Abstract
The titled compounds were examined as PPO inhibitors and antibrowning agents; their various mechanisms were investigated and discussed. All compounds reduced significantly both the browning process and PPO activity. Browning index gave strong correlation with PPO activity (r2 = 0.96, n = 19) indicating that the browning process is mainly enzymatic. Ascorbic acid could reduce the formed quinone instantly to the original substrate (catechol) at high concentration (>1.5 %) while at lower concentrations acted as competitive inhibitor (KI = 0.256 ± 0.067 mM). Cysteine, at higher concentrations (≥1.0 %), reacted with the resulted quinone to give a colorless products while at the low concentrations, cysteine worked as competitive inhibitor (KI = 1.113 ± 0.176 mM). Citric acid acted only as PPO non-competitive inhibitor with KI = 2.074 ± 0.363 mM. The products of PPO-catechole-cysteine reaction could be separation and identification by LC-ESI-MS. Results indicated that the product of the enzymatic oxidation of catechol, quinone, undergoes two successive nucleophilic attacks by cysteine thiol group. Cysteine was condensed with the resulted mono and dithiocatechols to form peptide side chains.
Keywords: Polyphenoloxidase (PPO), Antibrowning agents, Inhibition mechanisms, Ascorbic acid, Cysteine, Citric acid, ESI-MS
Introduction
Reducing the undesirable browning process has gained much interest since it lowers the quality and nutritive value of fresh-cut vegetables (Toivonen and Brummell 2008). The browning process has been attributed mainly to the action of PPO on polyphenols producing quinones that are responsible for the developed color (Queiroz et al. 2008; Constabel and Barbehenn 2008; Chang 2009). Most strategies of controlling the browning process are either inhibiting PPO activity or converting quinones to colorless materials. Sulfites are well known reducing agents and used to be from the most effective and frequently used antibrowning agents; however, their use has been restricted because of their adverse effects on human health (Oliphant et al. 2012; Stohs and Miller 2014); therefore, many other antibrowning agents with various mechanisms have been introduced. Ascorbic acid is a good antioxidant (Amorati et al. 2011; Ali et al. 2013) and the most antibrowning agent widely used today (Golan-Goldhirsh et al. 1992; Whitaker 1994; Rupasinghe et al. 2005). Some reports attributed its action to the reduction of the formed quinones to the original colorless diphenols (Nicolas et al. 1994; Son et al. 2001; Limbo and Piergiovanni 2006); others considered ascorbic acid as a PPO inhibitor (Altunkaya and Gökmen 2008); however, the condition required to possess each mechanism is not defined. Cysteine was also investigated as antibrowning agent and its activity was attributed to various mechanisms i.e. its nucleophilic reactivity towards quinones to give colorless adduct (Richard-Forget et al. 1992; Friedman and Bautista 1995; Ding et al. 2002; Peñalver et al. 2002; Garcia-Molina et al. 2005), its inhibitory effect towards PPO (Altunkaya and Gökmen 2008) or its ability to reduce o-quinones to their polyphenol precursors (Cilliers and Singleton 1990). Polycaboxylic acids e.g. citric, tartaric, malic and succinic acids act as PPO activity either by lowering the pH or chelating the copper at the enzyme active site (Sedaghat and Zahedi 2012). Other reported browning inhibitors are some flavonoids, kojic acid (Chang 2009), p-alkoxybenzoic acids, (Chen et al. 2005), salicylic acid (Kumar et al. 2011) various dipeptides (Girelli et al. 2004) and 1-methylcyclopropene (Watkins 2008).
This work differentiated among the various antibrowning mechanisms of the title compounds under given conditions by using simple UV–vis analysis. Although the mechanism of antibrowning activity of cysteine may involve nucleophylic attack towards quinones to form colorless products, the formed cysteine-catechol adducts have not been separated and characterized; the present work isolated, separated and identified some of the colorless reaction products by LC-ESI-MS. Recently, quinones have been used to detect free thiol group of cysteine in peptides and proteins by ESI-MS (Diedrich and Julian 2010).
Materials and methods
Chemicals, instrumentation and plant materials
All chemicals used were reagent grade obtained from Sigma or Fluka Chemical Companies. The absorbance was recorded by Shimatzu 160A UV-Visible spectrophotometer; when needed, full scan (200–800 nm) was recorded. The lettuce cultivar (Great lakes) was obtained from Kaha Experimental Farm, Qalubia governorate, Egypt.
Sample preparation and treatment
Heads of lettuce (Lactuca Sativa L. cv. Great Lakes) were harvested at the proper stage of maturity from Kaha Experimental Farm, Qalubia governorate, Egypt. Good heads were transported immediately to the laboratory of postharvest center, Horticulture Research Institute. All leaves were removed and stems were cut by a sharp knife into round slices (1 cm thickness × 2.5 diameter) then dipped for 5 min. in distilled water (control) or one of the following solutions (1 %): Na2SO3, NaHSO3, ascorbic acid, L-cysteine and citric acid. All samples were air dried and every five slices were packed in sealed polypropylene bags of size (15 cm × 10 cm and 30 μm thickness) to serve as replicates. Twelve pages each containing 5 pieces were prepared for each treatment. Samples were arranged in a complete randomized design and stored at 2 ± 1 °C and 95 % relative humidity for 2, 5 and 8 days. At each interval, samples from three bags of each treatment were taken as replicates for measuring color change on the cutting edge; the same samples were subjected to enzyme extraction and activity determination. Each determination was performed in 5 replicates.
Enzyme extraction and activity determination
PPO (EC 1.14.18.1) was extracted by homogenizing treated or untreated vegetable samples with 1.5-fold their weight sodium phosphate buffer (0.1 M, pH 6.5) containing 30 mM sodium ascorbate and 0.4 M sucrose at 25 ºC. The crude extraction was filtered and refrigerated till used within 24 h. Optimum pH was determined at various pH (4–8) in 0.1 M sodium phosphate buffer and catechol as a substrate. Catechol (3 mL, 80.0 mM) dissolved in the phosphate buffer was mixed with 1.0 mL of enzyme extract. All of the enzymatic reactions were kept at the optimum condition (substrate saturation, pH 6.5 and 25 °C). The increase in absorbance of 0.01 per min. at 410 nm at the specified condition was defined as one unit of PPO activity. The results were expressed as the activity percentage of the respective zero experiment (Dogăn et al. 2002).
Enzyme inhibition mechanism
Enzyme extract (1.0 mL) was added to 3.0 mL catechol solution; the absorbance was then recorded at the zero time (100 %). After 60 s., the increase in absorbance was measured, then 10 μL of inhibitor solution dissolved in 50 % aqueous ethanol at various concentrations (or 10 μL solvent in control experiment) was added and the absorbance was recorded again immediately at various intervals till the end of the experimental period (600 s.). The absorbance change was used to express the activity percentage.
Inhibition kinetics
Inhibition kinetics of PPO was performed at the unsaturation level of substrate (catechol) at various final concentrations (0.84–7.15 mM). Inhibitor, ascorbic acid, cysteine or citric acid, (0.3 mL) was added to the assay solution to reach a final concentration (0.03–0.7 mM), then the remaining enzyme activity was determined after 30 s. Lineweaver-Burk curves (Lineweaver and Burk 1934) were used to calculate Km and KI (Dixon 1953).
Analysis of PPO-catechol-cysteine reaction products
PPO-catechol-cysteine reaction was performed under the PPO assay condition by mixing 3 mL catechol, 1 mL enzyme extract and 250 μL cysteine (0.2 M). After incubation for10 min at 25 °C, the reaction mixture was clarified by centrifugation and the supernatant was subjected to LC-ESI-MS analysis by Waters, Acquity UPLC H-Class instrument equipped with TQ triple quadropole ESI-MS detector and Acquity UPLC BEH C18 2.1×50 mm column contains trifunctional C18 stationery phase (1.7 μm particle size, 185 m2/g surface area and 0.7 g/ml pore volume). Mobile phase was methanol water (1:1 volume ratio) at flow rate 0.5 ml/min. ESI-MS was performed at both ES- and ES + modes with scan 100–1,000 m/e.
Color measurements
Color changes in fresh-cut vegetables were recorded by (chromameter CR-400, Minolta, Japan), calibrated against a standard white tile provided by the manufacture. Tristimulus values according to International Commission of Illumination, CIE (L, a, b) were recorded for three pieces per sample and measured immediately after cutting to determine the initial color.
The total color difference, ∆E, (Misnawi et al. 2003) and the browning indexes, BI, (Palou et al. 1999) were calculated according to following equations
Where ∆ refers to the difference between final and initial measurements.
Where X = (a + 1.75 L) / (5.645 L + a - 3.012 b).
Statistical analysis
The independent t-test was used to examine the mean differences between the treated samples and their respective zero or control experiments at p ≤ 0.01 (high significant) or p > 0.01–≤0.05 (significant). Lineweaver and Burk curves were generated by regression analysis. SPSS package (version 16) was used in the statistical analysis.
Results and discussion
Effects of some antibrowning agents on browning process and PPO activity in lettuce-head fresh-cut
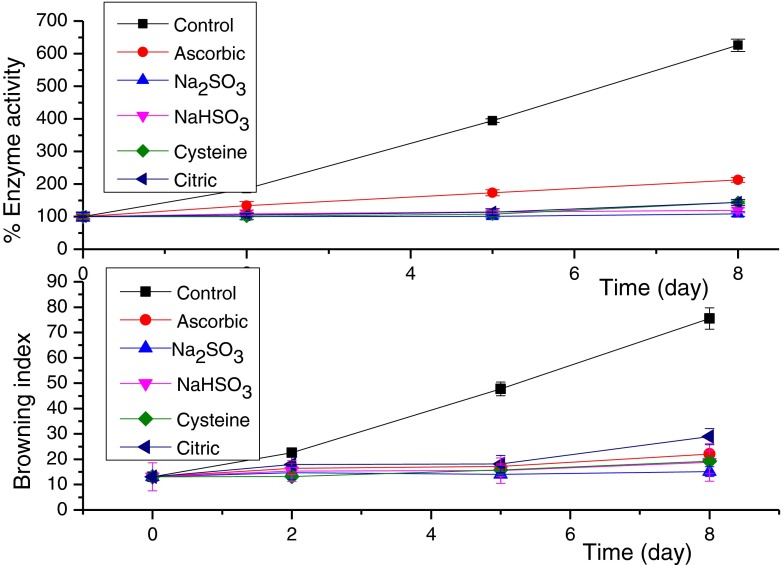
Optimum pH of lettuce-head polyphenoloxidase was determined to be used in enzyme activity determinations; the enzyme exhibited sensitivity towards the medium pH with the maximum activity at pH 6.5. Results presented in Table 1 and Fig. 1 showed the effect of the examined compounds on both PPO activity and the browning index (BI). The enzyme activity was considered 100 % at the zero time. The control experiment showed strictly a significant increase in the enzyme activity up to 625.66 % and in the browning index from 13.04 to 75.52 after 8 days. As shown in Table 1, all the examined compounds, cysteine, ascorbic acid, citric acid and sodium bisulfate, significantly reduced the PPO activity (p ≤ 0.01) to 143.6, 212.5, 144.0 and 119.3 %, and the browning index (p ≤ 0.05) to 19.2, 22.1, 28.9 and 18.8 respectively relative to the control group after 8 days.
Table 1.
Effect of storage period and browning inhibitors (1 %) on the browning process and PPO activity
| Storage period (day) | Compounds | PPO activity | Browning index | ∆E | Compounds | PPO activity | Browning index | ∆E |
|---|---|---|---|---|---|---|---|---|
| 0 | Control | 100.00 ± 8.33 | 13.04 ± 1.65 | Ascorbic Acid | 100.00 ± 6.25 | 13.04 ± 1.65 | ||
| 2 | 186.11 ± 9.62** | 22.54 ± 1.28** | 12.19 | 133.33 ± 13.01† | 16.38 ± 1.93 | 3.36 | ||
| 5 | 394.44 ± 5.39** | 47.78 ± 2.73** | 10.77 | 172.92 ± 9.46*‡ | 17.18 ± 1.11*‡ | 3.82 | ||
| 8 | 625.66 ± 18.92** | 75.52 ± 4.17** | 16.89 | 212.5 ± 7.8**‡ | 22.08 ± 0.53**‡ | 5.01 | ||
| 0 | Na2SO3 | 100.00 ± 12.5 | 13.04 ± 1.65 | L-Cysteine | 100.00 ± 7.69 | 13.04 ± 1.65 | ||
| 2 | 100.25 ± 9.46† | 14.64 ± 2.55‡ | 2.6 | 100.83 ± 10.32‡ | 13.14 ± 1.42† | 3.52 | ||
| 5 | 101.25 ± 5.2‡ | 14.02 ± 0.4‡ | 5.28 | 107.69 ± 7.69‡ | 15.73 ± 2.57‡ | 1.58 | ||
| 8 | 108.25 ± 7.22*‡ | 15.11 ± 1.97‡ | 3.32 | 143.58 ± 4.44*‡ | 19.22 ± 0.68*‡ | 4.48 | ||
| 0 | NaHSO3 | 100.00 ± 8.49 | 13.04 ± 1.65 | Citric acid | 100.00 ± 14.28 | 13.04 ± 1.65 | ||
| 2 | 108.36 ± 4.08 | 15.33 ± 1.65‡ | 11.41 | 104.76 ± 5.4‡ | 17.97 ± 0.61*‡ | 3.69 | ||
| 5 | 114.25 ± 4.99‡ | 15.52 ± 1.65**‡ | 1.75 | 113.25 ± 10.91‡ | 18.13 ± 3.32† | 3.57 | ||
| 8 | 119.25 ± 7.34‡ | 18.77 ± 2.47‡ | 3.08 | 143.98 ± 9.3*‡ | 28.94 ± 3.18**‡ | 6.17 |
*Significant different from Zero experiment at p > 0.01–≤0.05
**High significant different from Zero experiment at p ≤ 0.01
†Significant different from Control experiment at p > 0.01–≤0.05
‡High significant different from Control experiment at P ≤ 0.01
Fig. 1.
Effects of the browning agents on the lettuce PPO activity and browning process
The enzyme activity of treated samples correlated better with the browning index, BI, (r2 = 0.96, n = 19) than with the total color difference, ∆E, (r2 = 0.61, n = 18); it is reported that the browning index is an important parameter in determining the browning process (Palou et al. 1999). The correlation shows that the browning process depends mainly on the PPO activity and well represented by BI as shown by the following equation:
Mode of action of the examined antibrowning agents
To differentiate among the mechanisms of the examined antibrowning compounds, under given condition and crop, the antibrowning agents were added to the reaction mixture of enzyme extract and catechol after 60 s, i.e. after the quinone has been formed. The results presented in Fig. 2 showed that the sulfites immediately reduced the formed color which was not developed again till the end of the experimental period (600 s) even at low concentration (0.5 %).
Fig. 2.

Inhibition of PPO activity by various antibrowning agents at different concentrations
Ascorbic acid, showed different behavior depending on the concentration. At low conc. (0.5 %), ascorbic acid did not reduce the formed color but behaved as enzyme inhibitor where it lowered the color increase with time compared to the control (Fig. 2a). Gradually at higher concentrations (Fig. 2b and c), ascorbic acid behaved similar to sulfides where it could reduce instantly the formed color and acted as quinone reducer. A lag period was reported before any observed increase in absorbance when ascorbic acid (Altunkaya and Gökmen 2008; Dincer et al. 2002; Neves et al. 2009) or sulfites (Neves et al. 2009) were used in PPO assays, which could be attributed to their reducing power toward quinone. Cysteine effect was also concentration dependent, it could also remove the developed color at higher concentrations (≥1.0 %) but its effect appeared after 120 s, not instantly as in sulfides and ascorbic acid, indicating its reactivity towards quinone but in a slower reaction rate. On the other hand, citric acid did not remove the formed quinone color (Fig. 2d) but reduced the rate of developing color afterwards compared to the control experiment indicating their action as PPO inhibitors.
Another experiment was conducted to identify the different inhibition mechanisms; full scan of the UV–vis spectrum (200–800 nm) was recorded for the enzymatic reaction mixture in the absence or presence of an inhibitor. Fig. 3 indicated the following observations: (a) the reaction product of the enzymatic oxidation of catechol (Fig. 3a) shows the formation of a peak at 410 nm for the newly formed quinone product at various periods (2–10 min) in addition to the strong absorbance of catechol (214–280 nm). (b) Fig. 3b shows the spectra of catechol and ascorbic acid at the same assay concentrations. (c) Fig. 3c presents the spectra of the reaction mixture after 10 min and adding ascorbic acid or cysteine; both spectra show only the catechol peak and complete vanish of the 410 nm peak indicating the complete reduction of the formed quinone product to the original catechol or forming a colorless products. (d) Fig. 3d shows that addition of citric acid after 10 min of the assay reaction did not affect the formed quinone peak while when added at the zero time, the formation of quinone is almost completely inhibited; in other words, its action is mainly direct PPO inhibition.
Fig. 3.

UV–vis spectra of PPO assay under various conditions. a absorbance of PPO assay at various periods, b catechol and ascorbic acid spectra at the assay concentrations, c adding ascorbic acid or cysteine after 10 min, and d adding citric acid at the beginning or after 10 min
Lineweaver-Burk curves were used to assign the PPO inhibition mechanisms of the examined compounds at concentrations (0.03-0.70 mM). Km of uninhibited enzyme was 2.360 mM. Ascorbic acid and cysteine acted as PPO competitive inhibitors at the specified low concentrations while citric acid functions as non-competitive inhibitor with inhibition constant (KI) 0.256 ± 0.067, 1.113 ± 0.176 and 2.074 ± 0.363 mM respectively. Correlation coefficient of all regressions were ≥ 0.989.
Separation and identification of PPO-catechol-cysteine reaction products
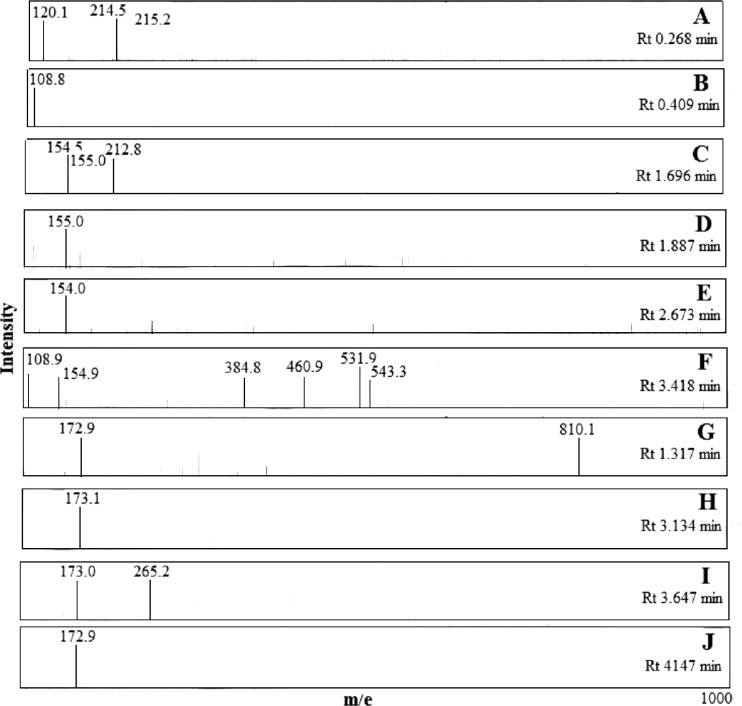
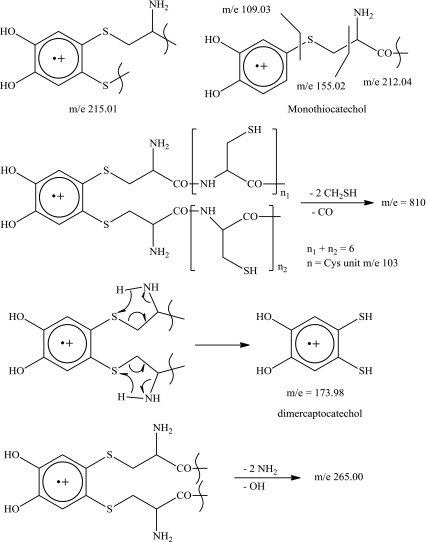
A model reaction of cysteine (Cys) and catechol (Cat) in the presence of PPO extract that oxidizes catechol to quinone was performed to identify some of the colorless reaction products. Negative LC-ESI-MS chromatogram (Fig. 4) detects two major peaks at 0.267 and 0.409 min and many minor products while positive mode gave similar chromatogram but could not resolve the major fractions and gave only one major peak at Rt 0.312 min; therefore, the mass spectra resulted from the negative mode were picked for further analysis. The main two fractions gave spectra shown in Fig. 5a and b respectively. The first spectrum (A) showed two stable fragments, the first fragment gave peak at m/e 120.1 for Cys-(H) moiety and the second fragment, for dithiocatechol (Scheme 1), gave two peaks at m/e 215.5 and 214.2 for M (m/e 215.01) and M-1 (m/e 214.00) respectively. Spectrum (B) showed a molecular ion and base peak at m/e 108.8 for M-1 of unreacted free catechol (M+ 110.04).
Fig. 4.
LC-ESI-MS (ES-) chromatogram of PPO-catechol-cysteine reaction products
Fig. 5.
Mass spectra of some LC-ESI-MS ,ES-) fractions of PPO-catechol-cysteine reaction products
Scheme 1.
Fragmentations and rearrangements of some PPO-catechol-cysteine reaction products
Fractions at Rt 1.696, 1.887 and 2.673 min gave spectra C, D and E respectively (Fig. 5); all spectra showed a base peak at m/e 155 while spectrum D showed also a peak at m/e 212.8. These fragmentations indicate the formation of monothiocatechol as presented in Scheme 1.
Fraction at Rt 3.418 min (spectrum F) showed a peak at m/e 108.9 indicating also a monothiocatechol product. The other significant peaks suggest condensation of catechol with up to 5 cys units where they appear at m/e 154.9 [Cat-SCH2], 384.8 [Cat-3Cys—(SH)], 460.9 [Cat-4Cys—(CO, 2NH2)], 531.9 [Cat-5Cys—(CO, CH2SH, OH)] and 543.3 [Cat-5Cys—(CH2SH, 2OH)].
Fraction at Rt 1.317 min gave spectrum G which showed two main peaks, one at m/e 810.1 suggesting the formation of dithiocatechol resulted from condensation of catechol with 8 cys units (Scheme 1). The second peak at m/e 172.9 is for M-1 of dimercaptocatechol (M + 173.98) resulted from rearrangement and hydrogen atom transfer process as presented in Scheme 1.
Fractions at Rt 3.134, 3.647 and 4.147 min (spectra H, I and J respectively) showed a strong or base peak at m/e 173 for the dimercaptocatechol discussed above (Scheme 1); spectrum H gave also a base peak at m/e 265.2 for condensation of catechol with two cys units (Scheme 1).
These results indicate that the reaction can be through one or two nucleophilic attacks of cysteine thiol group towards quinones, formed by oxidation of catechol in the presence of PPO enzyme, to give mono or dithiocatechol adducts respectively (Scheme 2); however, no tri- or tetrathiocatechol (m/e 205.95 and 237.92 respectively) were observed in any spectra under the examined condition. Peptide side chains were also detected. Crude enzyme extract is used to be close model to living cell; peptidases that may be present in the extract could catalyze the detected peptide side chains.
Scheme 2.
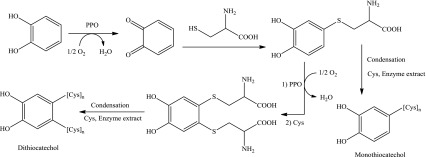
PPO-catechol-cysteine reactions
References
- Ali HM, Abo-Shady A, Sharaf Eldeen HA, Soror HA, Shousha WG, Abdel-Barry OA, Saleh AM. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem Cent J. 2013;7:53–61. doi: 10.1186/1752-153X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altunkaya A, Gökmen V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa) Food Chem. 2008;107:1173–1179. doi: 10.1016/j.foodchem.2007.09.046. [DOI] [Google Scholar]
- Amorati R, Pedulli GF, Valgimigli L. Kinetic and thermodynamic aspects of the chain-breaking antioxidant activity of ascorbic acid derivatives in non-aqueous media. Org Biomol Chem. 2011;9:3792–3800. doi: 10.1039/c1ob05334e. [DOI] [PubMed] [Google Scholar]
- Chang T-S. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q-X, Song K-K, Qiu L, Liu X-D, Huang H, Guo H-Y. Inhibitory effects on mushroom tyrosinase by p-alkoxybenzoic acids. Food Chem. 2005;91:269–274. doi: 10.1016/j.foodchem.2004.01.078. [DOI] [Google Scholar]
- Cilliers JJL, Singleton VL. Caffeic acid autooxidation and the effects of thiols. J Agric Food Chem. 1990;38:1789–1796. doi: 10.1021/jf00099a002. [DOI] [Google Scholar]
- Constabel CP, Barbehenn R (2008) defensive roles of polyphenol oxidase in plants. In: Schaller A (ed) Induced plant resistance to herbivory. Springer Science + Business Media BV, pp 253–269
- Diedrich JK, Julian RR. Site-selective fragmentation of peptides and proteins at quinone-modified cysteine residues investigated by ESI-MS. Anal Chem. 2010;82:4006–4014. doi: 10.1021/ac902786q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincer B, Colak A, Aydin N, Kadioglu A, Güner S. Characterization of polyphenoloxidase from medlar fruits (Mespilusgermanica L., Rosaceae) Food Chem. 2002;77:1–7. doi: 10.1016/S0308-8146(01)00359-4. [DOI] [Google Scholar]
- Ding C-K, Chachin K, Ueda Y, Wang CY. Inhibition of loquat enzymatic browning by sulfhydryl compounds. Food Chem. 2002;76:213–218. doi: 10.1016/S0308-8146(01)00270-9. [DOI] [Google Scholar]
- Dixon M. The determination of enzymeinhibition constants. Biochem J. 1953;55:170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogăn M, Aslan O, Dogăn S. Substrate specificity, heat inactivation and inhibition of polyphenol oxidase from different aubergine cultivars. Int J Food Sci Technol. 2002;37:415–423. doi: 10.1046/j.1365-2621.2002.00580.x. [DOI] [Google Scholar]
- Friedman M, Bautista FF. Inhibition of polyphenol oxidase by thiol in the absence and presence of potato tissue suspensions. J Agric Food Chem. 1995;43:69–76. doi: 10.1021/jf00049a014. [DOI] [Google Scholar]
- Garcia-Molina F, Penalver MJ, Rodriguez-Lopez JN, Garcia-Canovas F, Tudela J. Enzymatic method with polyphenol oxidase for the determination of cysteine and N-acetylcysteine. J Agric Food Chem. 2005;53:6183–6189. doi: 10.1021/jf050197k. [DOI] [PubMed] [Google Scholar]
- Girelli AM, Mattei E, Messina A, Tarola AM. Inhibition of polyphenol oxidases activity by various dipeptides. J Agric Food Chem. 2004;52:2714–2745. doi: 10.1021/jf0305276. [DOI] [PubMed] [Google Scholar]
- Golan-Goldhirsh A, Osuga DT, Chen AO, Whitker JR. Effect of ascorbic acid and copper on proteins. In: O´Souza VT, Feder J, editors. The bioorganic chemistry of enzymatic catalysis. Boca Raton: CRC Press; 1992. pp. 61–76. [Google Scholar]
- Kumar D, Mishra DS, Chakraborty B, Kumar P. Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbo S, Piergiovanni L. Shelf life of minimally processed potatoes part 1. Effects of high oxygen partial pressures in combination with ascorbic and citric acids on enzymatic browning. Postharvest Biol Technol. 2006;39:254–264. doi: 10.1016/j.postharvbio.2005.10.016. [DOI] [Google Scholar]
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. doi: 10.1021/ja01318a036. [DOI] [Google Scholar]
- Misnawi JS, Jamilah B, Nazamid S. Effects of incubation and polyphenol oxidase enrichment on colour, fermentation index, procyanidins and astringency of unfermented and partly fermented cocoa beans. J Food Sci Technol. 2003;38:285–295. doi: 10.1046/j.1365-2621.2003.00674.x. [DOI] [Google Scholar]
- Neves VA, Picchi DG, da Silva MA. Some biochemical properties of polyphenoloxidase from spearmint (Menthaarvensis) Braz Arch Biol Technol. 2009;52:1001–1010. doi: 10.1590/S1516-89132009000400025. [DOI] [Google Scholar]
- Nicolas JJ, Richard-Forget FC, Goupy PM, Amiot MJ, Aubert SY. Enzymatic browning reactions in apple and products. Crit Rev Food Sci Nutr. 1994;34:109–157. doi: 10.1080/10408399409527653. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Mitra A, Wilkinson M. Contact allergy to sodium sulfite and its relationship to sodium metabisulfite. Contact Dermatitis. 2012;66:128–130. doi: 10.1111/j.1600-0536.2011.02029.x. [DOI] [PubMed] [Google Scholar]
- Palou E, López-Malo A, Barbosa-Cánovas GV, Welti-Chanes J, Swanson BG. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J Food Sci. 1999;64:42–45. doi: 10.1111/j.1365-2621.1999.tb09857.x. [DOI] [Google Scholar]
- Peñalver MJ, Rodríguez-López JN, García-Molina F, García-Cánovas F, Tudela J. Method for the determination of molar absorptivities of thiol adducts formed from diphenolic substrates of polyphenol oxidase. Anal Biochem. 2002;309:180–185. doi: 10.1016/S0003-2697(02)00312-3. [DOI] [PubMed] [Google Scholar]
- Queiroz C, Lopes MM, Fialho E, Valente-Mesquita VL. Polyphenol oxidase: characteristics and mechanisms of browning control. Food Rev Int. 2008;24:361–375. doi: 10.1080/87559120802089332. [DOI] [Google Scholar]
- Richard-Forget FC, Goupy PM, Nicolas JJ. Cysteine as an inhibitor of enzymatic browning. 2. kinetic studies. J Agric Food Chem. 1992;40:2108–2113. doi: 10.1021/jf00023a014. [DOI] [Google Scholar]
- Rupasinghe HPV, Murr DP, DeEll JR, Odumeru J. Influence of 1-methylcyclopropene and NatureSeal on the quality of fresh-cut “Empire” and “Crispin” apples. J Food Qual. 2005;28:289–307. doi: 10.1111/j.1745-4557.2005.00035.x. [DOI] [Google Scholar]
- Sedaghat N, Zahedi Y. Application of edible coating and acidic washing for extending the storage life of mushrooms (Agaricus bisporus) Food Sci Technol Int. 2012;18:523–530. doi: 10.1177/1082013211433075. [DOI] [PubMed] [Google Scholar]
- Son SM, Moon KD, Lee CY. Inhibitory effects of various antibrowning agents on apple slices. Food Chem. 2001;73:23–30. doi: 10.1016/S0308-8146(00)00274-0. [DOI] [Google Scholar]
- Stohs SJ, Miller MJS. A case study involving allergic reactions to sulfur-containing compounds including, sulfite, taurine, acesulfame potassium and sulfonamides. ChemToxicol. 2014;63:240–243. doi: 10.1016/j.fct.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Toivonen PMA, Brummell DA. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol Technol. 2008;48:1–14. doi: 10.1016/j.postharvbio.2007.09.004. [DOI] [Google Scholar]
- Watkins CB. Overview of 1-methylcyclopropene trials and uses for edible horticultural crops. HortSci. 2008;43:86–94. [Google Scholar]
- Whitaker JR. Principals of enzymology for the food science. 2. New York: Marcel Dekker; 1994. [Google Scholar]