Abstract
Reverse micellar extraction (RME) was used for the separation and purification of bromelain from pineapple core and efficacy of RME purified bromelain (RMEB) in tenderization of beef meat was compared with that of commercial stem bromelain (CSB). RME resulted in reasonably high bromelain activity recovery (85.0 %) and purification fold (4.0). Reduction in meat toughness was higher in RMEB treated meat (52.1 %) compared to raw (control) and CSB treated (26.7 %). Significant increase in water holding capacity (WHC) was observed in RMEB treated meat (91.1 %) as against CSB treated (55.6 %) and control (56.6 %). No change in cooking loss was observed in RMEB treated meat, whereas the loss increased by nearly 14.0 % in case of CSB treated. While the meat color was retained, trichloroacetic acid (TCA) soluble protein content increased due to hydrolysis of protein in RMEB treated meat. Scanning electron microscopy (SEM) analysis revealed that RMEB treatment completely ruptures myofibril tissues, indicating a higher degree of tenderization.
Keywords: Reverse micellar extraction, Pineapple waste, Bromelain, Commercial stem bromelain, Meat tenderization
Introduction
Demand for bromelain is increasing because of its wide range of application in various fields like medical, food and cosmetics. Bromelain is particularly useful for reducing muscle and tissue inflammation, as a digestive aid and dietary supplement. Besides pharmacological application, bromelain is also employed in food industries, namely, breweries, meat processing and baking industry (Feijoo-Siota and Villa 2011; Fileti et al. 2009; Hebbar et al. 2011).
Bromelain is generally classified as stem bromelain (EC. 3.4.22.32) and fruit bromelain (EC. 3.4.22.33) depending on its source, with all commercially available bromelain being derived from the stem (Rowan et al. 1990). While bromelain extracted from pineapple stem is commercially known, presence of bromelain is also reported in the core, peel and crown of pineapple fruit (Hebbar et al. 2008). However, there are hardly any reports on the extraction of fruit bromelain from pineapple wastes and, in-depth studies on its suitability for food and pharmaceutical application. Pineapple core, which is slightly harder in texture, is one of the major wastes generated (15 % of the fruit) by the pineapple processing industry (Chaurasiya and Hebbar 2013). The extraction and purification of the bromelain from core will add value to wastes generated by pineapple processing industry. Commercially, precipitation method is employed for the extraction and primary purification of bromelain from pineapple stem. Harrach et al. (1998) reported a process involving separation and lyophilisation for the preparation of bromelain powder (40 % protein) from pineapple juice.
Reversed micelles (RM) are thermodynamically stable, optically transparent, nanometer size droplets of an aqueous solution stabilized in an apolar environment by the surfactant present at the interface. Some of the advantages of RME are, no loss of native function/activity, low interfacial tension, ease of scale up, integration with conventional downstream processing techniques and potential for continuous operation (Hebbar et al. 2011; Hebbar et al. 2012; Krishna et al. 2002). Since proteins get solubilized inside reverse micelles along with the bulk aqueous phase, its structural integrity and bioactivity can be retained. Reverse micellar extraction of proteins/enzymes by phase separation method involves two characteristic steps, namely, selective solubilization of proteins into reverse micelles, termed as forward extraction and, successive release of proteins from forward extracted organic phase into the fresh aqueous phase, called as back extraction. By suitably modifying the extraction conditions, RME can be used for selective extraction and concentration of protein/enzymes.
Tenderness is one of the major factors contributing to consumers’ perception of meat taste and tenderized meat can fetch a better price in the market (Miller et al. 2001Savell et al. 1989. Exogenous enzymes originating from plants, bacteria, and fungal sources have been used for centuries to improve tenderness by proteolytic activity. United States federal agencies recognize five exogenous enzymes – papain, ficin, bromelain, Aspergillus oryzae protease, and Bacillus subtilis protease – as generally recognized as safe (GRAS) to improve meat tenderness. Each of these has been shown to have varying degrees of activity against myofibrillar and collagenous proteins (Sullivan and Calkins 2010).
The major objective of this work is to study the efficacy of RMEB, obtained from pineapple core for tenderization of beef meat and comparison of its efficacy with that of CSB. In this work, effect of RMEB and CSB on warner–bratzler shear force (WBSF), pH of meat extract, moisture content, cooking loss, WHC, color, TCA soluble peptides, water soluble proteins (WSP), salt soluble proteins (SSP), microstructure of tissues and hydrolysis of proteins in beef meat tenderization was studied.
Materials and Methods
Materials
Raw material
Partially ripe pineapple fruits (Kew variety) were purchased from the local market in Mysore. Fruits with almost same degree of maturity were purchased from a single supplier in order to minimize the variation in quality. Beef meat cuts from thigh was selected and purchased from the local market in Mysore. The superficial fatty tissues were removed before processing.
Chemicals
Cetyltrimethylammonium bromide (CTAB), tris base from Himedia Mumbai, 1-hexanol (for synthesis), ethylene diamine tetra acetic acid (EDTA) disodium salt, glutaraldehyde, ethanol and 1-butanol (GR grades) from MERCK Mumbai were purchased. Iso-octane (HPLC grade), casein (Hammerstein grade), polyvinylpyrolidone (PVP) K-250, coomassie brilliant blue (R250), bromophenol blue, mercaptoethanol, TCA and l-cysteine hydrochloride from SRL Mumbai were used. Stem bromelain (30–50 % protein), l-tyrosine, acrylamide, bisacrylamide, ammonium persulphate, glycine, sodium dodecyl sulphate (SDS), sucrose and bovine serum albumin (BSA) were purchased from Sigma chemicals. All other chemicals of AR grade were used for the experiments and analysis.
Methods
Extraction of bromelain from pineapple core
Pineapple was thoroughly washed with tap water before separating the crown and peel. The core portion of the fruit was scooped out from the fruit and used for the extraction of bromelain according to the procedure described in our previous paper (Chaurasiya and Hebbar 2013). Optimized conditions (extraction: 0.01 M sodium phosphate buffer of pH 7.0; at 25 ± 3 °C; and centrifugation at 25±1 °C) were used to obtain the crude extract which was subjected to RME.
Reverse micellar extraction
Reverse micellar extraction was performed by the method described by Hebbar et al. (2011) with slight modification. Forward extraction was carried out by contacting known volume (10 mL) of organic phase consisting of 150 mM CTAB/80 % (v/v) iso-octane/5 % (v/v) 1-hexanol/15 % (v/v) 1-butanol with an equal volume of aqueous phase (crude enzyme extract of pH 8.0 with 0.1 M NaCl). Back extraction was carried out by mixing the reverse micellar phase obtained from forward extraction with fresh aqueous phase (acetate buffer of pH 4.2 and 0.5 M KBr). For both forward and back extractions phases were mixed thoroughly using magnetic stirrer in a glass container for 60 min followed by phase separation by density difference in a pear shape separating funnel. The aqueous phases after forward and back extractions were analyzed for bromelain activity and protein content. The phase mixing and separation were carried out at room temperature (25 ± 3 ºC).
Removal of salts
Aqueous phase after RME was subjected to either dialysis or ultrafiltration in order to remove buffer salts (Potassium bromide and sodium acetate) that are added during RME. Dialysis was performed by using cellulose tubing (Himedia, Mumbai) of 12–14 kDa cut-off. Sample was dialyzed against triple distilled water for overnight at 4 ºC with continuous stirring. Ultrafiltration was performed by using lab scale, tangential flow filtration system (Millipore Corporation, Germany) by using 10 kDa cut-off cassette (Pellicon XL, Millipore Corporation, Germany). Ultrafiltration was carried out at 25 ± 3 ºC, at the rate of 1.5 ml/min of permeate (Hebbar et al. 2012). Concentrated retentate was freeze dried and used for tenderization studies. Aqueous phase obtained from RME, dialysis and ultrafiltration was used for the freeze drying (LT5S, Lyophilization Systems, Inc. U.S.A.). The aqueous phase was cooled to −30 °C and freeze dried to obtain powder.
Meat tenderization
Meat tenderization was performed by the dipping method described by Bhaskar et al. (2006) with slight modification. Meat was manually cut into small slices of 6x2x2 cm and used for the study. Known concentration (200 U/g of meat) of RMEB and CSB powder was reconstituted into triple distilled water and slices of meat were dipped in this solution. Meat and water ratio was maintained as 1:1 (w/v) for all the samples. Meat was incubated at room temperature (25 ± 3 ºC) for 2 h, and after incubation period samples were washed, drained and used for analysis. Triple distilled water was used as control.
Analysis
Protein content
Protein content in crude and at different stages of RME was determined by spectrophotometric method at 280 nm (UV-1800, Shimadzu corporation, Kyoto, Japan) using BSA as standard (Layne 1957) because CTAB interferes with other protein estimation methods which are based on color development. In this method, air was used as a blank. For the meat samples, Lowry’s method was used by using BSA as standard (Lowry et al. 1951).
Bromelain activity
Bromelain activity was determined according to the casein digestion unit (CDU) method using hammersten grade casein (0.6 %) as substrate in the presence of cysteine and EDTA (Murachi 1976). The assay was based on proteolytic hydrolysis of the casein substrate. Bromelain breaks peptide bonds and releases free amino acids after hydrolysis with protein. In this case, l-tyrosine is released from casein after hydrolysis with bromelain enzyme. Unhydrolyzed substrate is precipitated with TCA and precipitated proteins were removed using whatman No. 1 filter paper. The absorbance of the clear filtrate (solubilized casein) was measured at 275 nm using a spectrophotometer. Enzyme blank was used to avoid the interference by enzyme solution. In the case of blank reaction stopping reagent (TCA) was added in substrate before enzyme solution. So all the protein got precipitated, and there was no reaction of bromelain with substrate. One unit of bromelain activity is defined as 1 μg of l-tyrosine released in 1 min per ml of sample when casein is hydrolyzed under the standard conditions of 37 °C at pH 7.0 for 10 min.
The enzyme activity was calculated using the equation given below:
| 1 |
Where, Et, Eb and Es are absorbance of enzyme sample, enzyme blank and standard l-tyrosine, respectively. Df is the dilution factor; Vr is the reaction volume and tr is the reaction time.
All readings were measured in triplicates and an average value of two experiments were reported.
The activity recovery (%) and purification fold were calculated according to the following equations (Eqn. 2 and 3)-
| 2 |
| 3 |
Warner–Bratzler shear force
Warner–Bratzler shear force was determined for the treated and control meat slices according to the method described by Bhaskar et al. (2006) with slight modification. Instron Universal Testing Machine (LR5K, Lloyed Materials Testing, West Sussex, UK) equipped with a 50 N load cell and 1 mm thick blade was used. Meat slices were cut against the muscles orientation and shear force was measured from the average peak force of two pieces.
pH of the extract
pH of the treated and untreated samples were measured by the pH Tutor, Cyberscan (EuTech Instruments, Singapore). A known quantity (4 g) of minced meat was homogenized (Ultra-Turrax T25 digital homogenizer, IKA India Pvt. Ltd., Bangalore, India) for 2 min at 3 000 rpm with chilled triple distilled water, and pH values were measured for all the samples at room temperature (25 ± 3 ºC).
Microstructure of meat slices
Microstructure analysis of the samples (treated and untreated) was performed using SEM (LEO-435 VP, LEO Electron Microscopy Ltd. Cambridge, UK). Slices of 2–3 mm thick, taken from the inner portion of meat was treated with 2.5 % (v/v) glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.0) for 4 h. The samples were rinsed twice with triple distilled water for 30 min each. Rinsed samples were dehydrated in ethanol with serial dilution ranging from 40 %–100 % (v/v) and stored in 100 % ethanol at 4 ºC till used for SEM (Ketnawa and Rawdkuen 2011). Samples were vacuum dried and coated with gold for 2 min. A specimen was observed at 1000X by using SEM.
Trichloro acetic acid soluble peptides
The TCA-soluble peptides content of the meat slices was measured according to the procedure described by (Benjakul et al. 2002). Minced meat (2 g) was homogenized for 2 min at 3 000 rpm with 18 ml of 5 % TCA (w/v) solution. Homogenized sample was stored at 4 ºC for 1 h followed by centrifugation (Sorvall RC5B plus, Thermo Fisher Scientific (India) Pvt. Ltd. Mumbai, India) at 8 000 g for 15 min. The supernatant was used for the analysis of protein.
Water and salt soluble proteins
For water soluble proteins, 2 g of minced meat was homogenized for 2 min at 3 000 rpm with 15 ml of chilled triple distilled water and kept at 4 ºC for 16 h. The homogenized sample was centrifuged at 8 000 g for 10 min and the supernatant was used for protein content analysis. Pellet obtained after WSP extraction was used for the extraction of SSP. Pellets were homogenized for 2 min at 3 000 rpm with chilled 0.67 N NaCl and kept at 4 ºC for 16 h. Homogenized sample was centrifuged to collect the supernatant for the estimation of protein content (Joo et al. 1999). Water soluble protein samples were also analyzed by HPLC.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) with 12 % gel was performed according to the method described by Laemmli (1970) with slight modification using electrophoresis unit (Genei, Bangalore, India). Two grams of minced samples (treated and untreated) were homogenized at 3 000 rpm for 2 min with 5 % SDS (w/v) followed by incubation for 1 h at 85 ± 2 ºC in a water bath. To obtain the clear supernatant samples were centrifuged at 8 000 g for 15 min without temperature control.
Supernatants were mixed and boiled with the 5x sample buffer (0.5 M Tris–HCl, pH 6.8 containing 5 % (w/v) SDS, 50 % (w/v) sucrose, 2.5 % (v/v) βME and 0.05 % (w/v) bromophenol blue). Then the samples were loaded to the gel and continuous power (70 V) was supplied till the completion. Gel was stained for 12 h by using staining solution (0.1 % (w/v) Coomassie Brilliant Blue (CBB) R-250 in 40 % (v/v) methanol, 10 % (v/v) glacial acetic acid and 50 % (v/v) water). Destaining was also performed by the same composition (without CBB) until the clear background.
High performance liquid chromatography analysis of protein patterns.
High performance liquid chromatography (HPLC) (LC 10, Shimadzu Corporation, KYT, Japan) analysis was performed for the WSP samples using PDA detector at (280 nm). All samples (treated and untreated) were filtered by 0.2 μm filter, and 20 μl was used for injection. TSK-Gel column (G4000SW, Tosoh Bioscience, Germany) of 7.5 mm ID and 30 cm long with a packing of silica based material (particle size 4–25 μm) were used. Mobile phase used was 0.3 M NaCl in 0.1 M sodium phosphate buffer (pH 7.0) at the flow rate of 1 ml/min.
Moisture content.
Moisture content of the samples were determined according to the gravimetric method (AOAC 1995). A known amount (3 g) of minced meat was kept at 105 ± 2 ºC in a hot air oven till the constant weight. Ratio between weight loss during drying and initial weight was expressed as moisture content (Eqn. 4) of meat.
| 4 |
To determine the WHC filter paper press method was used (Grau and Hamm 1953). A small amount of minced meat (200–400 mg) was pressed on a filter paper between two clear plastic plates by hydraulic press (Cenco, Central scientific co., Chicago, U.S.A.). Each sample was pressed for 1 min at 500 psi pressure. Because of the external pressure water was squeezed out and absorbed by the filter paper and two rings of the free juice and meat film were formed. Area of both the film was measured by planimeter (Digiplan, F.W. Breithaupt & sons, Gmbh & co. KG, Germany). Water holding capacity was calculated according to the equations given below (Eqn. 5 and 6)-
| 5 |
| 6 |
Cooking loss was measured by the weight difference before and after cooking the meat. Known quantity of the meat (20 g) was weighed into polypropylene bag and subjected to steam cooking for 15 min. Cooked meat samples were cool to room temperature (25 ± 3 ºC) and wiped with the filter paper to remove excess water on the surface (Bhaskar et al. 2006). Ratio of difference weight to initial weight was expressed as cooking loss in percentage (Eqn. 7).
| 7 |
Color measurement
Color was measured by a Konica Minolta Spectrophotometer (CM-5, Konica Minolta Sensing Singapore Pvt. Ltd., Singapore). Each sample was placed at the light port at 2° and measured for color values in triplicates. The instrument was initially calibrated with a white standard plate. The CIE values are expressed in three coordinates, namely L*-values (lightness), a*-values (redness), b*-values (yellowness) (Scheier et al. 2014).
Statistical analysis
Statistical analysis was performed using t-test tool in Microsoft 2 013 for two samples assuming unequal variances. Samples were tested at 95 % (P < 0.05) confidence limit and significant differences are expressed by superscripts a, b and c.
Results and Discussion
Reverse micellar extraction of bromelain
Extract obtained from pineapple core employing the conditions indicated in materials and methods, had pH value 4.2, protein content 15.5 mg/g of core with bromelain activity of 162.0 U/g of core. This extract was subjected to RME using conditions optimized and described in our previous study (Chaurasiya and Hebbar 2013). Reverse micellar extraction facilitated the selective separation of bromelain from crude extract, which contains polyphenol oxidase, peroxidase and cellulase. Bromelain activity recovery and purification fold were found to be as 85.0 % and 4.0, respectively.
Warner–Bratzler shear force
Palatability or eating quality of meat is mainly related to tenderness, juiciness, and flavor or odour. In most countries, people want the meat to be tender, though in African countries, the preference is to have meat that is chewy. In this study, there was a significant effect of treatment with bromelain on tenderness of the meat (P <0.05). RMEB treated meat resulted in maximum reduction in WBSF (52.1 %) whereas reduction was 26.8 % in CSB treated compared to control (Table 1). The reduction is attributed to action of bromelain on meat tissue, increasing tenderness. Earlier reports indicated that the application of different protease enzymes including bromelain decreased the WBSF of beef meat by 7–21 % (Stefanek et al. 2002; Sullivan and Calkins 2010).
Table 1.
Warner-Bratzler shear force and pH of meat
| Samples | Reduction in Warner–Bratzler shear force (%) | pH of the meat extract |
|---|---|---|
| Control | 100 ± 4.50 | 6.1 ± 0.01 |
| Treated with CSB | 26.8 ± 4.02a | 5.8 ± 0.01a |
| Treated with RMEB | 52.1 ± 5.34a,b | 5.7 ± 0.01a |
Means ± SD (n = 2), P < 0.05, a: significant difference from control, b: significant difference from CSB treated sample
pH of the meat extract
The pH value of meat product is highly important because it has a major influence on WHC, tenderness, and juiciness (Bendall and Swatland 1988). The pH values for the control and treated meat are presented in Table 1. The pH value reduced significantly (P <0.05) from 6.1 to 5.8 after treatment with CSB and the maximum significant reduction (P <0.05) of pH (5.7) was observed for the RMEB treated meat. Similar results had been reported by the Ketnawa and Rawdkuen (2011) for the tenderization of different meats by bromelain. This decrease in pH could be because of the release of amino acids by the proteolytic activity of bromelain on meat proteins. Gill and Newton (1981) reported that pH value below 5.8 increased the shelf life of vacuum packed meat and attaining pH lower than the above threshold value in the present study could result in extension of shelf life of meat. Because less pH inhibits the growth of the microbial species, which are responsible for the “greening” and development of off flavor in the packed meat.
Microstructure of meat
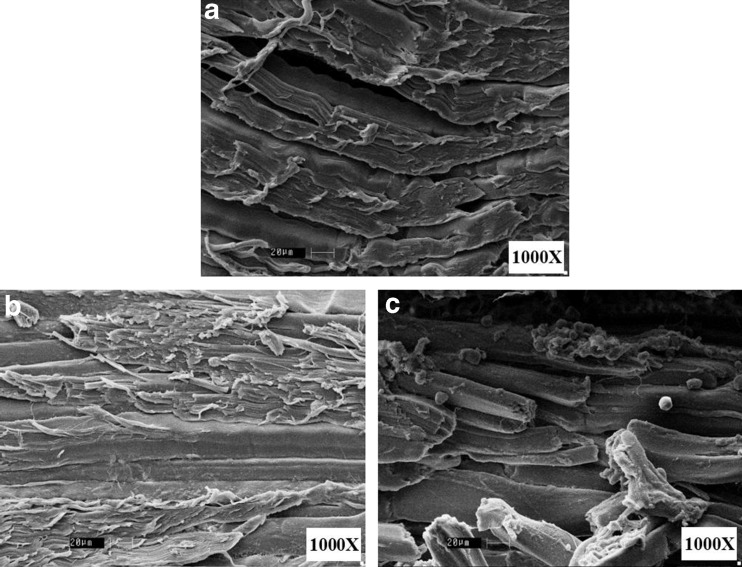
Microstructure of treated and untreated meat samples are shown in Fig. 1 (1000X). Well organized bundles of myofibril and sarcomere tissues are seen in the case of control (Fig. 1a). Myofibrils are long rod-like organelle found in skeletal and cardiac muscle that constitute approximately 80 % of the volume of the muscle cell. Individual myofibrils are made up of repeating sarcomeres, which works as basic functional unit for myofibrils. Contraction occurs via shortening of individual sarcomeres resulting in shortening of the overall myofibril and ultimately the muscle cell (Huff-Lonergan 2002). Whereas CSB treated samples shows the sharp cuts in the myofibril tissue bundles without any other effect like granulation or complete disruption of tissues (Fig. 1b). Complete rupturing of tissues was observed in RMEB treated meat and this could be attributed to higher degree of breaking down of myofibril tissues (Fig. 1c). Apart from the rupturing of tissues, granulation and aggregation of white color materials were also observed in case of RMEB treated meat that could be the collagen fibers (Fig. 2c). Chang et al. (2012) reported a significant change in collagenous fiber as denaturation, loos arrangement, granulation and aggregation of collagen fiber in the extracellular space, as a result of ultrasound treatment on beef muscles. These changes in collagen fiber were directly related to the texture of meat.
Fig. 1.
Microstructure of treated and untreated meat samples. a control, b treated with commercial stem bromelain, c treated with RME purified bromelain
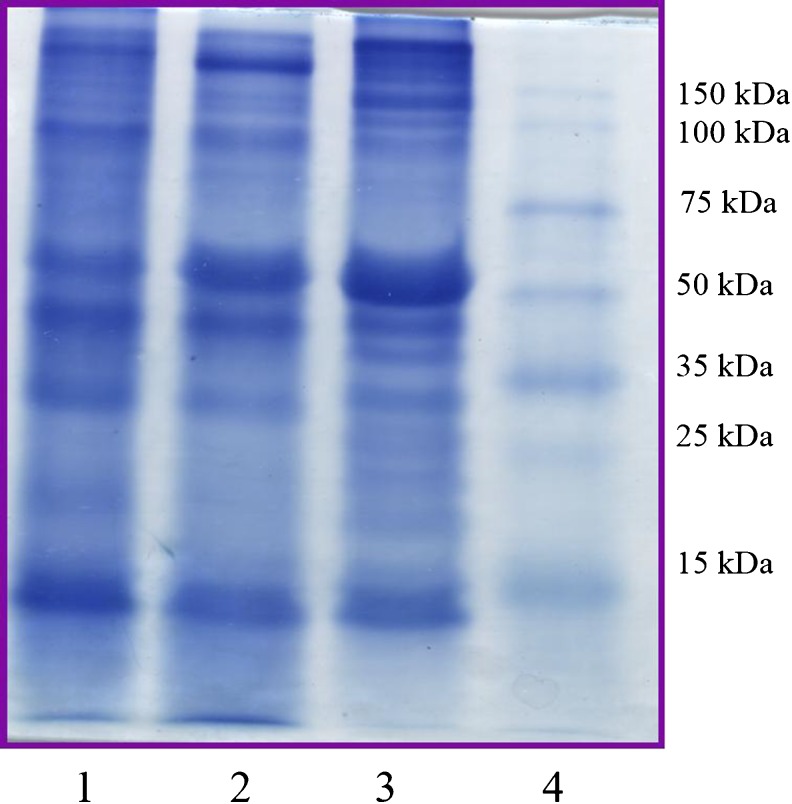
Fig. 2.
SDS-PAGE pattern of proteins in treated and untreated meat samples. Lane 1: treated with RME purified bromelain, Lane 2: treated with commercial stem bromelain, Lane 3: control, Lane 4: molecular weight marker
TCA soluble peptides
Table 2, shows the TCA soluble protein content in treated and untreated meat samples. The TCA soluble protein content increased (up to 5 fold) significantly (P <0.05) for both the treated samples as compared to control. A significant difference (P <0.05) between treated samples was observed with CSB treated meat having higher TCA soluble protein content (10.2 mg/g of meat) than that of RMEB treated meat (9.1 mg/g of meat). Ketnawa and Rawdkuen (2011) reported an increase of 27 % TCA soluble peptides when 20 % (w/w of meat) of the bromelain content was used for the treatment of chicken, beef and squid muscles. Results indicated that the high level of hydrolyzing activity of bromelain on muscle proteins, which was also reflected in SDS-PAGE analysis (Fig. 2) and HPLC chromatogram (Fig. 3).
Table 2.
Effect of tenderization on TCA, water and salt soluble proteins
| Samples | TCA soluble proteins (mg/g of meat) | Water soluble proteins(mg/g of meat) | Salt soluble proteins (mg/g of meat) |
|---|---|---|---|
| Control | 2.1 ± 0.13 | 19.4 ± 0.42 | 48.6 ± 1.27 |
| Treated with CSB | 10.2 ± 0.15 a | 27.7 ± 0.85 a | 51.1 ± 0.32 |
| Treated with RMEB | 9.1 ± 0.15 a,b | 31.4 ± 0.85 a | 49.6 ± 1.43 |
Means ± SD (n = 2), P < 0.05, a: significant difference from control, b: significant difference from CSB treated sample
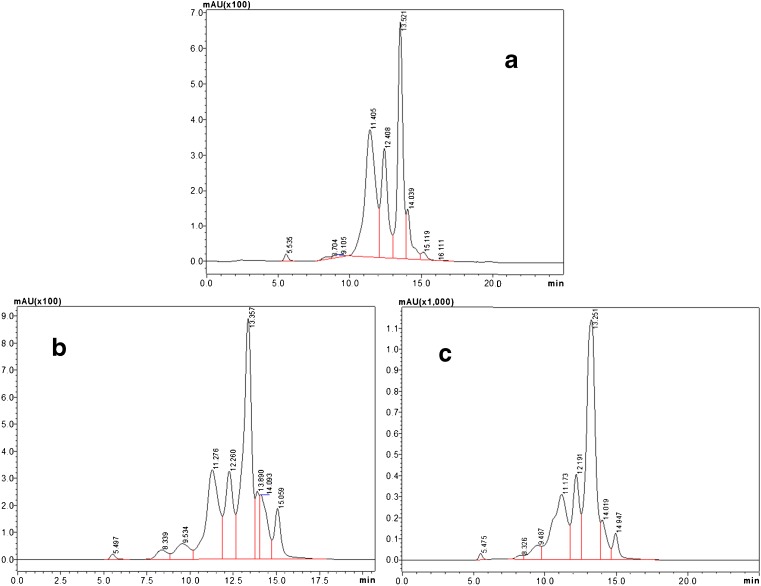
Fig. 3.
High performance liquid chromatogram for the water soluble proteins in treated and untreated meat samples. a control, b treated with commercial stem bromelain, c: treated with RME purified bromelain
Water and salt soluble proteins
Water and salt soluble proteins were analyzed to check the degree of proteolysis of meat proteins by CSB and RMEB. These treatments revealed that the bromelain has more impact on WSP than SSP (Table 2). Reverse micellar extraction purified bromelain had significant (P <0.05) effect on the WSP (31.4 mg/g of meat) than CSB (27.7 mg/g of meat), both of which were much higher than that of control (19.4 mg/g of meat). This result was in line with HPLC chromatograms (Fig. 3), where absorbance of the low MW protein increased in case of RMEB and CSB treated meat. Salt soluble proteins were used as an indicator of myofibrillar protein hydrolysis and results indicated a marginal rise in SSP content in RMEB treated meat (48.6 mg/g of meat) and CSB meat (51.1 mg/g of meat) over control (47.5 mg/g of meat). Sullivan and Calkins (2010) reported variation in WSP and SSP contents when meat was treated with different protease enzymes. In the present work the observed variation could be due to a different degree of effect of two types of bromelain on meat.
SDS-PAGE of meat extract
The SDS-PAGE electrophoresis was performed for the extract obtained from meat samples (Fig. 2). Fig. 2 shows the high intensity of major histocompatibility complex (MHC) band (above 150 kDa) in control whereas intensity has decreased in CSB and RMEB treated meat. Same trend was observed for the actin (45 kDa) also. Rawdkuen et al. (2011) reported that the intensity of high molecular weight proteins and number of bands decreased in Calotropis procerain protease tenderized beef, squid and giant catfish meat when compared to control.
Maximum degradation of MHC and actin proteins were observed in RME purified bromelain treated samples, which was clearly visible from the intensity and number of bands for small molecular weight proteins. Shin et al. (2008) reported degradation of MHC and actin proteins in beef meat when treated with papain and S. Aspratus extract (a plant protease). Gerelt et al. (2000), also reported the degradation of MHC protein into small molecular weight proteins of 140 and 90 kDa, in myofibrils treated with papain. Many researchers have reported a significant correlation between MHC fragments and tenderness of the meat, higher the fragments more will be the tenderness of meat (Sawdy et al. 2004; Shin et al. 2008).
High performance liquid chromatography of meat extract
There are only a few reports on establishing relationship between quality of meat and rate of proteolysis (Ketnawa and Rawdkuen 2011; Moya et al. 2001; Sawdy et al. 2004; Shin et al. 2008). For a better understanding and clarity about the hydrolysis of high MW proteins into low MW proteins, HPLC was performed for the WSP (Fig. 3). HPLC chromatograms indicated the cleavage of high MW proteins into low MW proteins which were supported by SDS-PAGE data (Fig. 2). Comparison was made on the basis of mili absorbance unit (mAU) for the three major peaks between 11.00-13.57 min retention times. Figure 3a shows the HPLC chromatogram of control sample, in which mAU of all three major peaks were found as 370, 320 and 675, respectively. For the CSB treated sample (Fig. 3b) the value of first peak decreased to 330, the third peak increased up to 890, while the second remained almost the same. In RMEB treated meat (Fig. 3c) the corresponding values were 305, 400 and 1150 mAU, respectively.Jorgova et al. (1989) reported a reduction in content of higher MW proteins and increase in low MW protein when bacterial proteolytic treatment was given to muscle. Bhaskar et al. (2006), also reported an increase in the level of low MW proteins by the proteolytic activity of ginger extract on high MW proteins in hen meat. Similar results have been observed in the present study.
Water holding capacity
Water holding capacity of the meat is an important property of meat, as it directly affects yield and quality of the meat, which has a direct bearing on economic value. Water holding capacity can be defined as the ability of meat to retain the inherent water even under external pressure. Juiciness of the meat is mainly related to the WHC of meat or low intramuscular fat level. In this study a significant (P <0.05) increase (91.1 %) was observed for the RMEB treated samples compared to CSB treated (55.6 %) and control (56.6 %) (Table 3). Similar increase in WHC (90.0 %) was observed by the Shin et al. (2008) when the meat samples were marinated with papain. Berge et al. (2001) reported that increase in WHC of the meat improved the tenderness of the meat for raw as well as cooked. This increase in WHC reduced the cooking loss as compared to CSB treated samples (Table 3). There are many other physical parameters such as color, pH, texture and firmness, which are partially dependent on WHC. Myofibrillar shrinkage/swelling and movement of water from the myofilament space into the extracellular space causes a reduction in WHC of meat. This study reflects the same trend as stated above. Huff-Lonergan and Lonergan (2005) reported that there are three main factors responsible for the shrinkage and/or swelled of the myofibrils: i) the onset of rigor mortis, ii) the extent of decline in pH and iii) protein fragmentation. This increase in WHC could be the combined effect of the pH decline (Table 1) and protein fragmentation (Fig. 3).
Table 3.
Effect of tenderization on initial moisture, free moisture, water holding capacity and cooking loss
| Samples | Initial moisture (%) | Free moisture after treatment (%) | Water holding capacity (%) | Cooking loss (%) |
|---|---|---|---|---|
| Control | 80.8 ± 0.02 | 43.4 ± 0.45 | 56.6 ± 0.45 | 49.7 ± 2.80 |
| Treated with CSB | 74.1 ± 0.13 a | 44.4 ± 0.01 | 55.6 ± 0.01 | 63.6 ± 1.81 a |
| Treated with RMEB | 77.1 ± 0.03 a,b | 8.9 ± 0.10 a,b | 91.1 ± 0.10 a,b | 47.2 ± 1.80 b |
Means ± SD (n = 2), P < 0.05, a: significant difference from control, b: significant difference from CSB treated sample
Cooking loss
Cooking loss of the treated and untreated samples is shown in the Table 3. There was no significant change in cooking loss in RMEB treated meat (47.2 %) compared to control (49.7 %), However significant difference was observed (P <0.05) between CSB treated (63.6 %) and control. High cooking loss could be because of the activity of CSB on hydrolysis of myofibril protein and providing more space for the movement of water and ultimately resulting in higher cooking loss. Whereas in RMEB treated meat, cooking loss has been reduced because of the higher level of WHC (Table 3). The probable reason for the above could be the presence of trace amount of salt (Potassium bromide 0.5 M) that comes along with bromelain during back extraction step. McGee et al. (2003) reported that when beef muscles were injected with the salt solutions such as sodium lactate, sodium tripolyphosphate and sodium chloride, cooking and reheating loss percentage decreased significantly. The cooking loss observed in the present study was much lower in case of RMEB treatment, than treatment of beef meat with bromelain extracted from peel reported by Ketnawa and Rawdkuen (2011).
Color of the meat
From the consumer acceptance point of view meat color is one of the major factors. Millions of dollars’ worth meat and meat product are being discarded because of discoloration of the meat, all over the world. In this study, a significant (P <0.05) decrease in CIE L* value was observed for the RMEB (31.8) treated sample compared to control (45.4). Pearson and Dutson (1985) reported that an increase in free water at the tissue surface could increase the surface lightness and lighter the appearance. Same results were observed for the control, and CSB treated samples where lightness of the meat increased (Table 4), and Table 3 supports the result because of high free moisture content. Whereas redness (CIE a* value) of the meat was increased in RMEB (9.5) treated meat compared to CSB (8.7) treated meat and control (7.9). Reducing activity of metmyoglobin can increase the color stability by reducing the metmyoglobin to myoglobin, and further oxidation of myoglobin maintains the red color of the meat. Apart from the reducing activity, in case of beef meat, lipid oxidation and pigment oxidation are also closely related to the meat discoloration (Faustman and Cassens 1990). pH also can influence the color stability of muscles. A small change in pH affects the stability of the meat, and lover pH favors the myoglobin oxidation. In this study lower pH was observed for the RME treated samples (Table 1) that could be a reason for stability of redness of the meat.
Table 4.
Effect of tenderization on color of meat
| Samples | L* | a* | b* |
|---|---|---|---|
| Control | 45.4 ± 0.34 | 7.9 ± 0.42 | 15.1 ± 0.34 |
| Treated with CSB | 42.4 ± 0.16 | 8.7 ± 0.32 | 13.7 ± 0.33 |
| Treated with RMEB | 31.8 ± 0.90 a,b | 9.5 ± 0.17 | 8.2 ± 0.54 a,b |
Means ± SD (n = 2), P < 0.05, a: significant difference from control, b: significant difference from CSB treated sample
Effect of salt in RMEB on tenderization of meat
As it was assumed from the results of WBSF, WHC, color and cooking loss, that the observed higher efficiency of RMEB is due to presence of salt in bromelain powder, dialysis and ultrafiltration was carried out after RME. McGee et al. (2003) reported that, injection of salt solution for tenderization of beef meat decreased the WBSF value and cooking loss and enhanced the sensory characteristics and color of the meat for consumer’s point of view. The present study revealed that the RMEB with salt reduced the WBSF up to 51.3 % whereas without salt (Dialyzed) reduced it to 29.4 % (Table 5) which is almost similar to CSB treated meat (Table 1). Similar trend was observed for WHC, color and cooking loss (Table 5 and 6). In the case of ultrafiltration, not much difference was observed in these parameters that could be because of the presence of salt in retentate of ultrafiltration, though in lesser quantity. This study proves that fruit bromelain, (without salt) purified by RME is equally effective as CSB but presence of salt increases the effectiveness of RMEB.
Table 5.
Effect of salt in RMEB on Warner-Bratzler shear force, pH and water holding capacity of meat
| Samples | Reduction in Warner–Bratzler shear force (percent) | pH of the meat extract | Water holding capacity (percent) |
|---|---|---|---|
| Control | 100 .0± 3.21 | 6.1 ± 0.01 | 50.6 ± 0.86 |
| RMEB | 51.3 ± 4.57 a,b | 5.8 ± 0.02 a | 88.7 ± 0.88 a,b |
| Dialyzed | 29.4 ± 3.96 a,c | 5.9 ± 0.01 a | 53.7 ± 0.60 |
| Ultrafiltered | 54.3 ± 6.01 a,b | 5.8 ± 0.01 a | 83.4 ± 0.25 a,b |
Means ± SD (n = 2), P < 0.05, a: significant difference from control, b: significant difference from dialyzed, c: Significant difference from RMEB, Note: All samples used in this study were RME purified followed by different operation before freeze drying
Table 6.
Effect of salt in RMEB on color of meat
| Samples | L* | a* | b* |
|---|---|---|---|
| Control | 46.0 ± 0.43 | 6.7 ± 0.11 | 13.0 ± 0.32 |
| RMEB | 34.1 ± 0.22 a,b | 8.5 ± 0.10 a,b | 10.7 ± 0.07 b |
| Dialyzed | 43.8 ± 0.28 a, | 7.5 ± 0.02 | 15.1 ± 0.29 a |
| Ultrafiltered | 37.1 ± 0.02 a,b,c | 7.7 ± 0.10 a,b | 12.2 ± 0.02 b,c |
Means ± SD (n = 2), P < 0.05, Means with different superscript are significantly different. All samples used in the study were purified followed by different operation before freeze drying
Conclusions
Reverse micellar extraction technique can be effectively used for the selective separation and purification of fruit bromelain from pineapple core. The purified bromelain obtained by this technique had good tenderization ability with meat showing better quality characteristics as compared to commercial stem bromelain. This study showed that there is good potential for value addition to pineapple wastes, particularly the core. Further studies are needed to characterize the RME purified bromelain, scale up of the process of extraction and also to extend the application to tenderization of meat from other sources.
Acknowledgments
The first author would like to thank CSIR, New Delhi, for the award of Senior Research Fellowship. Authors wish to thank Director, CSIR-CFTRI & Dr. KSMS Raghavarao, CFTRI for the encouragement and support.
Contributor Information
N. Bhaskar, Email: bhaskar@cftri.res.in
H. Umesh Hebbar, Phone: +9108212513910, Email: hebbar@cftri.res.in.
References
- AOAC . Official methods of analysis. Washington: Association of official analytical chemists; 1995. [Google Scholar]
- Bendall J, Swatland H. A review of the relationships of pH with physical aspects of pork quality. Meat Sci. 1988;24:85–126. doi: 10.1016/0309-1740(88)90052-6. [DOI] [PubMed] [Google Scholar]
- Benjakul S, Visessanguan W, Riebroy S, Ishizaki S, Tanaka M. Gel‐forming properties of surimi produced from bigeye snapper, Priacanthus tayenus and P. macracanthus, stored in ice. J. Sci Food Agr. 2002;82:1442–1451. doi: 10.1002/jsfa.1207. [DOI] [Google Scholar]
- Berge P, Ertbjerg P, Larsen LM, Astruc T, Vignon X, Møller AJ. Tenderization of beef by lactic acid injected at different times post mortem. Meat Sci. 2001;57:347–357. doi: 10.1016/S0309-1740(00)00110-8. [DOI] [PubMed] [Google Scholar]
- Bhaskar N, Sachindra N, Modi V, Sakhare P, Mahendrakar N. Preparation of proteolytic activity rich ginger powder and evaluation of its tenderizing effect on spent-hen muscles. J Muscle Foods. 2006;17:174–184. doi: 10.1111/j.1745-4573.2006.00043.x. [DOI] [Google Scholar]
- Chang H-J, Xu X-L, Zhou G-H, Li C-B, Huang M. Effects of characteristics changes of collagen on meat physicochemical properties of beef semitendinosus muscle during ultrasonic processing. Food Bioprocess Tech. 2012;5:285–297. doi: 10.1007/s11947-009-0269-9. [DOI] [Google Scholar]
- Chaurasiya RS, Hebbar UH. Extraction of bromelain from pineapple core and purification by RME and precipitation methods. Sep Purif Technol. 2013;111:90–97. doi: 10.1016/j.seppur.2013.03.029. [DOI] [Google Scholar]
- Faustman C, Cassens R. The biochemical basis for discoloration in fresh meat: A review. J Muscle Foods. 1990;1:217–243. doi: 10.1111/j.1745-4573.1990.tb00366.x. [DOI] [Google Scholar]
- Feijoo-Siota L, Villa T. Native and biotechnologically engineered plant proteases with industrial applications. Food Bioprocess Tec. 2011;4:1066–1088. doi: 10.1007/s11947-010-0431-4. [DOI] [Google Scholar]
- Fileti AMF, Fischer GA, Santana JCC, Tambourgi EB. Batch and continuous extraction of bromelain enzyme by reversed micelles. Braz Arch Biol Techn. 2009;52:1225–1234. doi: 10.1590/S1516-89132009000500021. [DOI] [Google Scholar]
- Gerelt B, Ikeuchi Y, Suzuki A. Meat tenderization by proteolytic enzymes after osmotic dehydration. Meat Sci. 2000;56:311–318. doi: 10.1016/S0309-1740(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Gill C, Newton K (1981) Microbiology of DFD beef. In: Hood DE and Tarrant P V (ed) The problem of dark-cutting in beef. Martinus Nijhoff, Den Haag, pp 305–327
- Grau R, Hamm R. Eine einfache methode zur bestimmung der wasserbindung im muskel. Naturwissenschaften. 1953;40:29–30. doi: 10.1007/BF00595734. [DOI] [Google Scholar]
- Harrach T, Eckert K, Maurer HR, Machleidt I, Machleidt W, Nuck R. Isolation and characterization of two forms of an acidic bromelain stem proteinase. J Protein Chem. 1998;17:351–361. doi: 10.1023/A:1022507316434. [DOI] [PubMed] [Google Scholar]
- Hebbar HU, Hemavathi A, Sumana B, Raghavarao KSMS. Reverse micellar extraction of bromelain from pineapple (Ananas comosus L. Merryl) waste: scale-up, reverse micelles characterization and mass transfer studies. Separ. Sci. Technol. 2011;46:1656–1664. [Google Scholar]
- Hebbar UH, Sumana B, Raghavarao KSMS. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresource Technol. 2008;99:4896–4902. doi: 10.1016/j.biortech.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Hebbar HU, Sumana B, Hemavathi A, Raghavarao KSMS. Separation and purification of bromelain by reverse micellar extraction coupled ultrafiltration and comparative studies with other methods. Food Bioprocess Tech. 2012;5:1010–1018. doi: 10.1007/s11947-010-0395-4. [DOI] [Google Scholar]
- Huff-Lonergan E (2002) Water-holding capacity of fresh meat. Fact Sheet (04669). National pork board, Des Moines, IA.
- Huff-Lonergan E, Lonergan SM. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Joo S, Kauffman R, Kim B, Park G. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999;52:291–297. doi: 10.1016/S0309-1740(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Jorgova V, Danchev S, Kostov A (1989) Effect of bacterial enzyme preparation on the solubility and electrophoretic properties of muscle proteins. Proceedings International Congress of Meat Science and Technology. pp 913–917
- Ketnawa S, Rawdkuen S. Application of bromelain extract for muscle foods tenderization. Food Nutri Sci. 2011;2:393–401. doi: 10.4236/fns.2011.25055. [DOI] [Google Scholar]
- Krishna SH, Srinivas N, Raghavarao K, Karanth N (2002) Reverse micellar extraction for downstream processing of proteins/enzymes. In: Scheper T (ed) History and Trends in Bioprocessing and Biotransformation (Advances in Biochemical Engineering/Biotechnol. Vol. 75). Springer Verlag, Berlin, pp 119–183 [DOI] [PubMed]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage. T4 Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layne E. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 1957;3:447–454. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McGee M, Henry K, Brooks J, Ray F, Morgan J. Injection of sodium chloride, sodium tripolyphosphate, and sodium lactate improves Warner–Bratzler shear and sensory characteristics of pre-cooked inside round roasts. Meat Sci. 2003;64:273–277. doi: 10.1016/S0309-1740(02)00189-4. [DOI] [PubMed] [Google Scholar]
- Miller M, Carr M, Ramsey C, Crockett K, Hoover L. Consumer thresholds for establishing the value of beef tenderness. J Anim Sci. 2001;79:3062–3068. doi: 10.2527/2001.79123062x. [DOI] [PubMed] [Google Scholar]
- Moya V-J, Flores M, Aristoy M, Toldra F. Pork meat quality affects peptide and amino acid profiles during the ageing process. Meat Sci. 2001;58:197–206. doi: 10.1016/S0309-1740(00)00152-2. [DOI] [PubMed] [Google Scholar]
- Murachi T. Bromelain Enzymes. In: Lorand L, editor. Methods in Enzymology Vol. New York: XLV. Academic Press; 1976. pp. 475–485. [DOI] [PubMed] [Google Scholar]
- Pearson AM, Dutson TR. Scientific basis for electrical stimulation. In: Pearson AM, Dutson TR, editors. Advances in Meat Research. Berlin: Springer Verlag; 1985. pp. 185–218. [Google Scholar]
- Rawdkuen S, Pintathong P, Chaiwut P, Benjakul S. The partitioning of protease from Calotropis procera latex by aqueous two-phase systems and its hydrolytic pattern on muscle proteins. Food Bioprod Process. 2011;89:73–80. doi: 10.1016/j.fbp.2010.02.001. [DOI] [Google Scholar]
- Rowan AD, Buttle DJ, Barrett AJ. The cysteine proteinases of the pineapple plant. Biochem J. 1990;266:869–875. [PMC free article] [PubMed] [Google Scholar]
- Savell J, Cross H, Francis J, Wise J, Hale D, Wilkes D, Smith G. National consumer retail beef study: Interaction of trim level, price and grade on consumer acceptance of beef steaks and roasts. J Food Quality. 1989;12:251–274. doi: 10.1111/j.1745-4557.1989.tb00328.x. [DOI] [Google Scholar]
- Sawdy J, Kaiser S, St-Pierre N, Wick M. Myofibrillar 1-D fingerprints and myosin heavy chain MS analyses of beef loin at 36 h postmortem correlate with tenderness at 7 days. Meat Sci. 2004;67:421–426. doi: 10.1016/j.meatsci.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Scheier R, Bauer A, Schmidt H. Early postmortem prediction of meat quality traits of porcine semimembranosus muscles using a portable raman system. Food Bioprocess Tech DOI. 2014 [Google Scholar]
- Shin H-G, Choi Y-M, Kim H-K, Ryu Y-C, Lee S-H, Kim B-C. Tenderization and fragmentation of myofibrillar proteins in bovinelongissimus dorsi muscle using proteolytic extract from Sarcodon aspratus. Food Sci. Technol LEB. 2008;41:1389–1395. doi: 10.1016/j.lwt.2007.08.019. [DOI] [Google Scholar]
- Stefanek J, Scanga J, Belk K, Smith G (2002) Effects of enzymes on beef tenderness and palatability traits. Colorado State University Animal Science, Department of Animal Science 61–66
- Sullivan GA, Calkins C. Application of exogenous enzymes to beef muscle of high and low-connective tissue. Meat Sci. 2010;85:730–734. doi: 10.1016/j.meatsci.2010.03.033. [DOI] [PubMed] [Google Scholar]