Abstract
Wheat germ oil (WGO) is beneficial for health since it is a rich source of omega-3, omega-6 and tocopherol. However, as it contains polyunsaturated fatty acids, it is prone to oxidation. The aim of this study was to encapsulate wheat germ oil and determine the effects of core to coating ratio, coating materials ratio and ultrasonication time on particle size distribution of emulsions and encapsulation efficiency (EE) and surface morphology of capsules. Maltodextrin (MD) and whey protein concentrate (WPC) at different ratios (3:1, 2:2, 1:3) were used as coating materials. Total solid content of samples was 40 % (w/w). Five core to coating ratios (1:8, 1:4, 1:2, 3:4, 1:1) were tried. Ultrasound was used at 320 W and 20 kHz for 2, 5, 10 min to obtain emulsions. Then, emulsions were freeze dried to obtain microcapsules. It was observed that, increasing WPC ratio in the coating resulted in higher encapsulation efficiency and smaller particle size. Microcapsules prepared with MD:WPC ratio of 1:3 were found to have higher EE (74.35–89.62 %). Increase in oil load led to decrease in EE. Thus 1:8 core to coating ratio gave better results. Increasing ultrasonication time also had a positive effect on encapsulation efficiency.
Keywords: Encapsulation efficiency, Microencapsulation, Wheat germ oil, Ultrasonication
Introduction
Functional foods can be defined as foods that contain bioactive compounds which have certain health benefits or potential to reduce the risk of some diseases. There is an increasing public demand to consume functional foods (Mark-Herbert 2004; Menrad 2003).
Wheat germ oil, which is derived from the germ part of wheat, contains omega-6 (between 44 and 65 %), omega-3 (in a lower proportion, 4–11 %) fatty acids and it is known to have the highest tocopherol content among other edible oils (Megahad and El Kinawy 2002; Wang and Johnson 2001). Due to its healthy ingredients, wheat germ oil can be incorporated into bread or cake formulations and consumed as a functional food.
However, high content of polyunsaturated fatty acids makes wheat germ oil highly prone to oxidation (Megahed 2011). Therefore, it is hard to consume oil in its free form. Oxidation can be prevented by using antioxidants. However, antioxidants may impart unacceptable color, taste and flavor to the product. Therefore, microencapsulation of oil may overcome this problem. By means of microencapsulation, oil is covered with coating material and thus it is expected to be protected from external factors such as light, heat, pH, etc. (Augustin and Hemar 2009).
Generally, microencapsulation consists of two main steps; emulsification and drying. More homogeneous emulsion can be obtained by using ultrasound as compared to mechanical process (Abismail et al. 1999) and ultrasound has been applied to different products successfully at laboratory scale (Wu et al. 2000; Mongenot et al. 2000; Kentish et al. 2008). For drying, spray and freeze drying methods are commonly used. Since freeze drying is applied at very low temperatures (e.g. −45 °C) and water is removed from the system by sublimation of ice using vacuum, oxidation and other chemical changes are limited (Longmore 1971).
Choosing suitable wall material for a specific core material is an important step in microencapsulation. Wall materials generally used for microencapsulation consist of carbohydrates which are modified starches, maltodextrins, cellulose derivatives and different gum types. Protein group used as wall material includes gluten, whey protein, caseinate and gelatin. Maltodextrins in combination with other materials have been preferred for encapsulation of oils (Kagami et al. 2003; Sheu and Rosenberg 1998; Toure et al. 2007) because it gives structural integrity to encapsulated powder, protects oil against oxidation and has low viscosity at high solids level. However, it lacks emulsifying property (Klinkesorn et al. 2004). Whey protein concentrate may be mixed with maltodextrin to obtain a good emulsion. Both whey protein concentrate and whey protein isolate have been used in encapsulation studies due to their surface active properties (Kim and Morr 1996; Hogan et al. 2001; Parthasarathi et al. 2013).
Wheat is one of the leading grain crops produced, consumed, and traded worldwide. In Turkey, wheat is the most produced grain and nearly 21,800,000 t of wheat was produced in 2011. During wheat milling process, wheat germ is separated from wheat and it is not commonly utilized as a part of daily diet. Encapsulation would enable wheat germ oil to be incorporated into food products and consumption of oil would be more common. However, there is no study in literature about the encapsulation of wheat germ oil.
The objective of this study is to encapsulate wheat germ oil by ultrasonication and freeze drying methods. The effects of different core to coating ratios, maltodextrin and whey protein concentrate ratios and ultrasonication time on the particle size distribution of emulsions and encapsulation efficiency and surface morphology of the encapsulated powder were studied.
Materials & methods
Materials
Whey protein concentrate (WPC) containing 80 % protein was purchased from Tunçkaya Kimyevi Maddeler (Tuzla, İstanbul). Maltodextrin (MD) having dextrose equivalent (DE) value of 4.5–8.5 (C*Dry MD 01955™) was obtained from Cargill Foods (Istanbul, Turkey) and wheat germ oil was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Hexane, hydrochloric acid, sodium hydroxide, dipotassium phosphate and potassium dihydrogen phosphate were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Merck Chemical Companies (Deisenhofen, Germany). They were analytical grade.
Encapsulation process
Encapsulation process was mainly composed of four main steps which were coating material preparation, coarse emulsion preparation, ultrasonication and freeze drying of emulsion.
Preparation of coating material solution
Hydrated solutions of MD at different concentrations (10 %, 20 %, 30 % by weight) were prepared by mixing MD with distilled water using magnetic stirrer (Heidolph MR 3001 K, Heidolph Instruments GmbH & Co, Schwabach, Germany). In the case of WPC, their solutions were prepared by dispersing the necessary amount of their powder into buffer solution (5 mM phosphate buffer, pH 7) to obtain different concentrations (10 %, 20 %, 30 % by weight) using magnetic stirrer. Mixing continued until the materials were dissolved. Then, pH of WPC solution was adjusted to 7.0 using 1 M HCl and 1 M NaOH if required.
MD and WPC solutions were prepared 1 day before the preparation of emulsion and kept for one night in a shaking water bath (GFL 1086, Burgwedel, Germany) at 25 °C and 90 rpm.
Coarse emulsion preparation
In order to get a stable emulsion with ultrasound, coarse emulsion preparation is a crucial step. Therefore, after preparation of coating material solutions separately, a pre-emulsion must be formed by mixing equal amounts (by weight) of MD and whey protein concentrate solutions at the required concentrations to obtain total solids content of 40 % (w/w) and MD:WPC ratios of 1:3, 2:2 and 3:1. Wheat germ oil is then added to obtain the desired core to coating ratios of 1:8, 1:4, 1:2, 3:4, 1:1 at 5,000 rpm and for 5 min using high-speed blender (IKA T25 digital Ultra-Turrax, Selangor, Malaysia). Mixing of 100 g solution was performed in 250 ml beaker.
Ultrasonication
Ultrasonic homogenizer (Sonic Ruptor 400, OMNI International the Homogenizer Company, GA, USA) equipped with titanium probe with diameter 25.4 mm was used for emulsification. Ultrasonic homogenization of 100 g solution was performed in 250 ml beaker using 80 % power for different times (2 min, 5 min, 10 min).
Freeze drying
Immediately after emulsification with ultrasound, samples were transferred to 100 ml beakers half fully and placed into freezer at about −4 C°. Frozen samples were then dried under vacuum at −50 C° and 0.007 atm for 48 h. After freeze drying process, sample was grinded by using a glass rod in order to transform it to powder form.
Particle size analysis of emulsions
Particle size analysis was performed for emulsions by laser light scattering method using Mastersizer 2000 (Malvern Instruments Limited, Worcestershire, UK). Particle refractive index value was 1.52 and dispersant refractive index value was 1.33. The absorption index was 0.1 for equipment during the measurement.
The particle size was described as surface-weighted mean diameter, D[3,2]. It was calculated according to Eq. (1). Width of the particle size distribution curve was defined by span and it was calculated from Eq. (2).
| 1 |
| 2 |
where, ni is the number and di is the diameter of particles; d(v, 90), d(v, 10), and d(v, 50) are diameters at 90 %, 10 %, and 50 % of cumulative volume, respectively. In other words, [d(v, 90) − d(v, 10)] is the range of the data and d(v, 50) is the median diameter.
Analysis of encapsulated powder
Encapsulation efficiency analysis
Encapsulation efficiency analysis was adapted from Millqvist-Fureby (2003). In order to calculate surface oil content, 5 g of powder was mixed with 50 ml n-hexane and shaken using magnetic stirrer at 200 rpm for 60 s. This suspension was allowed to stand for 10 min and then filtered through filter paper (No. 41, Whatman, Maidstone, UK). Then, the powder residue was washed with 2 × 5 ml hexane. Extracted oil was transferred to a beaker and solvent was evaporated under the fume hood. After evaporation of solvent, beaker was dried in an oven at 105 °C until constant weight (about 1 h) was reached. Weight of beaker containing extracted oil residue was subtracted from weight of initial clean and dry beaker and thus extracted oil amount was calculated. Encapsulation efficiency (EE) was calculated by the following equation:
| 3 |
Where, TO is the total amount of oil present in the encapsulated powder and SO is the amount of oil on the surface of the capsules.
Surface morphology of capsules
Scanning electron microscope was used to investigate the surface morphology and the microstructural properties of the freeze dried encapsulated powders. Samples were coated with a very thin layer of a gold-palladium (Au-Pd) alloy using HUMMLE VII Sputter Coating Device (ANATECH, Union city, CA, USA). Coated samples were analyzed using scanning electron microscopy (SEM) (JSM-6400 Electron Microscope, Jeol Ltd., Tokyo, Japan) equipped with NORAN System 6 X-ray Microanalysis System and Semafore Digitizer and operating at two different voltages which were 10 kV and 20 kV. Images were taken at ×50 magnification. The images of the encapsulated powders at microscopic scale were taken by the equipment’s software installed on a computer connected to the system.
Statistical analysis
The effects of MD:WPC ratio, core to coating ratio, and ultrasonication time on encapsulation efficiency and particle size were determined by using analysis of variance (ANOVA) using SAS software version 9.1 (SAS Institute Inc., NC, USA). If results were significantly different from each other, Duncan’s Multiple comparison test was used for comparisons (p ≤ 0.05). All the results were the average of two replications.
Results and discussion
In the first part of the study, five different core to coating ratios were tried which were 1:8, 1:4, 1:2, 3:4, 1:1. As coating material, MD and WPC mixed at different ratios, which were 3:1, 2:2 and 1:3, were used. For these experiments ultrasound power was 80 % and ultrasonication time was 2 min. After selecting the samples that had higher encapsulation efficiency, the effects of ultrasonication time (2 min, 5 min and 10 min) on encapsulation efficiency, particle size distribution and surface morphology were also studied in the second part of the study.
Encapsulation efficiency of encapsulated powder
Increase in the concentration of WPC, decreased surface oil content (Table 1) which means increased encapsulation efficiency (Fig. 1). Coating formulation that had higher WPC had better encapsulating property which can be due to good emulsifying property of WPC. Similarly, Jimenez et al. (2006) found that the surface oil contents of microcapsules prepared with gum arabic and blend of WPC and MD were higher than those prepared with WPC only. This result showed that WPC provided better encapsulation efficiency values compared to gum arabic and the blend of WPC and MD.
Table 1.
Surface oil content (g/g) of capsules prepared with different maltodextrin (MD): whey protein concentrate (WPC) ratios in the coating formulation and different core to coating ratios when 2 min ultrasonication was used in preparation of emulsions
| MD:WPC | Core:coating | Surface oil content (g/g) |
|---|---|---|
| 3:1 | 1:8 | 0.20 |
| 3:1 | 1:4 | 0.37 |
| 3:1 | 1:2 | 0.77 |
| 3:1 | 3:4 | 1.23 |
| 3:1 | 1:1 | 1.51 |
| 2:2 | 1:8 | 0.15 |
| 2:2 | 1:4 | 0.45 |
| 2:2 | 1:2 | 0.96 |
| 2:2 | 3:4 | 1.30 |
| 2:2 | 1:1 | 1.59 |
| 1:3 | 1:8 | 0.09 |
| 1:3 | 1:4 | 0.20 |
| 1:3 | 1:2 | 0.80 |
| 1:3 | 3:4 | 1.28 |
| 1:3 | 1:1 | 1.56 |
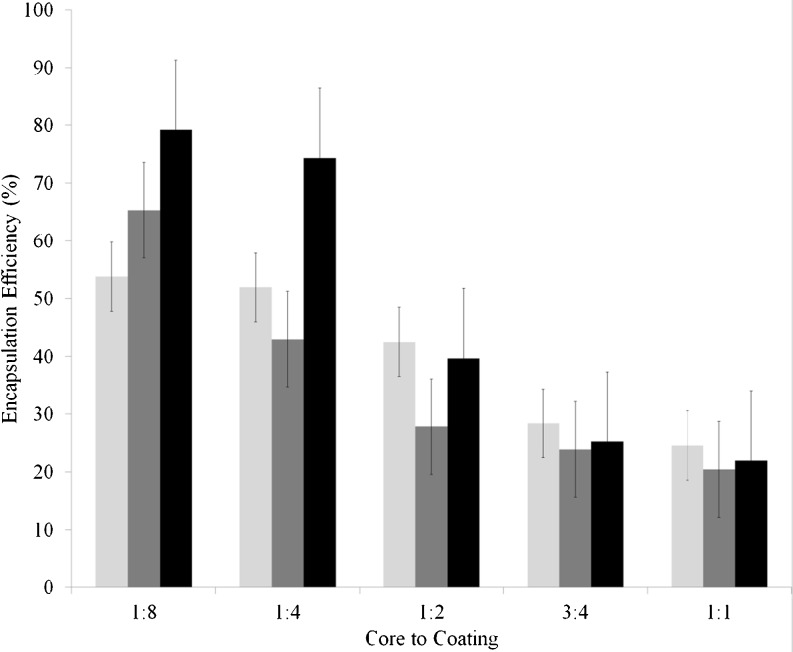
Fig. 1.
Variation of encapsulation efficiency of microcapsules prepared with 2 min ultrasonication as a function of core:coating ratio and MD:WPC ratio; light grey: MD:WPC ratio of 3:1, dark grey: MD:WPC ratio of 2:2, black: MD:WPC ratio of 1:3
It can be seen from Table 1 that as core to coating ratio increased, surface oil content increased for constant MD:WPC ratio which implied that EE decreased. It can be said that as the amount of oil increases, encapsulation becomes more difficult and more surface oil exists on encapsulated powder and thus EE decreases. This can also be seen in Fig. 1. The effect of core to coating ratio on encapsulation efficiency was statistically significant. The highest EE values (53.8 %, 65.35 % 79.2 %, for MD:WPC ratio of 3:1, 2:2 and 1:3, respectively) were obtained when core to coating ratio was 1:8. Similarly, in their study, Hogan et al. (2001) revealed that increasing soy oil: sodium caseinate ratio from 0.25 to 3.0 led to a decrease in encapsulation efficiency from 89.2 to 18.8 % during spray drying. Also, Tan et al. (2005) and Tonon et al. (2011) found that increasing oil load had a negative effect on microencapsulation efficiency. In consideration of these results, it would be preferable to keep oil in lower amounts in order to obtain higher encapsulation efficiency.
Encapsulation efficiency was quite satisfactory also when core to coating ratio was 1:4 and MD:WPC ratio was 1:3 (74.35 %) (Fig. 1). In the second part of the study, among 15 different formulations, core to coating ratio of 1:8 for all MD:WPC ratios and core to coating ratio of 1:4 for MD:WPC ratio of 1:3 which had the highest encapsulation efficiency values were chosen and the effects of longer ultrasonication time on encapsulation efficiency were studied.
In general, increasing ultrasonication time up to 10 min had a positive effect on encapsulation efficiency and it was seen that the effect of ultrasonication time was dependent on the composition of powders (Table 2).
Table 2.
Effect of ultrasonication time on encapsulation efficiency values
| MD:WPC | Core:coating | Ultrasonication time (min) | Encapsulation efficiency (%) |
|---|---|---|---|
| 3:1 | 1:8 | 2 | 53.80 Ea |
| 3:1 | 1:8 | 5 | 51.29 E |
| 3:1 | 1:8 | 10 | 75.10 BCD |
| 2:2 | 1:8 | 2 | 65.35 D |
| 2:2 | 1:8 | 5 | 70.30 CD |
| 2:2 | 1:8 | 10 | 81.59 ABC |
| 1:3 | 1:8 | 2 | 79.20 ABC |
| 1:3 | 1:8 | 5 | 85.56 AB |
| 1:3 | 1:8 | 10 | 85.37 AB |
| 1:3 | 1:4 | 2 | 74.35 BCD |
| 1:3 | 1:4 | 5 | 77.54 BC |
| 1:3 | 1:4 | 10 | 89.62 A |
aEncapsulation efficiency values having different letters are significantly different (p ≤ 0.05)
According to one way ANOVA, it was seen that for MD:WPC ratio of 3:1 and core to coating ratio of 1:8, 2 min and 5 min ultrasound applications were not significantly different but 10 min provided higher encapsulation efficiency value (Table 2). Cilek et al. (2012) studied microencapsulation of phenolic compounds by using ultrasonication and found that samples prepared by 5 and 10 min ultrasonication had lower encapsulation efficiency as compared to samples prepared by 15, 20 and 25 min of ultrasonication.
In the case of MD:WPC ratio of 2:2, core to coating ratio of 1:8 and MD:WPC ratio of 1:3 and core to coating ratio of 1:4, 2 min and 10 min ultrasonication times were significantly different from each other with respect to their effect on encapsulation efficiency. On the other hand, ultrasonication time had no significant effect on encapsulation efficiency when MD:WPC ratio of 1:3 and core to coating ratio of 1:8 was used in encapsulation.
Increasing concentration of WPC in coating, increased encapsulation efficiency when 2 min or 5 min ultrasonication was used (Table 2). However, increasing the amount of WPC in coating formulation had no significant effect on encapsulation efficiency when 10 min ultrasonication was applied.
Particle size distribution of emulsions
Particle size of emulsions is an important aspect for retention of core material within the capsules. Therefore, this analysis was performed in emulsions. Particle size distribution of emulsions were compared in terms of Sauter mean diameter (D[3,2]) and span. Effect of different ultrasonication times on particle size can be analysed when the same coating formulation and core to coating ratios are used in encapsulation. For MD:WPC ratio of 3:1 and core:coating ratio of 1:8 mean particle size expressed as Sauter mean diameter (D[3,2]) was larger for 2 min ultrasound application although there was no significant difference between 5 and 10 min ultrasonication (Table 3). In the case of MD:WPC ratio of 2:2 and core:coating ratio of 1:8, the same trend was observed. For MD:WPC ratio of 1:3, in the case of core:coating ratio of both 1:4 and 1:8, there was significant difference between D[3,2] values of encapsulated products for different ultrasonication times and as time increased mean particle size decreased. In Fig. 2, the difference between particle size distribution of encapsulated powders for different ultrasonication times can be clearly seen for MD:WPC ratio of 1:3 and core:coating ratio of 1:4. As ultrasonication time increased, the particle size distribution curve shifted to the left meaning that microcapsules with smaller particle size were obtained. This is similar to the results found in literature. In the study of Jafari et al. (2007a) sonication time was found to have a significant effect (P < 0.05) on D[3,2] up to 60s, that is as sonication time increased, D[3,2] decreased. Kentish et al. (2008) applied 200 W ultrasonic power to emulsion of flaxseed oil, water and Tween 40 and as sonication time increased from 1 to 3 min, D[3,2] of emulsion decreased from 0.35 to 0.15 μm. Cucheval and Chow (2008) used ultrasound for preparation of emulsion made of soybean oil, water and tween 80 and found that droplet size decreased sharply between 0 and 3 min. Abismail et al. (1999) showed that D[3,2] of oil-in-water emulsion which consists of water, kerosene (oil) and polyethoxylated sorbitan monostearate (surfactant) decreased with sonication time from 5 to 30 s.
Table 3.
Particle size analysis results of emulsions
| MD:WPC | Core:coating | Ultrasonication time (min) | Particle size (D[3,2]) (μm) | SSAa (m2/g) | Span |
|---|---|---|---|---|---|
| 3:1 | 1:8 | 2 | 2.43Ab | 2.46 | 1.63 |
| 3:1 | 1:8 | 5 | 2.02CB | 2.96 | 1.47 |
| 3:1 | 1:8 | 10 | 2.01CB | 2.98 | 1.27 |
| 2:2 | 1:8 | 2 | 2.15B | 2.79 | 1.59 |
| 2:2 | 1:8 | 5 | 1.82DC | 3.28 | 1.71 |
| 2:2 | 1:8 | 10 | 1.68D | 1.50 | 3.57 |
| 1:3 | 1:8 | 2 | 1.60D | 3.57 | 4.26 |
| 1:3 | 1:8 | 5 | 1.21E | 4.95 | 21.07 |
| 1:3 | 1:8 | 10 | 0.41F | 15.59 | 18.60 |
| 1:3 | 1:4 | 2 | 1.68D | 3.75 | 1.60 |
| 1:3 | 1:4 | 5 | 1.00E | 4.75 | 24.06 |
| 1:3 | 1:4 | 10 | 0.40F | 52.87 | 11.70 |
aSpecific surface area
bParticle size values having different letters are significantly different (p ≤ 0.05)
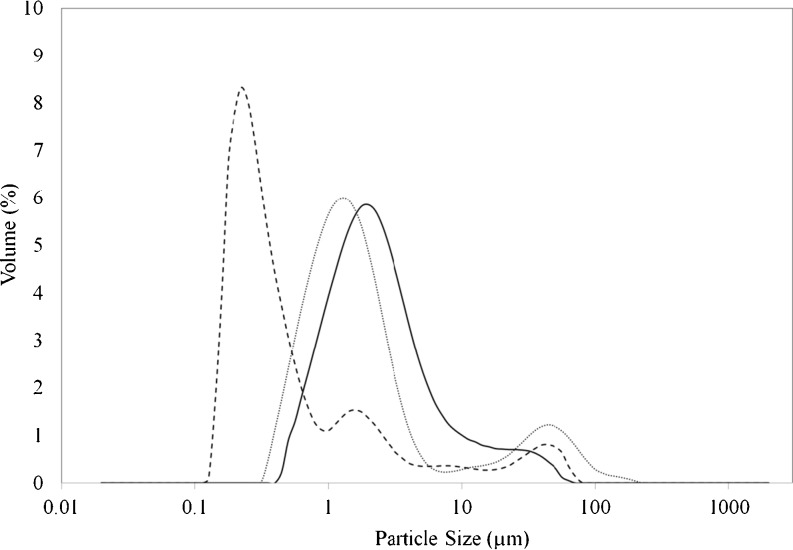
Fig. 2.
Particle size distribution of emulsions prepared with MD:WPC ratio of 1:3, core to coating ratio of 1:4, and different ultrasonication times; dash: 10 min; round dot: 5 min; straight line: 2 min
As WPC concentration increased in the coating formulation, the mean diameter of the particles decreased significantly (Table 3). This can also be seen in Fig. 3, in which particle size distribution curve shifted to the left (smaller size) when WPC concentration increased. This could be explained by the good emulsifying property of WPC related to the many hydrophobic and hydrophilic parts of WPC. Higher amount of WPC could lead to better emulsification and oil globules could disperse finely and as a result of this particle size of emulsion could be smaller. It was also observed that as WPC concentration and ultrasonication time increased, specific surface area increased (Table 3).
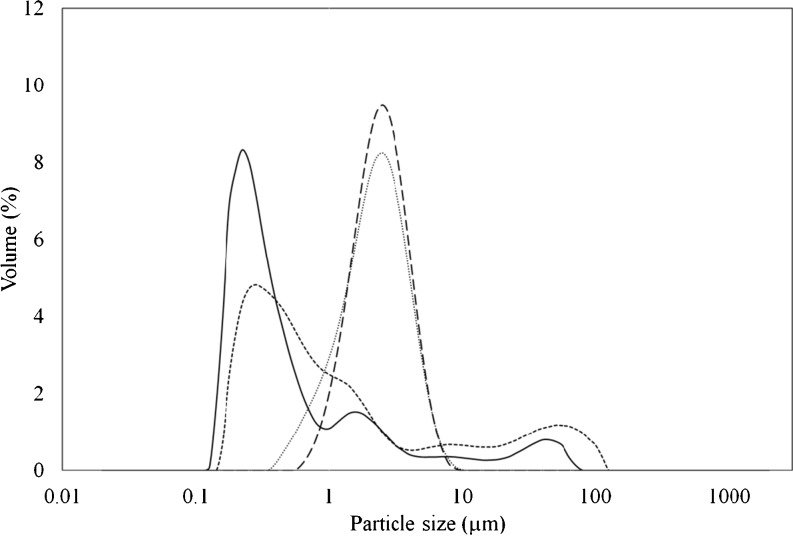
Fig. 3.
Particle size distribution of emulsions prepared with 10 min ultrasonication and different MD:WPC and core:coating ratios; long dash: MD:WPC of 3:1, core:coating ratio of 1:8; round dot: MD:WPC of 2:2, core:coating ratio of 1:8; straight line: MD:WPC of 1:3, core:coating ratio of 1:4; dash: MD:WPC of 1:3, core:coating ratio of 1:8
On the other hand, increasing WPC in the coating, increased span of the particle size distribution significantly, especially for 5 min and 10 min ultrasonication times (Table 3). It can also be seen from Fig. 3 that the span of the samples prepared with MD:WPC ratio of 1:3 as a coating material were wider when compared to the ones prepared using 3:1 and 2:2 MD:WPC ratios. Wider span indicates that there are both small and large particles in the emulsion. The reason for these larger particles can be aggregation of some amount of WPC in emulsion. It is predicted that aggregation occurs due to higher protein concentration and WPC denaturation. Euston et al. (2000) studied about aggregation kinetics of heated WPC stabilized emulsion. They put forward a hypothesis that aggregation takes place between adsorbed protein at the emulsion droplet surface and non-adsorbed heat denatured protein. They also observed that aggregation proceeded more rapidly at higher WPC concentration which is consistent with our study.
Increasing the amount of oil as compared to its coating did not have any significant effect on mean particle size. According to Table 3, for the same ultrasonication time, Sauter mean diameter (D[3,2]) of samples having MD:WPC ratio of 1:3 as a coating formulation but different core to coating ratios were not significantly different.
There was an inverse correlation between Sauter mean diameter and EE (r = 0.8). As can be seen in Tables 2 and 3, the lower the size, the higher was the EE. This result is similar to the findings of Goula and Adamopoulas (2012). It is stated that enclosing small emulsion droplets within the coating material is more efficient.
Surface morphology of encapsulated powder
In this part of the study, it was aimed to observe outer surface properties of encapsulated powders and to check differences between encapsulated powders that were produced under different conditions.
It is known that freeze dried powder has generally irregular shape, very light, highly porous structure as compared to the ones obtained using other drying techniques such as spray drying (Anwar and Kunz 2011). It is seen that freeze dried powders have larger surface area. SEM results obtained in this study are in accordance with these properties, additionally they had slab like shapes, some of them had layer by layer structure.
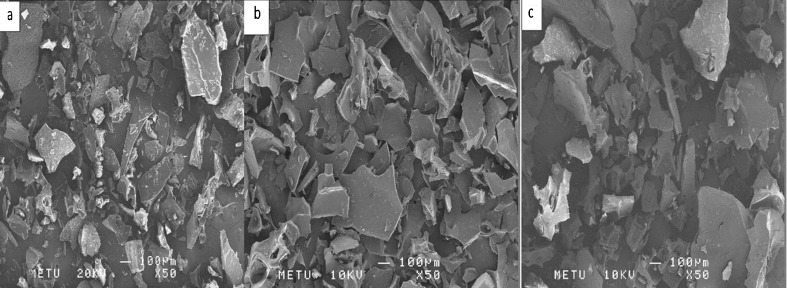
In Fig. 4, it can be seen that particle size of samples that were treated with 5 and 10 min ultrasonication was smaller than samples prepared with 2 min ultrasonication for MD:WPC ratio of 2:2 and core to coating ratio of 1:8. This result is supported also by our particle size values (Table 3). As ultrasonication time increased from 2 to 5 min, particle size was decreased but there was no significant difference between 5 and 10 min application.
Fig. 4.
SEM images of microcapsules prepared with core to coating ratio of 1:8, MD:WPC ratio of 2:2 and different ultrasonication times with ×50 magnification a 2 min b 5 min c 10 min
In Fig. 5, it can be seen that for the same time of ultrasonication, the surface of powder having higher concentration of WPC (Fig. 5c) was smoother whereas surface of powder that had higher MD concentration (Fig. 5a) was rougher. In literature, there are some studies which show that the structures of microcapsules are affected by the ratio of coating formulation. Sheu and Rosenberg (1998) reported that when whey protein isolate:MD ratio was increased from 1:19 to 3:1, encapsulated powder surface became smoother and surface cracks were decreased. Moreover, Jafari et al. (2007b) observed that addition of WPC into emulsion composition had changed the structure and surface morphology of the encapsulated oil by decreasing surface dents and increasing smoothness. They suggested that this could be due to drying of wall matrix at a slower rate with WPC samples and probably WPC provides elasticity to wall systems. The results observed from SEM images can be related to encapsulation efficiency. Smoother surface of microcapsules prepared with higher WPC concentration provided lower surface oil content (Table 1) and higher encapsulation efficiency (Fig. 1). Sheu and Rosenberg (1998) found that at a whey protein isolate:MD ratio of 1:9 or 1:1, microcapsules had fewer surface dents than those at a 1:19 ratio. Surface indentation can be correlated with extractable surface oil amount. When surface oil is extracted from capsules having more dents, more oil would be extracted because solvent will reach inner surfaces, extract more oil and this will decrease encapsulation efficiency.
Fig. 5.
SEM images of microcapsules prepared with 5 min ultrasonication time, 1:8 core to coating ratio and different MD:WPC ratios a 3:1 b 2:2 c 1:3 (×50 magnification)
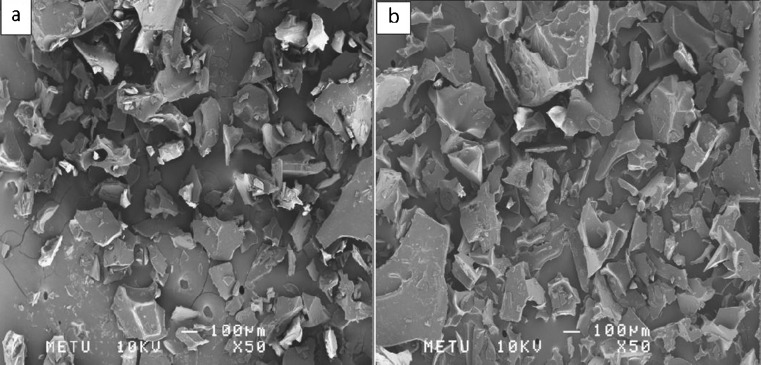
When different core to coating ratios are compared at the same ultrasonication time it was seen that there was no significant difference between SEM images (Fig. 6).
Fig. 6.
SEM images of microcapsules prepared with 10 min ultrasonication time, MD:WPC ratio of 1:3 and different core to coating ratios a 1:4 b 1:8
Conclusion & recommendations
In this study, wheat germ oil was encapsulated by using ultrasonication and freeze drying methods and MD and WPC at different ratios were used as a coating material. Among five core to coating ratios, 1:8 gave the best result in terms of encapsulation efficiency. As a common trend, it was observed that increasing WPC ratio in coating resulted in both higher encapsulation efficiency and smaller particle sizes. Increasing ultrasonication time also had a positive effect on encapsulation efficiency.
As a conclusion, microcapsules that were coated with MD:WPC ratio of 1:3 with core to coating ratio of 1:8 and prepared by ultrasonication for 10 min can be utilized as a functional food because this formulation had the highest encapsulation efficiency and the smallest particle size.
As a recommendation, further study can be done for determining the oxidative stability of encapsulated powder during storage at different relative humidity and temperatures for a certain time period. Incorporation of encapsulated powder to foods such as cake or bread can also be studied and thermal stability and bioavailability of those functional foods can be analyzed.
Acknowledgments
Cargill Foods (Istanbul, Turkey) is greatly acknowledged for providing maltodextrin for this study.
References
- Abismail B, Canselier JP, Wilhelm AM, Delmas H, Gourdon C. Emulsification by ultrasound: drop size distribution and stability. Ultrason Sonochem. 1999;6:75–83. doi: 10.1016/S1350-4177(98)00027-3. [DOI] [PubMed] [Google Scholar]
- Anwar SH, Kunz B. The influence of drying methods on the stabilization of fish oil microcapsules: comparison of spray granulation, spray drying and freeze drying. J Food Eng. 2011;105:367–378. doi: 10.1016/j.jfoodeng.2011.02.047. [DOI] [Google Scholar]
- Augustin MA, Hemar Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev. 2009;38:902–912. doi: 10.1039/B801739P. [DOI] [PubMed] [Google Scholar]
- Cilek B, Luca A, Hasirci V, Sahin S, Sumnu G. Microencapsulation of phenolic compounds extracted from sour cherry pomace: effect of formulation, ultrasonication time and core to coating ratio. Eur Food Res Technol. 2012;235(4):587–596. doi: 10.1007/s00217-012-1786-8. [DOI] [Google Scholar]
- Cucheval A, Chow RCY. A study on the emulsification of oil by power ultrasound. Ultrason Sonochem. 2008;15:916–920. doi: 10.1016/j.ultsonch.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Euston SR, Finnigan SR, Hirst RL. Aggregation kinetics of heated whey protein-stabilized emulsions. Food Hydrocoll. 2000;14:155–161. doi: 10.1016/S0268-005X(99)00061-2. [DOI] [Google Scholar]
- Goula AM, Adamopoulas KG. A method for pomegranate seed application in food industries: seed oil encapsulation. Food Bioprod Process. 2012;90:639–652. doi: 10.1016/j.fbp.2012.06.001. [DOI] [Google Scholar]
- Hogan SA, McNamee BF, O’riordan ED, O’Sullivan M. Microencapsulating properties of whey protein concentrate 75. J Food Sci. 2001;66(5):675–680. doi: 10.1111/j.1365-2621.2001.tb04620.x. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82:478–488. doi: 10.1016/j.jfoodeng.2007.03.007. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Optimization of nano-emulsions production by microfluidization. Eur Food Res Technol. 2007;255:733–741. doi: 10.1007/s00217-006-0476-9. [DOI] [Google Scholar]
- Jimenez M, Garcia HS, Beristain CI. Spray-dried encapsulation of conjugated linoleic acid (CLA) with polymeric matrices. J Sci Food Agric. 2006;86:2431–2437. doi: 10.1002/jsfa.2636. [DOI] [Google Scholar]
- Kagami Y, Sugimura S, Fujishima N, Matsuda K, Kometani T, Matsumura Y. Oxidative stability, structure and physical characteristics of microcapsules formed by spray drying of fish oil with protein and dextrin wall materials. J Food Sci. 2003;68(7):2248–2255. doi: 10.1111/j.1365-2621.2003.tb05755.x. [DOI] [Google Scholar]
- Kentish S, Wooster TJ, Ashokkumar M, Balachandran S, Mawson R, Simons L. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg. 2008;9:170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- Kim YD, Morr CV. Microencapsulation properties of gum arabic and several food proteins: spray-dried orange oil emulsion particles. J Agric Food Chem. 1996;44:1314–1320. doi: 10.1021/jf9503927. [DOI] [Google Scholar]
- Klinkesorn U, Sophanodora P, Chinachoti P, McClements DJ. Stability and rheology of corn oil-in-water emulsions containing maltodextrin. Food Res Int. 2004;37:851–859. doi: 10.1016/j.foodres.2004.05.001. [DOI] [Google Scholar]
- Longmore AP. Advances in vacuum and freeze drying. Food Process Ind. 1971;40:46–49. [Google Scholar]
- Mark-Herbert C. Innovation of a new product category functional foods. Technovation. 2004;24:713–719. doi: 10.1016/S0166-4972(02)00131-1. [DOI] [Google Scholar]
- Megahad AO, El Kinawy SO. Studies on the extraction of wheat germ oil by commercial hexane. Grasas Aceites. 2002;53(4):414–418. doi: 10.3989/gya.2002.v53.i4.339. [DOI] [Google Scholar]
- Megahed MG. Study on stability of wheat germ oil and lipase activity of wheat germ during periodical storage. Agric Biol J N Am. 2011;2(1):163–168. doi: 10.5251/abjna.2011.2.1.163.168. [DOI] [Google Scholar]
- Menrad K. Market and marketing of functional food in Europe. J Food Eng. 2003;56:181–188. doi: 10.1016/S0260-8774(02)00247-9. [DOI] [Google Scholar]
- Millqvist-Fureby A. Characterisation of spray-dried emulsions with mixed fat phases. Colloids Surf B. 2003;31(1–4):65–79. doi: 10.1016/S0927-7765(03)00044-4. [DOI] [Google Scholar]
- Mongenot N, Charrier S, Chalier P. Effect of ultrasound emulsification on cheese aroma encapsulation by carbohydrates. J Agric Food Chem. 2000;48:861–867. doi: 10.1021/jf990494n. [DOI] [PubMed] [Google Scholar]
- Parthasarathi PN, Ezhilarasi BS, Jena BS, Anandharamakrishnan C. A comparative study on conventional and microwave-assisted extraction for microencapsulation of Garcinia fruit extract. Food Bioprod Process. 2013;91:103–110. doi: 10.1016/j.fbp.2012.10.004. [DOI] [Google Scholar]
- Sheu TY, Rosenberg M. Microstructure of microcapsules consisting of whey proteins and carbohydrates. J Food Sci. 1998;63:491–494. doi: 10.1111/j.1365-2621.1998.tb15770.x. [DOI] [Google Scholar]
- Tan LH, Chan LW, Heng PWS. Effect of oil loading on microspheres produced by spray drying. J Microencapsul. 2005;22(3):253–259. doi: 10.1080/02652040500100329. [DOI] [PubMed] [Google Scholar]
- Tonon RV, Grosso RFC, Hubinger MD. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res Int. 2011;44(1):282–289. doi: 10.1016/j.foodres.2010.10.018. [DOI] [Google Scholar]
- Toure A, Xiaoming Z, Jia CS, Zhijian D. Microencapsulation and oxidative Stability of ginger essential oil in maltodextrin-whey protein isolate. Int J Dairy Sci. 2007;2(4):387–392. doi: 10.3923/ijds.2007.387.392. [DOI] [Google Scholar]
- Wang T, Johnson L. Refining high-free fatty acid wheat germ oil. J Am Oil Chem Soc. 2001;78(1):71. doi: 10.1007/s11746-001-0222-2. [DOI] [Google Scholar]
- Wu H, Hulbert GJ, Mount JR. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov Food Sci Emerg. 2000;1:211–218. doi: 10.1016/S1466-8564(00)00020-5. [DOI] [Google Scholar]