Abstract
The oil in mackerel muscle was extracted using an environmental friendly solvent, supercritical carbon dioxide (SC-CO2) at a semi-batch flow extraction process and an n-hexane. The SC-CO2 was carried out at temperature 45 °C and pressures ranging from 15 to 25 MPa. The flow rate of CO2 (27 g/min) was constant at the entire extraction period of 2 h. The highest oil extracted residues after SC-CO2 extraction was used for activity measurement of digestive enzymes. Four digestive enzymes were found in water soluble extracts after n-hexane and SC-CO2 treated samples. Amylase, lipase and trypsin activities were higher in water soluble extracts after SC-CO2 treated samples except protease. Among the four digestive enzymes, the activity of amylase was highest and the value was 44.57 uM/min/mg of protein. The water soluble extracts of SC-CO2 and n-hexane treated mackerel samples showed same alkaline optimum pH and pH stability for each of the digestive enzymes. Optimum temperature of amylase, lipase, protease and trypsin was 40, 50, 60 and 30 °C, respectively of both extracts. More than 80 % temperature stability of amylase, lipase, protease and trypsin were retained at mentioned optimum temperature in water soluble extracts of both treated samples. Based on protein patterns, prominent protein band showed in water soluble extracts after SC-CO2 treated samples indicates no denaturation of protein than untreated and n-hexane.
Keywords: Digestive enzymes, Enzyme activity, Mackerel muscle, pH stability, Temperature stability
Introduction
For higher efficiency of enzyme isolation, lipid removal is needed from the sample. Conventional methods for removal of oil from fish involve cooking, pressing, and/or liquid extraction. Removal of lipids with organic solvents causes protein denaturation and loss of functional properties (Pariser et al. 1978). Supercritical fluid extraction (SFE) is an efficient alternative for the extraction of natural substances from foods (Mendes et al. 2003; Sun and Temelli 2006). In recent years, the use of SFE for the removal of organic compounds from different liquid and solid matrices has been attracted much attention. This technique has some advantages over more conventional separation techniques, largely due to the unique physical properties of SFs. A SF separation process using carbon dioxide as the solvent offers potential advantages because it is non-flammable, non-toxic, inert to most materials, inexpensive, and can be used under mild operational conditions (Ge et al. 2002). Supercritical carbon dioxide has been used for extraction of lipid from different marine organisms (Yamaguchi et al. 1986; Temelli et al. 1995; Park et al. 2008). But attentions are getting increased on the protein after lipid separation using SC-CO2 extraction.
Mackerel belongs to the family of Scombridae, and it is abundant in cold and temperate shelf areas. It contains a lot of protein, essential amino acid, lipid and many kinds of biological active compound. Various digestive enzymes are naturally present in fish. In recent years, recovery and characterization of enzymes from fish and aquatic invertebrates has gained importance and this has led to the emergence of some interesting new applications of these enzymes in food processing (Shahidi and Janak-Kamil 2001; Uddin et al. 2009; Abdelkarder et al. 2012).
Enzymes are large biological molecules which can function uniquely to control process time, enhance flavour, improve texture, extend shelf life and decrease the use of chemical food additives. Enzymes have been used as processing aids in various food related industries for a long time (Simpson et al. 1991; Vilhelmsson 1997). Digestive enzymes have also many other important applications for different food related industries. Amylase is commonly used in brewing and wine making, processed foods and other commercially available food supplements (Kubrak 2007). Lipolytic enzymes are currently attracting for their biotechnological potentiality. They constitute the most important group of biocatalysts for biotechnological applications. Some of the industrially important chemicals manufactured from fats and oils by chemical processes could be produced by lipases with greater rapidity and better specificity under mild conditions (Vulfson 1994). Proteases execute a large variety of functions and have important biotechnological applications and widely used in detergent industries, dairy industries and meat industries. The applications of proteases in medical industries are also growing widely (Djamel et al. 2009). Trypsin is a well-studied serine-protease having a lysine or arginine residue (Perona and Craick 1995) which is used in the food industries as a baking enzyme to improve the workability of dough, to improve the texture of fish products and to control aroma formation in cheese and milk products.
Therefore, the aim of this study was to measure the digestive enzyme activities and characterizes the digestive enzymes from de-oiled mackerel muscle after lipid extraction using SC-CO2 and n-hexane.
Materials and methods
Materials
Mackerel was collected from Busan Cooperative Fish Market (Seo-gu, Busan, Korea). The muscle was separated by mechanically and washed thoroughly with cold distilled water in the laboratory. Pure carbon dioxide (99.99 %) was supplied by KOSEM (Sangbuk-myeon Yangsan, Korea). All other chemicals used in this study were of analytical or HPLC grade.
Sample preparation
The mackerel muscle was dried in a freeze-dryer for about 72 h. The dried samples were crushed by a mechanical blender (PN, SMKA-4000, Ansan, Korea). These samples called freeze dried mackerel muscle were then stored at −20 °C prior to SC-CO2 and n-hexane extraction.
SC-CO2 extraction
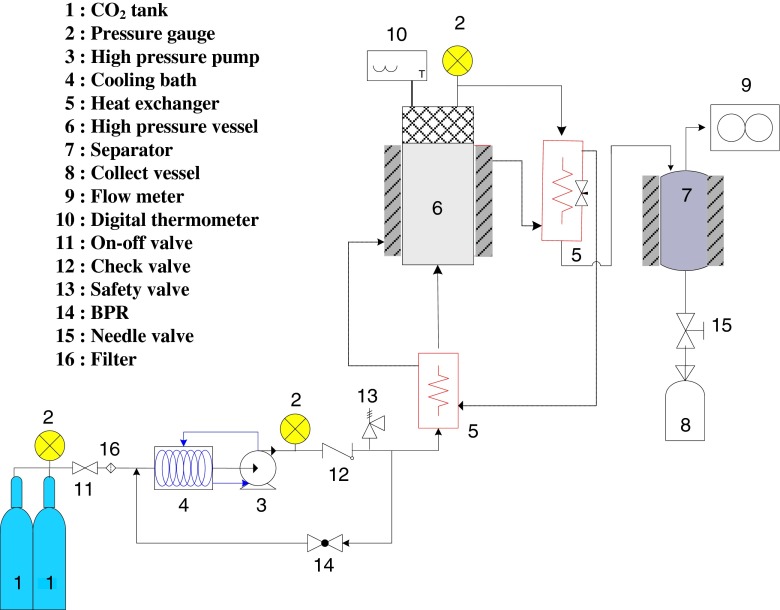
The set up of a laboratory scale of supercritical fluid extraction (SFE) process is shown in Fig. 1. Exactly, 20 g of freeze dried raw mackerel muscle was loaded into the stainless steel extraction vessel which was 200 mL in volume. A thin layer of cotton was placed at the bottom of the extraction vessel. Before plugging with cap another layer of cotton was used at the top of the sample. CO2 was pumped at constant pressure into the extraction vessel by high pressure pump (Milroyal, Milton Roy, USA) up to the desired pressure. A back pressure regulator was used to control the pressure of CO2. The extraction temperature was maintained by connecting the extraction vessel with water bath. Flow rates and accumulated gas volume passing through the apparatus were measured using a gas flow meter (Shinagawa, Tokyo, Japan). After SC-CO2 extraction, the mackerel muscle residues remaining in the vessel was stored at −20 °C until further use and analysis. Mackerel muscle was extracted at temperature 45 °C and pressure ranging from 15 to 25 MPa for 2 h using SC-CO2. The flow rate of CO2 was kept constant at 27 g/min for all extraction conditions.
Fig. 1.
Schematic diagram of SC-CO2 extraction process
n-Hexane extraction
The extraction was carried out using hexane as solvent. Exactly, 40 g of freeze dried raw mackerel muscle with 200 mL hexane was placed into the beaker and stirred 20 h by a magnetic stirrer at 45 °C and 300 rpm. After extraction, the hexane solution was filtered by a filter paper and then evaporated in a rotary vacuum evaporator (EYELA N-1100, Tokyo, Japan) at 40 °C. The remaining residue was dried using dry oven at 40 °C for 6 h and then residues and oil was stored at −20 °C until further use and analysis.
Water soluble extract preparation
The highest oil extracted mackerel muscle residues after SC-CO2 extraction and residues after n-hexane extraction were homogenized in cold distilled water (1 g sample/7 mL of water) by mechanical stirring at 4 °C for 2 h. The samples were then centrifuged at 7,500 rpm for 15 min at 4 °C. The supernatants were collected and stored at −20 °C. These samples called water soluble extracts which were used for protein estimation, digestive enzyme activity measurement and their characterization.
Measurement of protein content in water soluble extract
The protein content of the water soluble extract was assayed according to the method of Lowry et al. (1951). Bovine serum albumin (mg/mL) as a standard was used to construct a standard calibration curve.
Digestive enzyme assay of water soluble extract
Amylase assay
Amylase activity was determined by the dinitrosalicylic (DNS) acid method (Miller 1959). The test tube containing 0.5 mL of water soluble extract which is equilibrated at 37 °C for 5 min and 0.5 mL of 1.0 % (w/v) potato starch (Sigma Chemical Co., St. Louis, Mo., USA) in 0.016 M sodium acetate buffer (pH 6.0) was incubated at 37 °C for 10 min. After incubation, 1 mL of DNS solution was added in each tube. All tubes were heated at boiling water bath for 5 min to stop the reaction. After cooling, 10 mL of distilled water was added and mixed well. Absorbance was taken at 540 nm. One unit of amylase activity was defined as the amount of enzyme that released 1 μmol (uM) of reducing end groups per minute. D-glucose was used to construct a standard calibration curve.
Lipase assay
Lipase activity was assayed by the modified method of Uddin et al. (2009). The substrate emulsions were prepared by drop wise addition of 0.2 mL solution A (40 mg of p-nitrophenyl laurate was dissolved in 12.0 mL of isopropanol) into 3.0 mL of solution B (0.4 g Triton X-100 and 0.1 g Gum Arabic were dissolved in 90 mL of 0.1 M potassium phosphate buffer, pH 7.5) under intense vortexing. These emulsions were stable for 1 h at room temperature. 0.2 mL of water soluble extract was added to 3.2 mL of the substrate emulsion and the mixture was incubated for 10 min in a shaking water bath at 37 °C. The reaction was terminated by boiling for 5 min. After centrifugation at 3,000 rpm for 15 min, the absorbance of the clear supernatant was measured at 410 nm. The mixture with 0.2 mL of the inactivate enzyme extract (heated at 100 °C for 5 min) was used as a control. One unit of enzyme activity was defined as the amount of enzyme required for the liberation of 1 μmol (uM) p-nitrophenol from p-nitrophenyl laurate per minute under the assay conditions.
Protease assay
Protease activity was assayed by the casein Folin-Ciocalteau method (Oda and Murao 1974) with slight modification. 1.14 % casein solution in 0.1 M glycine-NaOH buffer (pH 9.6) was used as a substrate. Water soluble extract (0.5 mL) was mixed with 2.0 mL of substrate and it was incubated for 10 min at 37 °C. The reaction was stopped by the addition of 2.5 mL of 0.44 M trichloroacetic acid (TCA) solution and settled down for 20 min. Then the sample was centrifuged for 10 min at 3,000 rpm. The supernatant was mixed with 2.5 mL of 0.5 M Na2CO3 and 0.5 mL of 2 N Folin-Ciocalteu reagents. The solution was kept in incubator at 37 °C for 20 min for colour developed and absorbance was measured at 660 nm (UVIKON 933, Kontron Instruments). One unit of protease activity was defined as the amount of enzyme required for liberating 1 μmol of tyrosine per min from casein. Tyrosine was used to construct a calibration curve.
Trypsin assay
Trypsin activity was measured by the modified method of Bergmeyer et al. (1974). 0.25 mM Nα-benzoyl-L-arginine ethyl ester in 67 mM sodium phosphate buffer, pH 7.6 was used as substrate. The water soluble extract (0.2 mL) was added to 3.0 mL of the substrate solution and the mixture was incubated at 35 °C for 10 min in a water bath. The reaction was terminated by boiling for 5 min. After cooling, the sample was centrifuged at 3,000 rpm for 10 min and the absorbance of the clear supernatant was measured at 253 nm. 0.2 mL of 1 mM hydrochloric acid solution instead of crude extract was used as a blank. One unit of enzyme activity was defined as the amount of enzyme required for the liberation of 1 μmol (uM) Nα-benzoyl-L-arginine from Nα-benzoyl-L-arginine ethyl ester per minute under the assay conditions.
Optimum pH and pH stability of amylase, lipase, protease and trypsin
Different buffers of wide range of pH values were used to evaluate the effect of pH on digestive enzyme activity. The buffers used were 0.1 M citric acid-sodium citrate (pH 4 ~ 6), 0.1 M potassium phosphate (pH 7 ~ 8.5) and 0.1 M glycine-NaOH (pH 10 ~ 12). The pH stability was measured by 12 h pre-incubation of the water soluble enzyme extract in buffers that had the same ionic concentrations at different pH values ranging from 4.0 to 12.0 at 4 °C. The enzyme activities were measured immediately after this treatment with the standard methods as mentioned above.
Optimum temperature and temperature stability of amylase, lipase, protease and trypsin
For the effect of optimum temperature on digestive enzyme in water soluble extract used different buffers such as potassium phosphate (0.1 M, pH 8.5) buffer for amylase, lipase and protease and 0.1 M glycine-NaOH (pH 10) buffer for trypsin. Enzyme activity was determined by performing the standard assay as mentioned within a temperature range of 20 ~ 80 °C. Temperature stability was measured by incubation of water soluble enzyme extract at temperature ranging from 20 to 80 °C for 2 h in a constant temperature of water bath. After treatment, the residual enzyme activities were analyzed under standard assay conditions as mentioned above.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE of water soluble extracts were carried out by the method of Laemmli (1970) using 4.4 % (w/v) stacking gel and 10 % (w/v) separating gel. Water soluble extracts were mixed with sample buffer at the ratio of 1:2 (v/v). Then 10 μL of the sample was loaded in each well. Electrophoresis was performed using a Mini-Protein III cell module (Bio-Rad Laboratories, CA, USA) at a constant voltage (30 mA for 1.5 h). Molecular weight markers (Sigma Chemical Co., St. Louis, Mo., USA) were used to estimate the molecular weight of protein.
Statistical analysis
All experiments were carried out in triplicate. The data were expressed to analysis of variance (ANOVA) and the differences between means were evaluated by Duncan’s multiple range test. SPSS statistics programme (SPSS version 15.0 for windows, SPSS Inc., Chicago, IL, USA) was used for data analysis.
Results and discussion
Total oil extraction
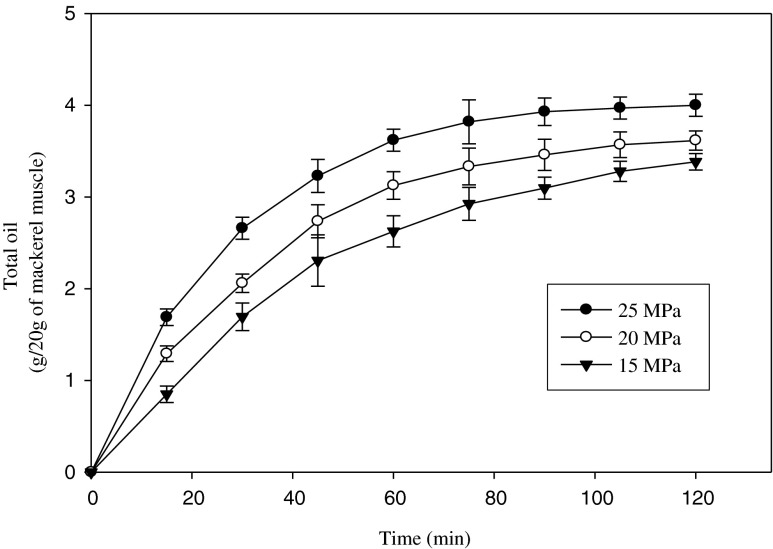
The extraction curves of mackerel muscle oil by SC-CO2 at temperature 45 °C and pressure ranging from 15 to 25 MPa are shown in Fig. 2. The highest oil obtained by SC-CO2 extraction was 4.00 ± 0.11 g/20 g of mackerel muscle at temperature 45 °C and pressure 25 MPa. At constant temperature, the amount of oil extracted from mackerel muscle was increased with increasing pressure. Due to the increase in pressure, the density of SC-CO2 was increased and hence the solvating power. The effect of pressure can be attributed to the increase in solvent power and by the strengthening of intermolecular physical interactions (Morita and Kajimoto 1990). The total amount of oil obtained by SC-CO2 extraction was 20.00 ± 0.54 % at 45 °C and 25 MPa while the oil obtained from mackerel muscle by hexane was 25.62 ± 0.62 % (data not shown). The observed difference in maximum yield may have been due to variations in the processing unit, operating conditions and so on.
Fig. 2.
SC-CO2 extracted oil from mackerel muscle at temperature 45 °C and pressure ranging from 15 to 25 MPa. Bars represents the standard deviation (n = 3)
Protein yield in water soluble extract
The protein content in water soluble extract from freeze dried, SC-CO2 extracted at temperature 45 °C and pressure 25 MPa and hexane extracted residues are shown in Table 1. It was found that the water soluble extract of SC-CO2 extracted residues contained more protein than freeze dried residues and hexane extracted residues. These occurrences can be happened that presence of lipid in the raw materials made them less accessible to water and due to the long time hexane extraction, protein concentration in residues may be reduced. Pariser et al. (1978) reported that protein denaturation is occurred by the removal of lipids with conventional liquid solvents. The protein yield in water soluble extract from freeze dried, SC-CO2 and hexane extracted residues were 5.02 ± 0.07, 9.28 ± 0.09 and 6.53 ± 0.06 mg/mL, respectively.
Table 1.
Protein yield of water soluble extract from freeze dried, SC-CO2 extracted and hexane extracted residues
| Protein concentration (mg/mL) | |
|---|---|
| Water soluble extract after freeze dried | 5.02 ± 0.07c |
| Water soluble extract after SC-CO2 extraction | 9.28 ± 0.09a |
| Water soluble extract after hexane extraction | 6.53 ± 0.06b |
Means ± SD (n = 3). Different small letters in the same column indicate significant differences (P < 0.05)
Digestive enzyme activities
The activities of amylase, lipase, protease and trypsin of SC-CO2 and hexane treated water soluble extracts of mackerel muscle are shown in Fig. 3a–d. Among the four digestive enzymes, the activity of amylase was highest. Protease activity was slightly higher in n-hexane treated sample than SC-CO2 and the activities of amylase, lipase and trypsin were higher in SC-CO2 treated samples compared to n-hexane treated samples. This may have resulted from a loss of digestive enzyme activity in mackerel samples by the n-hexane treatment. This means enzymes are not stable in the presence of organic solvent and are susceptible to denaturation. Some authors reported similar observation (Ogino et al. 1994).
Fig. 3.
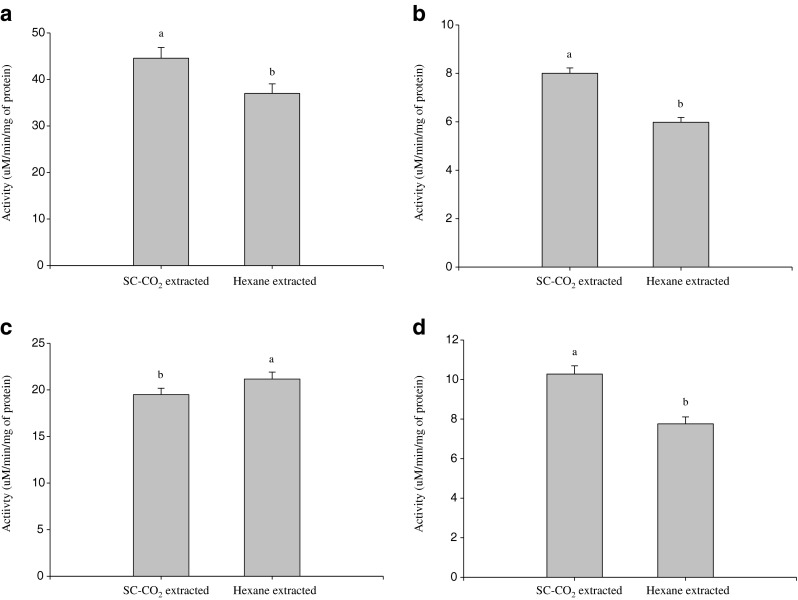
Activities of digestive enzymes in SC-CO2 and n-hexane treated mackerel muscle residues extract a Amylase, b Lipase, c Protease and d Trypsin. Bars represents the standard deviation (n = 3). Different small letters on the top of column in each figure indicate significant differences (P < 0.05)
Optimum pH
The optimum pH of amylase, lipase, protease and trypsin are shown in Fig. 4a–d. The activities of amylase in the SC-CO2 and hexane treated water soluble extracts of mackerel muscle were maximal at pH 8.5. An optimum pH of 7 ~ 11 was reported for amylase obtained from the Siamese fighting fish (Karun et al. 2010). The water soluble extract of both treated mackerel was found optimum lipolytic activity at pH 8.5. The optimum pH 7 ~ 11 for lipase activities were reported for fish and other sources (Kumar et al. 2005; Uddin et al. 2009; Karun et al. 2010). The highest proteolytic activity of water soluble extracts after SC-CO2 and hexane treated mackerel was found at alkaline pH 8.5. High protease activities at pH ranging from 8.0 to 10.0 have also been reported in several fish species (Eshel et al. 1993; Hidalgo et al. 1999; Poonsuk and Thiraratana 2008). Low protease activities were found in acidic pH. Among the acidic pH, the protease activity was high at pH 6. This indicates that the water soluble extracts of mackerel contained both acidic and alkaline proteases. Similar results were found by Natalia et al. (2004) from carnivorous ornamental fish and Uddin et al. (2009) from squid viscera. The trypsin in water soluble extracts showed maximal hydrolytic activity at alkaline pH 10 from both treated mackerel residues. The activity of trypsin decreased at pH 11 and 65 % of activity was remained of that pH, probably as a result of the denaturation of enzyme. The alkaline proteinase, trypsin from the intestine of nile tilapia and bigeye snapper showed high activity at pH range of 7 ~ 10 and 8 ~ 11, respectively (Bezerra et al. 2005; Pham and Soottawat 2006). In the water soluble extracts of SC-CO2 and hexane treated mackerel residues, pH differences effect on digestive enzyme activities were not significant.
Fig. 4.
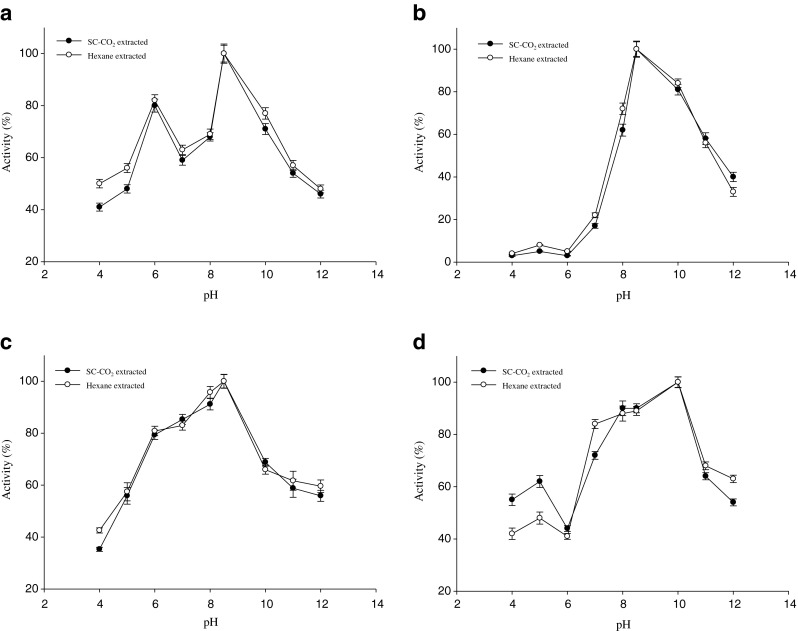
Optimum pH of digestive enzymes in SC-CO2 and n-hexane treated mackerel muscle residues extract a Amylase, b Lipase, c Protease and d Trypsin. Bars represents the standard deviation (n = 3)
pH stability
Figure 5a–d shows the pH stability of digestive enzymes of water soluble extract from SC-CO2 and n-hexane treated mackerel residues. Amylase activity was retained more than 88 % activity at pH 6.0 and 8.5–10.0 from mackerel extracts. Noman et al. (2006) and Uddin et al. (2009) reported that more than 80 % and almost 90 % amylase activity was retained at the pH range of 6.0 ~ 8.0 and 6.0 ~ 8.5, respectively of Pachyrhizus erosus L. tuber and squid viscera, which was almost similar to mackerel muscle residues. Lipase was stable within a pH range of 8.5–10.0 in water soluble mackerel extract, where more than 85 % activity was retained. Kumar et al. (2005) and Aryee et al. (2007) reported that the stability of lipase was at pH range of 8.0 ~ 10.5 with 75 % activity of Bacillus coagulans BTS-3 and pH of 7.0 ~ 10.0 with 70 % activity of grey mullet, respectively. From water soluble extract of mackerel, protease activities were found more than 86 % of its original value, in the range of pH 7.0 ~ 8.5 and then decreased with increasing pH. The pH stability of water soluble extracts of tuna and squid viscera were reported to be in the range of 9.0 ~ 11.0 and 8.0 ~ 10.5, where more than 90 % activity was retained (Parsertsan et al. 2001; Uddin et al. 2009). Trypsin stability of mackerel extract was in alkaline pH range of 8.0 ~ 10.0, above 85 % activity were retained of that pH. However, pH stability was decreased in acidic conditions. At acidic pHs, the conformational changes of enzyme took place and enzyme could not bind to the substrate properly (Klomklao et al. 2006). Trypsin from pyloric caeca of bigeye snapper was stable in the neutral and alkaline pH range of 7 ~ 12 with more than 90 % activity (Pham and Soottawat 2006). However, pH stability of all digestive enzymes in mackerel muscle extract from SC-CO2 and hexane treated was identical.
Fig. 5.
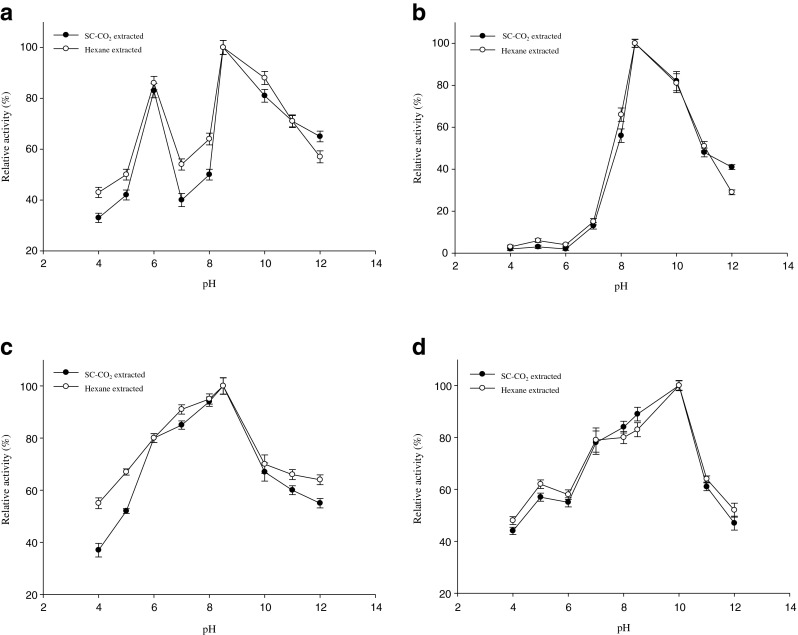
pH stability of digestive enzymes in SC-CO2 and n-hexane treated mackerel muscle residues extract a Amylase, b Lipase, c Protease and d Trypsin. Bars represents the standard deviation (n = 3)
Optimum temperature
The maximum activities of amylase, lipase, protease and trypsin in both SC-CO2 and n-hexane treated mackerel water soluble extracts were found at the temperature of 40, 50, 60 and 30 °C, respectively shown in Fig. 6a–d. The optimum temperature for amylase in the water soluble extract of tuber and squid viscera was 37 °C (Noman et al. 2006; Uddin et al. 2009) which is slightly lower than mackerel extract. Karun et al. (2010) reported that optimum lipase activity was found at 40 °C from Siamese fighting fish which was lower than mackerel extract. The optimum temperature of protease from tampaqui waste was 60 °C (Espósito et al. 2009) which was similar compared to mackerel protease. The optimal temperature of trypsin in mackerel muscle was lower than those of cod, capelin, anchovy and sardine, which ranged from 40 to 45 °C (Murakami and Noda 1981; Simpson and Haard 1984; Martinez et al. 1988). The differences in temperature optima may be due to several factors including different living temperatures, extracts from different sources, the different substrates used for measurements since different substrate may exhibit temperature activity differences with enzymes.
Fig. 6.
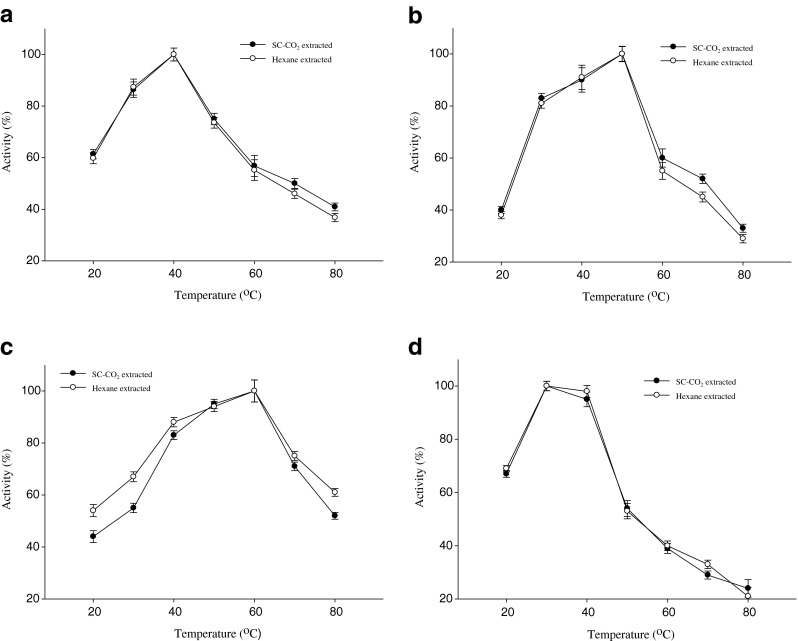
Optimum temperature of digestive enzymes in SC-CO2 and n-hexane treated mackerel muscle residues extract a Amylase, b Lipase, c Protease and d Trypsin. Bars represents the standard deviation (n = 3)
Temperature stability
Temperature stability of amylase, lipase, protease and trypsin in SC-CO2 and n-hexane treated mackerel extracts are shown in Fig. 7a–d. For amylase, more than 90 % of activities in mackerel extract were found up to 40 °C. Noman et al. (2006) and Uddin et al. (2009) reported that temperature stability of α-amylase from tuber and squid viscera were 80 % up to 40 °C which was lower compared to mackerel amylase. The water soluble extracts of mackerel retained above 90 % lipase activity up to 50 °C. Above 70 % of lipase activity in viscera of grey mullet was retained up to 50 °C (Aryee et al. 2007). At same temperature residual lipase activity was high in extract of mackerel residues than grey mullet. This might be happened due to very little change of conformation or properties of active site by temperature and high pressure during extraction by SC-CO2. The protease activities of SC-CO2 and n-hexane treated mackerel extracts were remained above 86 % up to 60 °C. Uddin et al. (2009) and Espósito et al. (2009) reported that the protease from fish waste retained about 85 and 86 % of activities up to 60 °C which was similar to mackerel residues. More than 80 % trypsin activity was retained up to 40 °C in mackerel extracts but lost its activity rapidly at temperatures above 40 °C. At high temperature, the enzyme possibly underwent denaturation and lost its activity. Abdelkarder et al. (2012) reported similar observation. Trypsin from the Atlantic blue crab was stable at a temperature ranging from 30 to 50 °C for 30 min (Dendinger and O’Connor 1990). Similar temperature stability of all digestive enzymes was observed from SC-CO2 and hexane treated mackerel muscle extract.
Fig. 7.
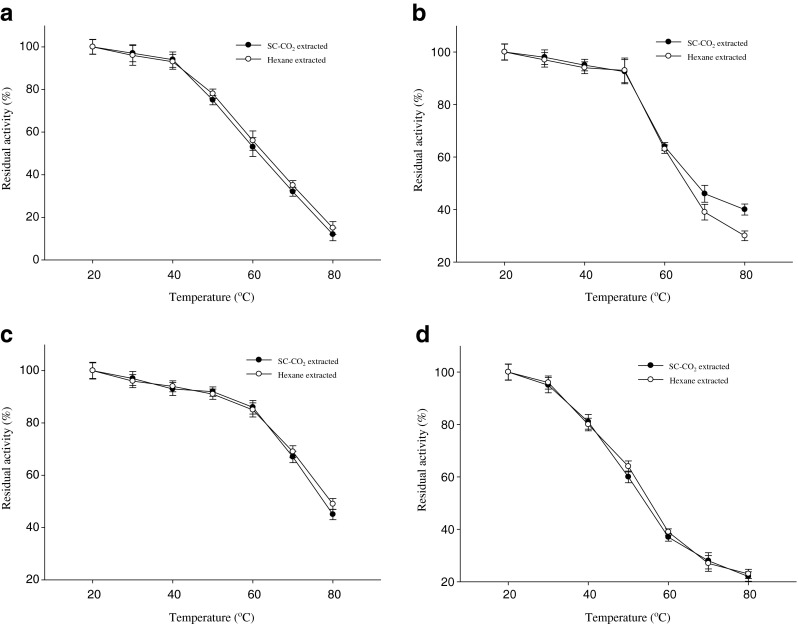
Temperature stability of digestive enzymes in SC-CO2 and n-hexane treated mackerel muscle residues extract a Amylase, b Lipase, c Protease and d Trypsin. Bars represents the standard deviation (n = 3)
SDS-PAGE
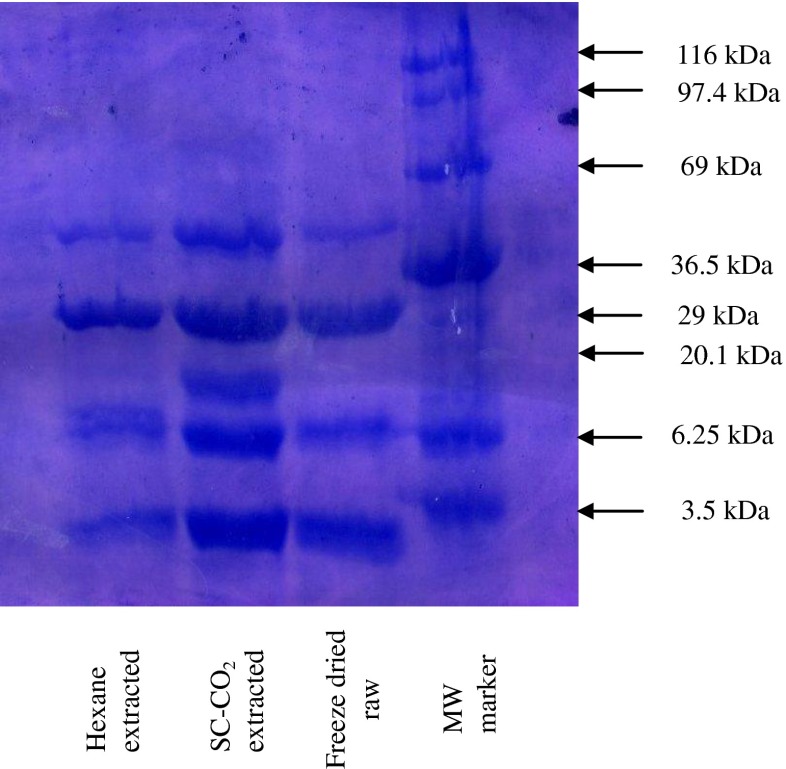
The gel electrophoresis of marker protein and the water soluble extract of freeze dried raw, SC-CO2 and hexane treated mackerel muscle are shown in Fig. 8. Different size of molecular weight of protein was observed after SDS-PAGE. The proteins in freeze dried raw mackerel were almost similar in subunit composition to SC-CO2 and hexane treated residues. After observing the gel banding patterns, protein band was more and prominent in SC-CO2 treated samples than freeze dried raw and n-hexane treated mackerel residues. From this observation it can be concluded that protein concentration in the solution was high after removal of lipid using SC-CO2 and protein denaturation was not found in SC-CO2 treated mackerel sample. Similar results have been reported in SC-CO2 extracted squid viscera residues and krill residues (Uddin et al. 2009; Abdelkarder et al. 2012).
Fig. 8.
SDS-PAGE electrophoresis of water soluble extract protein of freeze dried raw, SC-CO2 and n-hexane extracted mackerel muscle residues
Conclusion
Protein concentration and digestive enzyme activities of SC-CO2 treated mackerel muscle residues increased as compared to n-hexane treated residues. Enzyme activities were changed using different pH and temperature. Alkaline optimum pH and pH stability, high optimum temperature and higher temperature stability showed all of the digestive enzymes. Denaturation of protein was not found in SC-CO2 treated samples. Thermally stable compounds are highly attractive for economical purposes. Therefore, SC-CO2 treated digestive enzymes can be used as a food ingredient in the food related industries.
Acknowledgments
This work (Grants No. C0019011) was supported by Business for Academic-industrial Cooperative establishments funded Korea Small and Medium Business Administration in 2012
References
- Abdelkarder AN, Kim SB, Lee YB, Chun BS. Digestive enzymes characterization of krill (Euphausia superba) residues deoiled by supercritical carbon dioxide and organic solvents. J Ind Eng Chem. 2012;18:1314–1319. doi: 10.1016/j.jiec.2012.01.026. [DOI] [Google Scholar]
- Aryee ANA, Simpson BK, Villalonga R. Lipase fraction from the viscera of grey mullet (Mugil cephalus): isolation, partial purification, and some biochemical characteristics. J Enzym Microb Technol. 2007;40:394–402. doi: 10.1016/j.enzmictec.2006.07.009. [DOI] [Google Scholar]
- Bergmeyer HU, Gawehn K, Grassi M. In: Methods of enzymatic analysis. Bergmeyer HU, editor. New York: Academic Press, Inc.; 1974. pp. 515–516. [Google Scholar]
- Bezerra RS, Lins EJF, Alencar RB, Paiva PMG, Chaves MEC, Coelho LCBB, Carvalho LB. Alkaline proteinase from intestine of nile tilapia (Oreochromis niloticus) J Process Biochem. 2005;40:1829–1834. doi: 10.1016/j.procbio.2004.06.066. [DOI] [Google Scholar]
- Dendinger JE, O’connor KL. Purification and characterization of a trypsin-like enzyme from the midgut gland of the Atlantic blue crab, Callinectes sapidus. J Comp Biochem Physiol B. 1990;95:525–530. [Google Scholar]
- Djamel C, Ali T, Nelly C. Acid protease production by isolated species of penicillium. Eur J Sci Res. 2009;25(3):469–477. [Google Scholar]
- Eshel A, Lindner P, Smirnoff P, Newton S, Harpaz S. Comparative study of proteolytic enzymes in the digestive tracts of the European sea bass and hybrid striped bass reared in freshwater. J Comp Biochem Physiol A. 1993;106:627–634. doi: 10.1016/0300-9629(93)90371-A. [DOI] [Google Scholar]
- Espósito TS, Amaral IPG, Buarque DS, Oliveira GB, Carvalho LB, Jr, Bezerra RS. Fish processing waste as a source of alkaline proteases for laundry detergent. J Food Chem. 2009;112:125–130. doi: 10.1016/j.foodchem.2008.05.049. [DOI] [Google Scholar]
- Ge Y, Ni Y, Chen Y, Cai T. Optimization of the supercritical fluid extraction of natural vitamin E from wheat germ using response surface methodology. J Food Sci. 2002;67:239–243. doi: 10.1111/j.1365-2621.2002.tb11391.x. [DOI] [Google Scholar]
- Hidalgo MC, Urea E, Sanz A. Comparative study of digestive enzymes in fish with different nutritional habits: proteolytic and amylase activities. J Aquac. 1999;170:267–283. doi: 10.1016/S0044-8486(98)00413-X. [DOI] [Google Scholar]
- Karun T, Uthaiwan K, Arunee E, Krisna RT. Temperature and pH characteristics of amylase and lipase at different developmental stages of Siamese fighting fish (Betta splendens regan, 1910) Kasetsart J (Nat Sci) 2010;44:210–219. [Google Scholar]
- Klomklao S, Benjakul S, Visessnguan W, Kishimura H, Simpson BK. Purification and characterization of trypsin from the spleen of tongol tuna (Thunnus tonggol) J Agric Food Chem. 2006;54:5617–5622. doi: 10.1021/jf060699d. [DOI] [PubMed] [Google Scholar]
- Kubrak OI. Microbe amylases: characteristic, properties and practical use. J Microb Z. 2007;69(6):56–76. [PubMed] [Google Scholar]
- Kumar S, Kikon K, Upadhyay A, Kanwar SS, Gupta R. Production, purification, and characterization of lipase from thermophilic and alkaliphilic Bacillus coagulans BTS-3. J Protein Expr Purif. 2005;41:38–44. doi: 10.1016/j.pep.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Roserbrough NJ, Farr AW, Randall RJ. Protein measurements with pholin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Martinez A, Olsen RL, Serra JL. Purification and characterization of two trypsin-like enzymes from the digestive tract of anchovy Engraulis encrasicholus. J Comp Biochem Physiol B. 1988;91:677–684. doi: 10.1016/0305-0491(88)90191-5. [DOI] [PubMed] [Google Scholar]
- Mendes RL, Nobre BP, Cardoso MT, Pereire AP, Palavre AF. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. J Inorg Chim Acta. 2003;356:328–334. doi: 10.1016/S0020-1693(03)00363-3. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicyclic acid reagent for the determination of reducing sugar. J Anal Chem. 1959;31:426–429. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Morita A, Kajimoto O. Solute solvent interaction in nonpolar supercritical fluid: a clustering model and size distribution. J Phys Chem. 1990;94:6420–6425. doi: 10.1021/j100379a048. [DOI] [Google Scholar]
- Murakami K, Noda M. Studies on proteinases from the digestive organs of sardine-purification and characterization of three alkaline proteinases from the pyloric ceca. J Biochim Biophys Acta B. 1981;65:17–26. doi: 10.1016/0005-2744(81)90245-X. [DOI] [PubMed] [Google Scholar]
- Natalia Y, Hashim R, Ali A, Chong A. Characterization of digestive enzymes in a carnivorous ornamental fish, the Asian bony tongue Scleropages for mosus (Osteoglossidae) J Aquacultur. 2004;233:305–320. doi: 10.1016/j.aquaculture.2003.08.012. [DOI] [Google Scholar]
- Noman ASM, Hoque MA, Sen PK, Karim MR. Purification and some properties of amylase from post-harvest Pachyrhizus erosus L. tuber. J Food Chem. 2006;99:444–449. doi: 10.1016/j.foodchem.2005.07.056. [DOI] [Google Scholar]
- Oda K, Murao S. Purification and properties of carboxyl proteinase in basidiomycetes. J Agric Biol Chem. 1974;38:2435–2437. doi: 10.1271/bbb1961.38.2435. [DOI] [Google Scholar]
- Ogino H, Miyamoto K, Ishikawa H. Organic solvent tolarent bacteria which secretes organic solvent stable lipolytic enzyme. J Appl Env Microb. 1994;60(10):3884–3886. doi: 10.1128/aem.60.10.3884-3886.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariser ER, Wallerstei, MB, Corkery CJ, Brown NL (1978) Fish protein concentrate: Panacea for world Malnutrition. MIT Press, Boston, ISBN: 10: 0262160692, pp 256
- Park JY, Lee MK, Uddin MS, Chun BS. Removal of off flavors and isolation of fatty acids from boiled anchovies using supercritical carbon dioxide. J Biotechnol Bioproc Eng. 2008;13:298–303. doi: 10.1007/s12257-007-0024-x. [DOI] [Google Scholar]
- Parsertsan P, Jitbunjerdkul S, Trairatananukoon Prachumratana T. Production of enzyme and protein hydrolyzate from fish processing waste. In: Roussos S, Soccol CR, Pandey A, Augur C, editors. New horizons in biotechnology. India: Kluwer Academic Publisher; 2001. pp. 63–72. [Google Scholar]
- Perona JJ, Craick SC. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4(3):337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham V, Soottawat B. Purification and characterization of trypsin from pyloric caeca of bigeye snapper (pricanthus macracanthus) J Food Biochem. 2006;30:478–495. doi: 10.1111/j.1745-4514.2006.00089.x. [DOI] [Google Scholar]
- Poonsuk P, Thiraratana P. Properties of protease and lipase from whole and individual organ of viscera from three tuna species. Songklanakarin J Sci Technol. 2008;30:77–86. [Google Scholar]
- Shahidi F, Janak-Kamil YVA. Enzymes from fish and aquatic invertebrates and their application in the food industry. J Trends Food Sci Technol. 2001;12:435–464. doi: 10.1016/S0924-2244(02)00021-3. [DOI] [Google Scholar]
- Simpson BK, Haard NF. Trypsin from Greenland cod, Gadus ogac. Isolation and comparative properties. J Comp Biochem Physiol B. 1984;79:613–622. doi: 10.1016/0305-0491(84)90375-4. [DOI] [PubMed] [Google Scholar]
- Simpson BK, Smith JP, Haard NF. Marine enzymes. In: Hui YH, editor. Encyclopedia of food science and technology. New York: Wiley; 1991. pp. 1645–1653. [Google Scholar]
- Sun M, Temelli F. Supercritical carbon dioxide extractions of carotenoids from carrot using canola oil as a continuous co-solvent. J Supercrit Fluids. 2006;37:397–408. doi: 10.1016/j.supflu.2006.01.008. [DOI] [Google Scholar]
- Temelli F, Leblanc E, Fu L. Supercritical carbon dioxide extraction of oil from Atlantic Mackerel (Scomber scombrus) and protein functionality. J Food Sci. 1995;60:703–706. doi: 10.1111/j.1365-2621.1995.tb06210.x. [DOI] [Google Scholar]
- Uddin MS, Ahn HM, Kishimura H, Chun BS. Comparative study of digestive enzymes of squid (Todarodes pacificus) Viscera after supercritical carbon dioxide and organic solvent extraction. J Biotechnol Bioproc Eng. 2009;14:338–344. doi: 10.1007/s12257-008-0271-5. [DOI] [Google Scholar]
- Vilhelmsson O. The state of enzyme biotechnology in the fish processing industry. J Trends Food Sci Technol. 1997;8:266–270. doi: 10.1016/S0924-2244(97)01057-1. [DOI] [Google Scholar]
- Vulfson EN. Industrial applications of lipases. In: Wooley P, Petersen SB, editors. Lipases. Cambridge: Cambridge University Press; 1994. pp. 271–288. [Google Scholar]
- Yamaguchi K, Murakami M, Nakano H, Konosu S, Kokura T, Yamamoto H, Kosaka M, Hata K. Supercritical carbon dioxide extraction of oils from Antarctic krill. J Agric Food Chem. 1986;34:904–907. doi: 10.1021/jf00071a034. [DOI] [Google Scholar]