Abstract
To observe the neuroprotective and antioxidant activities of the grass carp protein hydrolysates (GPH) obtained from grass carp (Ctenopharyngodon idella) skin by enzymatic hydrolysis. GPH prepared using Protamex, at different (5, 10, 15, 20 and 30 %) degrees of hydrolysis (DH) were investigated. The DPPH radial scavenging, reducing power and inhibition of linoleic acid oxidation activities of GPH were significantly improved by a low DH (5 %) compared with those of GPH with a higher DH (p < 0.05). A low degree of enzymatic hydrolysis was appropriate to obtain GPH with improved neuroprotective activities. These results suggest that the control of the DH may be an effective strategy to modify specific neuroprotective and antioxidant activities of GPH, and GPH has potential as a functional food ingredient for related functional and health benefits.
Keywords: Ctenopharyngodon idella, Protein hydrolysates, Neuroprotective activity, Antioxidant activity
Introduction
The prevalence of neurodegenerative diseases such as Alzhermer’s disease (AD) and Parkinson’s disease (PD) is growing fast along the population ages. Accumulating lines of evidence have demonstrated that oxidative stress plays a crucial role in the pathogenesis of neurodegenerative diseases (Canet-Aviles et al. 2004; Hu et al. 2011; Reed 2011). Although a living system possesses several natural defense mechanisms, such as antioxidants and enzymes, oxidative stress resulting from the imbalance of prooxidant/antioxidant homeostasis still leads to the generation of reactive oxygen species (ROS), which cause extensive damage to lipids, proteins and DNA (Tian et al. 2011). Particularly, the brain is fragile to oxidative stress damage because of its high energy use, high metabolic demands, high cellular contents of lipids and protein, and low levels of endogenous scavengers (Erickson and Barnes 2003; Gao et al. 2012).
More evidence suggests that the dietary intervention may prevent such disease as neurodegenerative disease. It is well known that in protein, its hydrolysates exhibited the biological activity. Bioactivities of protein hydrolysates have been described, containing blood pressure-lowering, antimicrobial, immunomodulatory, and antioxidative effects, etc. (Aluko and Monu 2003; Slizyte et al. 2009; Jamdar et al. 2010). Moreover, some protein hydrolysates can protect neutron cells against cell death caused by hydroxyl radical-induced oxidative damage (Ko et al. 2012; Gao et al. 2012).
With the rapid development of the freshwater fish processing industry in China, large quantities of by-products are generated accounting for 50–70 % of the original raw material. Grass carp, native to Asia, is one of the “four major freshwater cultured fish species” along with bighead carp, black carp and silver carp grown in China (Ladislaus et al. 2007). Grass carp skin has been known as a good source of high quality collagen that can be utilized for producing functional food, medicine and cosmetic products (wasswa et al. 2007). Thus, effective utilization of these by-products is a promising method to protect the environment and produce high value-added products.
At present, no information is available about the neuroprotective and antioxidant activities of protein hydrolysates from freshwater fish skin. Therefore, the purpose of the present study was to investigate the effects of the degree of enzymatic hydrolysis on the neuroprotective and antioxidant activities of protein hydrolysates from grass carp skin.
Materials and methods
Materials
Protamex (from B.licheniformis, EC 3.4.24.28, 100,000 U/g) were purchased from Nanning Pangbo Biological Engineering Co. Ltd (Nanning, China). Linoleic acid, β-carotene, 1,1-diphenyl-2-picrylhydrazyl (DPPH), fetal bovine serum, penicillin-streptomycin, 6-hydroxydopamine (6-OHDA), dimethyl sulfoxide (DMSO), Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Sigma Chemical Co. (St. Louis, USA). 3-(4,5)-dimethylthiahiazo- (−z-y1)-3,5-diphenytetrazoliumromide (MTT) was purchased from Applygen Technologies Inc. (Beijing, China). MES 23.5 and SH-SY5Y cells were purchased from Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences (Shanghai, China). All of the chemicals used in the experiments were of analytical grade.
Live farmed grass carp (Ctenopharyngodon idella), with weights ranging from 2 to 3.5 kg, were obtained in the spring from a local market in Jinzhou, Liaoning Province, China. They were transported to the laboratory within 30 min, and after that, the fish were stunned by a sharp blow to the head with a wooden stick. Then, the skins were manually removed with a filleting knife and washed with cold distilled water. The clean skins were cut into small pieces (0.5 × 0.5 cm2) using a scissor, and were kept on ice prior to enzymatic hydrolysis.
Preparation of protein hydrolysates from grass carp skin
Grass carp skin was precisely weighed and water was added by a skin/water ratio (1:5). The pH was adjusted to 7.0 and the reaction mixture was heated to 50 °C. The hydrolysis was started by the addition of enzyme to give the desired enzyme/substrate ratio (1:500). The hydrolysates with varying degrees of hydrolysis, namely 5, 10, 15, 20, and 30 %, were obtained by incubation at 50 °C for 10, 20, 40, 70, and 330 min, respectively. At the end of the hydrolysis the enzyme was inactivated by heating at 90 °C for 20 min, cooled down to room temperature, and centrifuged at 4,000 rpm for 20 min. The supernatant containing soluble protein hydrolysates was adjusted to pH 7.0, frozen, lyophilised and stored at −20 °C until further analysis. The DH during the enzymatic hydrolysis was determined by the pH-stat method, as described by Adler-Nissen (1986). The DH was defined as the ratio of the number of peptide bonds cleaved (h) to the total number of peptide bonds per weight unit (htot) and calculated from the consumption of alkali (NaOH). The DH (%) was calculated by the following equation:
where B represents the volume (ml) of NaOH consumed during the proteolysis of the substrate, Nb is the molar concentration of NaOH, Mp is the mass of protein being hydrolysed (g), α and htot are constants, including α is the average degree of dissociation of the α-NH2 groups in the protein substrate (0.858), and htot is the total content of peptide bonds available for proteolytic hydrolysis (7.8).
Compositional analysis
The chemical composition of the fish skin protein hydrolysates were analysed according to the AOAC method (AOAC 1997). The dry matter was determined gravimetrically after drying at 100 °C until a constant weight was achieved. The protein content was determined using the Kjeldahl method. The fat content was determined by extracting samples with petroleum ether using a Soxhlet apparatus. The ash content was determined by incineration in a muffle furnace maintained at 600 °C until a constant final weight was achieved. The carbohydrate content was determined using enzymatic-gravimetric method.
Cell culture and MTT assay on MES 23.5 cells
MES 23.5 cells were maintained in Dulbecco’s modified Eagle’s medium, supplemented with 5 % fetal bovine serum, 100 units/mL penicillin-streptomycin in an atmosphere of 5 % CO2 at 37 °C. Then, MES 23.5 cells (200 μL) were seeded in a 96-well plate at a density of 2 × 105 cells/well for 24 h prior to experimentation. Subsequently, the cells were divided into three groups: (1) Control group: cells were treated with serum-free medium for 24 h. (2) 6-OHDA group: cells were treated with 6-hydroxydopamine (6-OHDA) (100 μM) in a serum-free medium for 24 h; (3) experimental groups: cells were treated with 6-OHDA (100 μM) and various hydrolysates at two concentrations (1 mg mL−1 and 0.1 mg mL−1) in a serum-free medium for 24 h. After the removal of medium from the wells, 10 μL of MTT (5 mg mL−1 suspended in 0.01 M phosphate buffer solution (PBS)) was added to each well. After 4 h of incubation in an atmosphere of 5 % CO2 at 37 °C, dimethyl sulfoxide (DMSO) (200 μL) was added and the absorbance was measured at 490 nm. The following equation was used to calculate cell vitality: Cell vitality (%) = (Asample − Ablank)/(Acontrol − Ablank) × 100. Ablank means the absorbance of 0.01 M PBS.
Cell culture and MTT assay on SH-SY5Y cells
Digested SH-SY5Y cells were cultured in DMEM/F12 medium containing 10 % fetal bovine serum. Then, SH-SY5Y cells (100 μL) were seeded in a 96-well plate at a density of 2.5 × 105 cells/well in an atmosphere of 5 % CO2 at 37 °C for 24 h. Then, the old medium was removed, and new medium (100 μL) containing 10 μL of different concentrations (0.5 mg mL−1 and 0.05 mg mL−1) of fractions (experimental groups) and solvents (control group and 30 μM 6-OHDA group) was applied. After 2 h, 0.3 mM 6-OHDA (10 μL) was added to the cells in the experimental groups and 6-OHDA group for another 24 h. Then, 10 μL MTT (5 mg mL−1) was added, and the cells were incubated for an additional 3 h. The medium was discarded, 100 μL of DMSO was added to each well, and the plates were shaken to dissolve the formazan crystals. Finally, the absorbance was measured at 490 nm. The following equation was used to calculate cell vitality: Cell vitality (%) = (Asample − Ablank)/(Acontrol − Ablank) × 100. Ablank means the absorbance of 0.01 M PBS.
Antioxidant activities
DPPH radical scavenging activity
The DPPH radical scavenging assay is not specific to any particular antioxidant component but rather reflects the overall antioxidant capacity of a sample. The determination of scavenging activity was based on the method described by Lopez et al. (2010) with minor modifications. A 2-mL sample solution at different concentrations (0–6 mg mL−1) was added to 2.5 mL of 0.02 mM DPPH dissolved in methanol. The reaction mixture was shaken and left for 30 min in the dark at room temperature. The absorption of the sample at 517 nm was measured with a spectrophotometer against a blank sample of methanol. The results were expressed as percentage of DPPH radical inhibition (%) using the following equation:
The reducing power
The reducing power of fish skin hydrolysates was measured by the method developed by Zhu et al. (2006) with minor modifications. A sample (0–25 mg) was added to 2.5 mL of 0.2 mol L−1 phosphate buffer solution (pH 6.6) and mixed with 2.5 mL of 1 % potassium ferricyanide solution. The mixture was incubated at 50 °C for 20 min. The solution was cooled rapidly, after which 2.5 mL of 10 % trichloroacetic acid (TCA) was added to the mixture. The reaction mixture was centrifuged at 5,000 rpm for 10 min. An aliquot of the supernatant (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL 0.1 % ferric chloride solution. The absorbance was measured at 700 nm after a 10 min reaction. An increase in absorbance indicates a higher reducing power.
β-Carotene-linoleic acid assay
Antioxidant activity was also evaluated with the β-carotene bleaching method described by Koleva et al. (2002), with some modifications. Two milligrams of β-carotene was dissolved in 4 mL of chloroform. An aliquot (1 mL) of the solution was added to a conical flask containing 20 mg of linoleic acid and 200 mg of Tween-40. Chloroform from the mixture was evaporated with a rotary evaporator at 50 °C. Distilled water (80 mL) was added slowly to the β-carotene emulsion and vigorously agitated to form a stable emulsion. An aliquot (2.5 mL) of the β-carotene emulsion and 0.5 mL of aqueous solution of protein hydrolysate at different concentrations (0–3 mg mL−1) were mixed in a tube, and the absorbance at 470 nm was measured immediately. The tube was then placed in a water bath at 50 °C for 110 min. Oxidation of the β-carotene emulsion was monitored by measuring the absorbance at 470 nm after the incubation period. The control consisted of 2.5 mL of the β-carotene emulsion in 0.5 mL of distilled water. The antioxidant activity was expressed as the percentage of inhibition relative to the control using the following equation:
where As,0 and As,110 are the absorbance of the sample at 0 and 110 min, respectively, while Ac,0 and Ac,110 are the absorbance of the control at 0 and 110 min, respectively.
Statistical analysis
The experiment followed a completely randomized design (n = 3). Data were subjected to one-way analysis of variance (ANOVA). Mean separations were performed by Duncan’s multiple range test (SAS version 8.1). Differences at p < 0.05 were considered significant.
Results and discussion
Compositional analysis of GPH
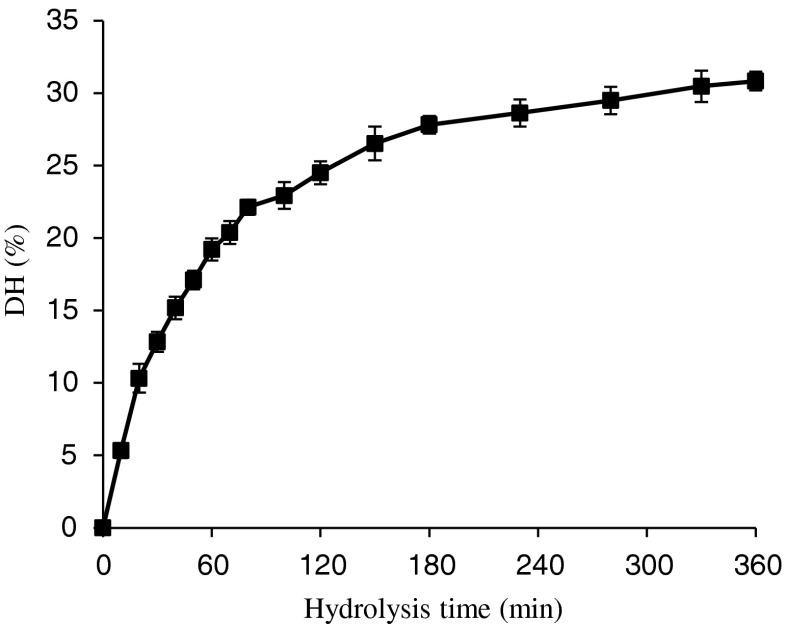
The chemical composition of the protein hydrolysates from the grass carp skin is presented in Table 1. The protein contents of GPH were found to be 95.0–96.1 %, and the fat content of GPH is low, about 1 %. The grass carp skin protein was subjected to hydrolysis by Protamex. The progress of hydrolysis was monitored in terms of degree of hydrolysis, which is presented in Fig. 1. As shown in the figure, the hydrolysis progressed rapidly during the first 0–2 h and the rate subsequently decreased, which is similar to the typical hydrolysis curve reported by Klompong et al. (2007) and Jamdar et al. (2010). A relatively high degree of hydrolysis (28 %) was obtained within the initial 3 h. The grass carp protein hydrolysate (GPH) at different degrees of hydrolysis, viz., 5 % (5.33 ± 0.5 %), 10 % (10.3 ± 1.0 %), 15 % (15.2 ± 0.8 %), 20 % (20.4 ± 0.8 %) and 30 % (30.5 ± 1.1 %) were prepared, using a degree of hydrolysis versus hydrolysis time curve.
Table 1.
Compositional analysis of protein hydrolysates from the grass carp skin. Each value represents the mean ± standard deviation of three replicates
| Dry matter (%) | Protein (%, DM) | Fat (%, DM) | Ash (%, DM) | Carbohydrate (%, DM) | |
|---|---|---|---|---|---|
| DH-5 | 96.9 ± 0.51 | 95.7 ± 0.53 | 1.22 ± 0.06 | 2.81 ± 0.36 | 0.25 ± 0.29 |
| DH-10 | 97.0 ± 0.52 | 96.1 ± 0.40 | 1.07 ± 0.23 | 2.56 ± 0.20 | 0.29 ± 0.03 |
| DH-15 | 96.6 ± 0.62 | 95.0 ± 0.29 | 1.11 ± 0.17 | 3.24 ± 0.20 | 0.65 ± 0.13 |
| DH-20 | 97.3 ± 0.95 | 95.5 ± 0.50 | 1.19 ± 0.14 | 2.77 ± 0.33 | 0.56 ± 0.08 |
| DH-25 | 97.1 ± 0.56 | 95.4 ± 0.62 | 1.08 ± 0.12 | 2.89 ± 0.43 | 0.60 ± 0.15 |
Fig. 1.
Hydrolysis curve of grass carp skin treated with protamex. The reaction was performed at pH 7.0 and 50 °C with an enzyme to substrate ratio (1:500) on the weight basis. Vertical bars represent standard deviations from triplicate measurements
Neuroprotective effects of protein hydrolysates from grass carp skin
Fish protein, being different from other dietary proteins, is probably to contain many specific and potent bioactive sub-sequences in nutritional perspective for their competitive and aggressive living conditions (Kim and Mendis 2006; Duarte et al. 2006). So if the potent bioactive compounds were extracted and the functions were revealed, it would open a new perspective for functional food development.
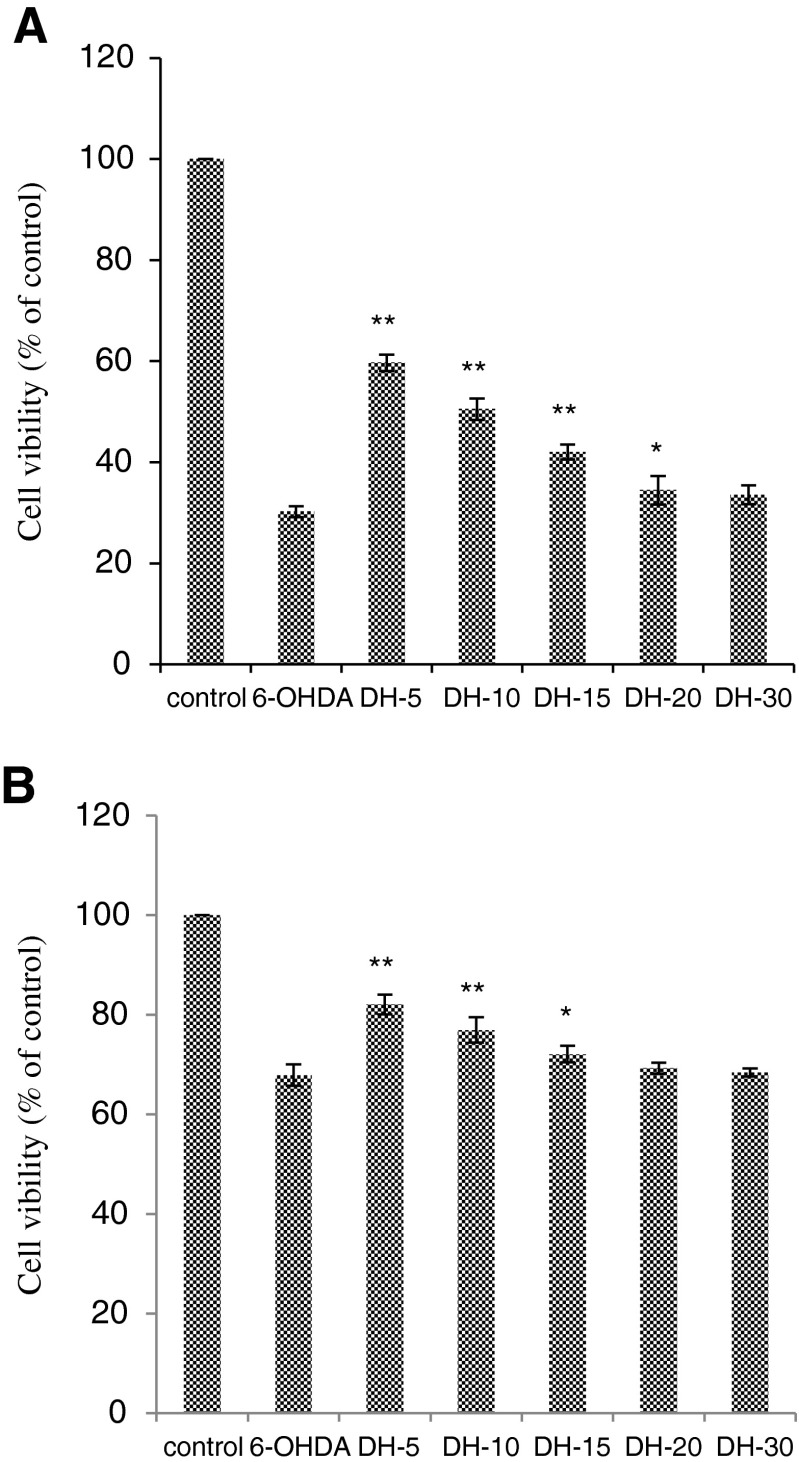
The protective blood–brain barrier prevents some chemicals, including large molecules and highly charged particles, from transferring from the blood to the brain. So, we tried to degrade dietary proteins from fish processing by-product into low molecular weight hydrolysates using specific enzyme to further confirm the neuroprotective effects of protein hydrolysates. Figure 2a showed that DH-5, DH-10, DH-15 and DH-20 exhibit significant (p < 0.05) neuroprotective activity at the concentration of 1 mg mL−1, while they do not have neuroprotective activity at the concentration of 0.1 mg mL−1. However, DH-30 did not exhibit this activity at either of the concentration used. Figure 2b showed that DH-5, DH-10 and DH-15 exhibit significant neuroprotective activity at the concentration of 0.5 mg mL−1, while they do not have neuroprotective activity at the concentration of 0.05 mg mL−1. However, neither DH-20 nor DH-30 exhibited neuroprotective activities at either concentration used.
Fig. 2.
Neuroprotective effects of the protein hydrolysates on 6-OHDA-induced neurotoxicity in MES 23.5 cells (a) and SH-Y5Y cells (b). The results are represented as the mean ± SD. **p < 0.01, compared with the 6-OHDA group; *p < 0.05, compared with the 6-OHDA group
Scavenging activity of DPPH radicals
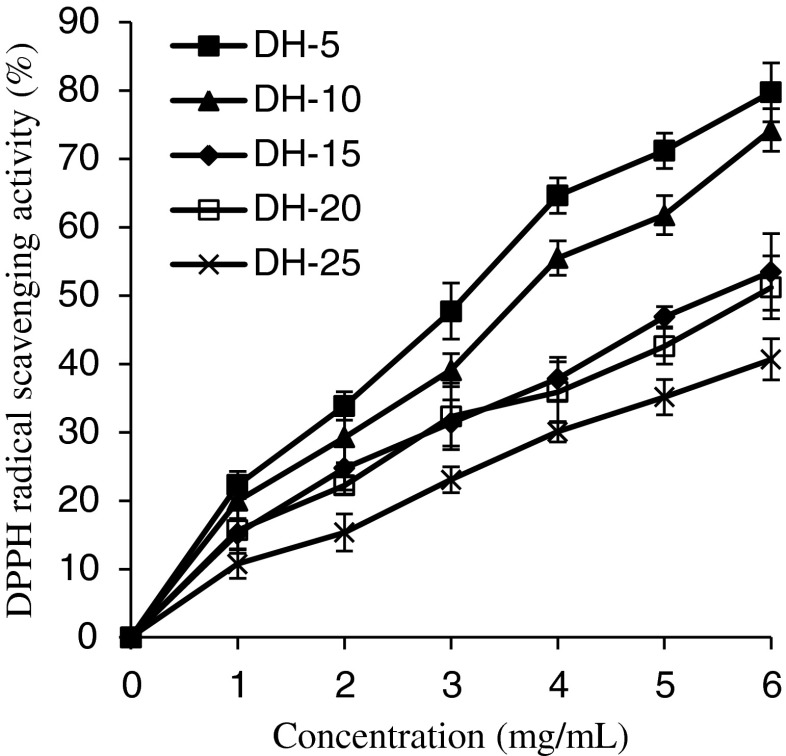
The DPPH radical scavenging activities of GSH at various concentrations (0–6 mg mL−1) are shown in Fig. 3. The DPPH radical assay had been widely used to evaluate the ability of some substances to act as free radical scavengers or hydrogen donors. The dose-dependent relationship exhibited in Fig. 3 suggests that the GSH have the ability to react as electron donors to scavenge DPPH radical. DH-5 was the best at scavenging DPPH radicals (p < 0.05), followed by DH-10. There was no significant difference in the DPPH radical scavenging ability between DH-15 and DH-20, and DH-30 showed the lowest effect on scavenging DPPH radicals. Further increases in the DH significantly reduced the activity. The result was similar to the report of Klompong et al. (2007) for yellow stripe trevally protein hydrolysates, but in contrast with that of porcine collagen hydrolysates reported by Li et al. (2007), who showed an increase in DPPH radical scavenging activity with increased DH.
Fig. 3.
The DPPH radical scavenging ability of grass carp protein hydrolysates (GPH). Vertical bars represent standard deviations from triplicate measurements
Reducing power
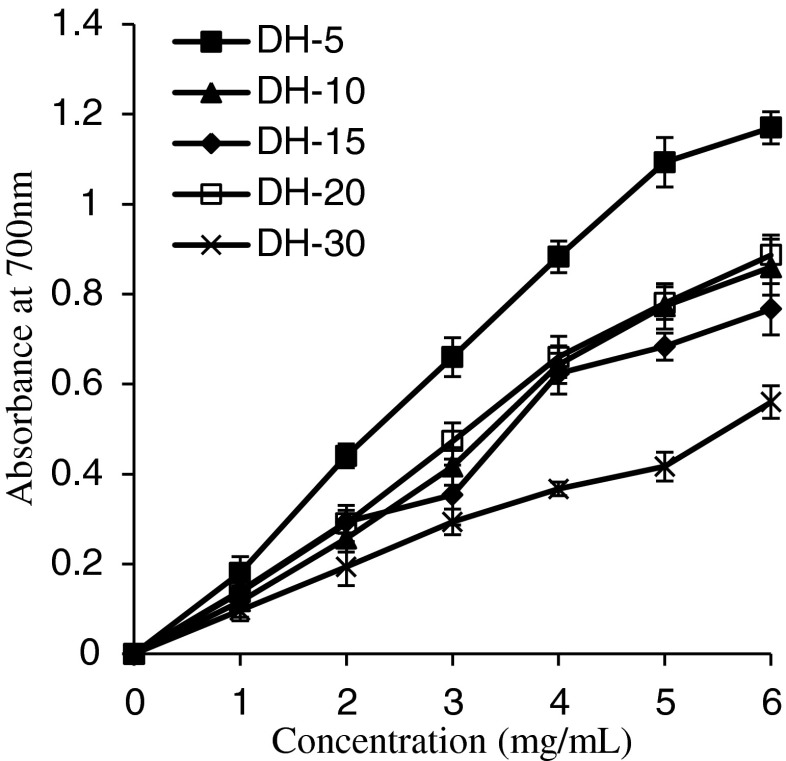
The reducing power assay determined the reducing power for Fe3+/ferricyanide and offered an important index of potential antioxidant activity. High absorbance means stronger reducing power of samples (Pulido et al. 2000). As shown in Fig. 4, the reducing power of the GSH increased with the concentrations, displaying a linear correlation, decreased when hydrolysis was performed and was negatively related with the DH. Among the samples, the highest antioxidant activity was found in GSH with DH of 5 %, which exhibited a significantly (p < 0.05) higher reducing power than other hydrolysates. There was no significant difference in the reducing power between DH-10 and DH-20. However, the reducing power of GSH with DH of 5–20 % at 6 mg mL−1 was in the range of 0.77–1.17, which is higher than that of some plant protein hydrolysates (Li et al. 2008; Pan et al. 2011). The results showed that GSH is a good electron donor and inhibits free radicals, indicating that it has strong potential for use as a natural antioxidant.
Fig. 4.
The reducing power of grass carp protein hydrolysates (GPH). Vertical bars represent standard deviations from triplicate measurements
β-Carotene-linoleic acid assay
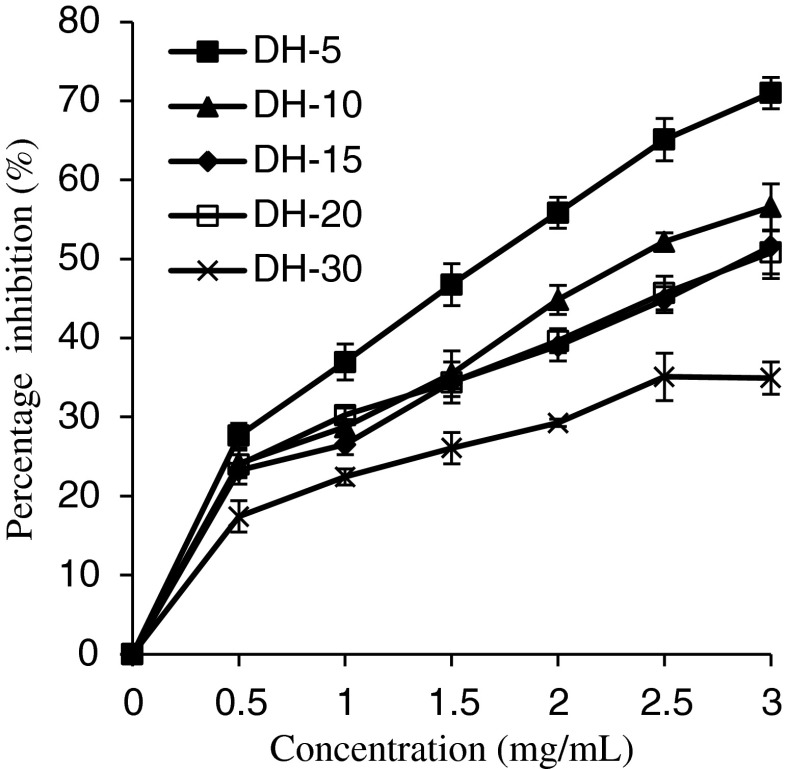
Figure 5 shows the antioxidant activity of GSH determined by the β-carotene-linoleic acid system. The antioxidant assay using the discoloration of β-carotene is widely used, because β-carotene is extremely susceptible to free-radical-mediated oxidation. In the absence of antioxidant β-carotene is rapidly bleached in the test solution; however, presence of antioxidant suppresses the extent of bleaching by neutralizing the linoleic free radical formed. All the GSH suppressed the discoloration of β-carotene at different DH and showed increase in antioxidant activity with increasing concentration. Among the samples, the highest antioxidant activity was found in GPH with a DH of 5 %, which exhibited a significantly (p < 0.05) higher ability to prevent β-carotene bleaching than other hydrolysates. However, further increase in the DH (10–30 %) did not show any increase in antioxidant activity. Thus, the result reveals that suppression of discoloration of β-carotene decreased with increase in the DH and only a low degree of hydrolysis with Protamex improved the ability of the GSH to inhibit β-carotene oxidation. The observation is in agreement with Jamdar et al. (2010), who have shown a slight decrease in oxidation inhibition with increase in DH for peanut protein hydrolysates.
Fig. 5.
The inhibition of linoleic acid oxidation of grass carp protein hydrolysates (GPH). Vertical bars represent standard deviations from triplicate measurements
Conclusions
Grass carp protein hydrolysates (GPH) were obtained from grass carp skin by hydrolysis with Protamex. The impact of the degree of hydrolysis (DH) on the neuroprotective and antioxidant activities of GPH was investigated. A low DH significantly improved the DPPH radical scavenging ability, the reducing power and inhibition of linoleic acid oxidation of GPH. DH-5 and DH-10 exhibited significant (p < 0.01) neuroprotective activity in MTT assay. It was concluded that the neuroprotective activities of samples correlated with the antioxidant activity. This study also suggests that GPH has the potential to be utilized as a new functional food ingredient, such as the natural antioxidant and neuroprotective agent, for use in health food products.
Acknowledgement
This study was supported by a grant from the National Key Technologies R&D Program of China during the 12th Five-Year Plan Period (2012BAD29B06).
Contributor Information
Luyun Cai, Phone: +86 416 3400008, Email: lycai515@163.com.
Jianrong Li, Email: lijr6491@163.com.
References
- Adler-Nissen J. Enzymatic hydrolysis of food proteins. New York: Elsevier Applied Science Publishers; 1986. Methods in food protein hydrolysis; pp. 110–130. [Google Scholar]
- Aluko RE, Monu E. Functional and bioactive properties of quinoa seed protein hydrolysates. J Food Sci. 2003;68:1254–1258. doi: 10.1111/j.1365-2621.2003.tb09635.x. [DOI] [Google Scholar]
- AOAC (1997) Official methods of analysis. Arlington, VA: American Association of Analytical Chemists (AOAC official method 930.15 for dry matter; 968.06 for protein; 920.39 for fat; 942.05 for ash; 991.43 for carbohydrate)
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J, Vinderola G, Ritz B, Perdigon G, Matar C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology. 2006;211:341–350. doi: 10.1016/j.imbio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38:61–69. doi: 10.1016/S0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Gao Y, Dong C, Yin J, Shen J, Tian J, Li C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell Mol Neurobiol. 2012;32:523–529. doi: 10.1007/s10571-011-9792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XQ, Huang YY, Dong QF, Song LY, Yuan F, Yu RM. Structure characterization and antioxidant activity of a novel polysaccharide isolated from pulp tissues of litchi chinensis. J Agri Food Chem. 2011;59:11548–11552. doi: 10.1021/jf203179y. [DOI] [PubMed] [Google Scholar]
- Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- Kim SK, Mendis E. Bioactive compounds from marine processing byproducts – a review. Food Res Int. 2006;39:383–393. doi: 10.1016/j.foodres.2005.10.010. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidant activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Ko S, Kim D, Jeon Y. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem Toxicol. 2012;50:2294–2302. doi: 10.1016/j.fct.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Koleva II, Van Beek TA, Linssen JPH, Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Analysis. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Ladislaus MK, Yan X, Yao WL, Sun DH, Qian H. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresource Technol. 2007;98:3338–3343. doi: 10.1016/j.biortech.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Li B, Chen F, Wang X, Ji B, Wu Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2007;102:1135–1143. doi: 10.1016/j.foodchem.2006.07.002. [DOI] [Google Scholar]
- Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–450. doi: 10.1016/j.foodchem.2007.04.067. [DOI] [Google Scholar]
- Lopez J, Uribe E, Vega-Galvez MM, Vergara J, Gonzales E, Di Scala K. Effect of air temperature on drying kinetics, vitamin C, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety O’Neill. Food Bioprocess Tech. 2010;3:772–777. doi: 10.1007/s11947-009-0306-8. [DOI] [Google Scholar]
- Pan M, Jiang TS, Pan JL. Antioxidant activities of rapeseed protein hydrolysates. Food Bioprocess Tech. 2011;4:1144–1152. doi: 10.1007/s11947-009-0206-y. [DOI] [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agri Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radical Biol Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Slizyte R, Mozuraityte R, Martinez-Alvarez O, Falch E, Fouchereau-Peron M, Rustad T. Functional, bioactive and antioxidant properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 2009;44:668–677. doi: 10.1016/j.procbio.2009.02.010. [DOI] [Google Scholar]
- Tian L, Zhao Y, Guo C, Yang X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr Polym. 2011;83:537–544. doi: 10.1016/j.carbpol.2010.08.023. [DOI] [Google Scholar]
- Wasswa J, Tang J, Gu XH, Yuan XQ. Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2007;104:1698–1704. doi: 10.1016/j.foodchem.2007.03.044. [DOI] [Google Scholar]
- Zhu KX, Zhou HM, Qian HF. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006;41:1296–1302. doi: 10.1016/j.procbio.2005.12.029. [DOI] [Google Scholar]