different contributions of hydrophobic interactions and hydrogen bonds, which can affect both the network structure and the rheological behavior (Brandão and Andrade, 1999). The intensity of these interactions depends on the sucrose concentration and an acidic environment and will be responsible for the greater or smaller amounts of free water available in the systems and for the lower or higher velocity of the freezing front advance.
Conclusions
Passion fruit pulps containing added pectin (in all combinations) and subjected to air–blast freezing at -20 °C demonstrated a pseudoplastic behavior before freezing and after thawing. The Ostwald-de Waele equation (power law) provided good fits to the experimental data for shear stress as a function of shear rate in the passion fruit pulp systems with added pectin and sucrose.
In this study, the pectin was solubilized in the passion fruit pulp at ambient temperature without previous thermal treatment. Potential gelation phenomena in these ambient conditions were not evaluated. A study of the rheological behavior involving oscillatory shear perturbations would be necessary to clarify this aspect.
However, the results show that the addition of pectin and sucrose to the passion fruit pulp at low pH influenced the rheological behavior of the systems before freezing and after thawing. The results show increases in the consistency index and reductions in the flow index after thawing compared to the unfrozen samples. The degree of variation in these parameters was dependent on the sucrose and pectin concentrations. This suggests that there are concentrations of these additives where hydrophobic interactions had increased the amounts of free water in the systems, thus favoring the freezing front advance. These results have applications in specifying the formulations of frozen products to which pectin may be added, for example, in sherbets and fruit ice pops, where an increase of viscosity during thawing is desirable.
Acknowledgments
The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG- Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES - Brazil) for financial support for this research.
References
- Blanshard JMV, Franks F. Ice Crystallization and its Control in Fozen-Food Systems. In: Blanshard JMV, Lilford P, editors. Food Structure and Behavior. 4. London: Academic; 1987. pp. 51–65. [Google Scholar]
- Bourne M (2002) Food Texture and Viscosity – Concept and Measurements. Food Sciences and Technology, International Series. Academic Press, London, UK.
- Brandão EM, Andrade CT. Influência de fatores estruturais no processo de gelificação de pectinas de alto teor de metoxilação. Polímeros: Ciência e Tecnologia. 1999;9(3):38–44. doi: 10.1590/S0104-14281999000300008. [DOI] [Google Scholar]
- Briggs JL, Steffe JF. Using Brookfield data and the Mitschka method to evaluate Power Law foods. Journal of Texture Studies. 1997;28(5):517–522. doi: 10.1111/j.1745-4603.1997.tb00134.x. [DOI] [Google Scholar]
- Cabral RAF, Orrego-Alzate CE, Gabas AL, Telis-Romero J. Rheological and thermophysical properties of blackberry. Ciência e Tecnologia de Alimentos. 2005;25(1):121–126. doi: 10.1590/S0101-20612005000100020. [DOI] [Google Scholar]
- Cabral MFP, Queiroz AJM, Figueiredo RMF. Comportamento reológico da polpa de cupuaçu (Theobroma grandiflorum Schum) peneirada. Revista Brasileira de Produtos Agroindustriais, Campina Grande. 2002;4(1):37–40. doi: 10.15871/1517-8595/rbpa.v4n1p37-40. [DOI] [Google Scholar]
- Conceição MC, Fernandes TN, Prado MET, Resende JV. Effect of sucrose and pectin addition on physical, chemical, thermal and rheological properties of frozen/thawed pineapple pulps. Korea-Australia Rheology Journal. 2012;24(3):229–239. doi: 10.1007/s13367-012-0028-8. [DOI] [Google Scholar]
- De Marchi R, Monteiro M, Benato EA, Silva CAR. Uso da cor da casca como indicador de qualidade do maracujá amarelo (Passiflora edulis Sims. f. flavicarpa Deg.) destinado à industrialização. Ciência e Tecnologia de Alimentos. 2000;20(3):381–387. doi: 10.1590/S0101-20612000000300017. [DOI] [Google Scholar]
- Fernandes TN, Resende JV, Cruvinel RSR, Reno MJ. Relação entre o comportamento reológico e dinâmica do congelamento e descongelamento de polpa de morango adicionada de sacarose e pectina. Ciência e Tecnologia de Alimentos. 2010;30(1):188–204. doi: 10.1590/S0101-20612010000100029. [DOI] [Google Scholar]
- Ferreira GM, Queiroz AJM, Conceição RS, Gasparetto CA. Efeito da temperatura no comportamento reológico das polpas de caju e goiaba. Revista Ciências Exatas e Naturais, Guarapuava. 2002;4(2):175–184. [Google Scholar]
- George RM. Freezing Processes Used in the Food Industry. Trends in Food Science & Technology. 1993;4(5):134–138. doi: 10.1016/0924-2244(93)90032-6. [DOI] [Google Scholar]
- Haminiuck CWI, Sierakowski MR, Branco IG, Maciel GM, Masson ML. Rheological study of ternary mixtures and pectic gels of red fruit pulps. International Journal of Food Science and Technology. 2007;42(6):629–639. doi: 10.1111/j.1365-2621.2006.01311.x. [DOI] [Google Scholar]
- Krokida, MK., Maroulis, ZB; Saravacos, GD (2001) Rheological properties of fluid fruit and vegetable puree products: compilation of literature data. International Journal of food properties v.4, n.2, p.179–200
- Pelegrine DH, Silva FC, Gasparetto CA. Rheological behavior of pineapple and mango pulps. LWT- Food Science and Technology. 2002;35(8):645–648. doi: 10.1006/fstl.2002.0920. [DOI] [Google Scholar]
- Raimundo K, Magri RS, Simionato EMRS, Sampaio AC. Avaliação física e química da polpa de maracujá congelada comercializada na região de Bauru. Revista Brasileira de Fruticultura. 2009;31(2):539–543. doi: 10.1590/S0100-29452009000200031. [DOI] [Google Scholar]
- Sato ACK, Cunha RL. Influência da temperatura no comportamento reológico da polpa de jabuticaba. Ciência e Tecnologia de Alimentos. 2007;27(4):890–896. doi: 10.1590/S0101-20612007000400033. [DOI] [Google Scholar]
- Sila DN, Van Buggenhout S, Duvetter T, Fraeye I, De Roeck A, Van Loey A, Hendrickx M. Pectins in processed fruits and vegetables: Part II – Structure- Function relationships. Comprehensive Reviews in Food Science and Food Safety. 2009;8(2):86–104. doi: 10.1111/j.1541-4337.2009.00071.x. [DOI] [Google Scholar]
- Silva FC, Guimarães DHP, Gasparetto CA. Reologia do suco de acerola: efeitos da concentração e temperatura. Ciência e Tecnologia de Alimentos. 2005;25(1):121–126. doi: 10.1590/S0101-20612005000100020. [DOI] [Google Scholar]
- Steffe JF. Rheological methods in food process engineering. 2. Michigan: Freeman Press; 1996. p. 418. [Google Scholar]
- Van Buggenhout S, Sila DN, Duvetter T, Van Loey A, Hendrickx M. Pectins in processed fruits and vegetables: Part III – Texture engineering. Comprehensive Reviews in Food Science and Food Safety. 2009;8(2):105–117. doi: 10.1111/j.1541-4337.2009.00072.x. [DOI] [Google Scholar]
- Wang CCH, Chang KC. Beet pulp and isolated pectin physicochemical properties as related to freezing. Journal of Food Science. 1994;9(6):113–1154. [Google Scholar]