Abstract
In this study, the effect of ohmic heating technique on electrical conductivity, water evaporation rate, heating rate, colour parameters, pH and energy consumption of tomato samples was investigated. Ohmic heating was accomplished till the moisture content of the tomato samples reduced from initial moisture content of as 9.33 (dry basis) to a safer level of 2.2. The results of the nonlinear mathematical model including the effects of voltage gradient level and the temperature on the electrical conductivity changes had good agreement (R ≥ 0.955) with the experimental data. Also, it was observed that the electrical conductivity increased along with concentration of tomato samples. The range of electrical conductivity during ohmic heating was 3.19–8.95 (S/m). It was found that processing time decreased from 28.32 to 4.3 min over the voltage gradient range studied (6 to 14 V/cm), which resulted in decreased specific energy consumption from 4.63 to 3.05 (MJ/kg water). Due to increasing of heating rate and water evaporation rate at high voltage gradient, the change of the pH was limited. Samples processed in high voltage gradient had higher L*, b* and hue angle (h), lower a* and Chroma (C) values as compared to low voltage gradient. The optimum value of processing time, pH, colour, specific energy consumption was obtained at 14 V/cm voltage gradient level.
Keywords: Colour, Energy consumption, Electrical conductivity, Ohmic heating, Tomato, Voltage gradient
Introduction
Conventional thermal methods have been to date the most common processing method employed for tomato products. During conventional processing in cans or aseptic processing systems for particulate foods, significant product quality damage may occur due to slow conduction or convection heat transfer (Zell et al.(2009); Sarimeseli (2011); Al-Harahsheh et al. (2009)).
Development of new technologies for thermal food treatment is still of great industrial and scientific interest. Ohmic heating is one of these new technologies. Ohmic heating, also called electrical resistance heating, is a heating process where an alternating current is passed through food materials thereby leading to heat generation (Zell et al. (2010); Darvishi et al. (2012a, b).
In recent years, the world’s food industry has focused increasing attention on ohmic heating of pump-able foods (Icier and Ilicali (2005a)). It is a highly attractive technique for continuous food processing. It can be used as a continuous in-line heater for cooking and sterilization of viscous and liquid food products. The rate of heat generation in ohmic heating depends strongly on the electrical conductivity of the food (Icier and Ilicali (2005a, b); Shirsat et al. (2008); Darvishi et al. (2012a, b). Therefore, electrical conductivity and heating rate should be known in order to ensure the proper design of the process from a product safety and quality point of view (Zareifard et al. (2003)). The use of ohmic heating in food products has offered a number of advantages such as faster heating, less power consumption and safer product (Bozkurt and Icier (2010); Chen et al. (2010); Allali et al. (2008)). Also ohmic heating has been shown to enhance drying rates (Sarang et al. (2008); Zhong and Lima. (2003)).
A large number of applications exist for ohmic treatments including heating, cooking, thawing, blanching, evaporation, dehydration, fermentation and extraction (Sarang et al. (2008)). There are several published patents about the usage of ohmic treatments on several food materials such as; strawberry (Allali et al. (2008); Sarang et al. (2008); Castro et al. (2004)); red apple, golden apple, peach, pear and pineapple (Sarang et al. (2008)); tomato soup (Jun and Sastry (2005)); apricot and peach purees Icier and Ilicali (2005a)); sour cherry juice (Icier Icier and Ilicali (2004)); tylose (Icier and Ilicali (2005b)); seawater (Assiry et al. (2010a); Assiry (2011)); lemon juice (Darvishi et al. (2011)); milk (Stancl and Zitny. 2010; Bansal and Chen. 2006); carrot and radish (Zhu et al. (2010)); potato (Marra et al. (2009); Zhu et al. (2010); Ye et al. (2004)); apple, orange, and pineapple juices (Amiali et al. (2006)); beetroot (Lima et al. (2001)); grape juice (Icier et al. (2008)); egg products (Darvishi et al. (2012a, b)); Icier and Bozkurt (2009); chicken (Tulsiyan et al. (2008)); Sarang et al. (2008)), beef and meat (Zhu et al. (2010); Bozkurt and Icier (2010); Icier and Ilicali (2005a)); Sarang et al. (2008)).
However, there was no information in literature about either the change of the electrical conductivity of tomato during ohmic cooking and the effect of ohmic heating method on quality of tomato samples. Therefore; the main objectives of this work were (i) to investigate the effect of voltage gradients applied during ohmic heating on some quality properties of tomato samples; such as the colour and pH, (ii) to obtain the electrical conductivity and ohmic heating rate; and (iii) to investigate the energy consumption.
Materials and methods
Material
Tomato used in this study was purchased from a local market. The whole tomatoes were washed, crushed and mixed in a way that a red less-viscous liquid obtained (Fig. 1). This liquid is considered as tomato samples in the remainder of the article. Tomato samples were stored at 4 ± 0.5 °C before experiments in order to slow down the respiration, physiological and chemical changes. The average moisture content of the tomato samples was as 9.33 ± 0.16 (dry basis), as determined by vacuum drying at 104 ± 1 °C for 24 h. Cooking was carried out until the final moisture content reaches to a level less than 2.2 ± 0.15 (dry basis).
Fig. 1.

preparing tomato samples include of peeling, mixing and measuring of Ph
Experimental equipment and procedure
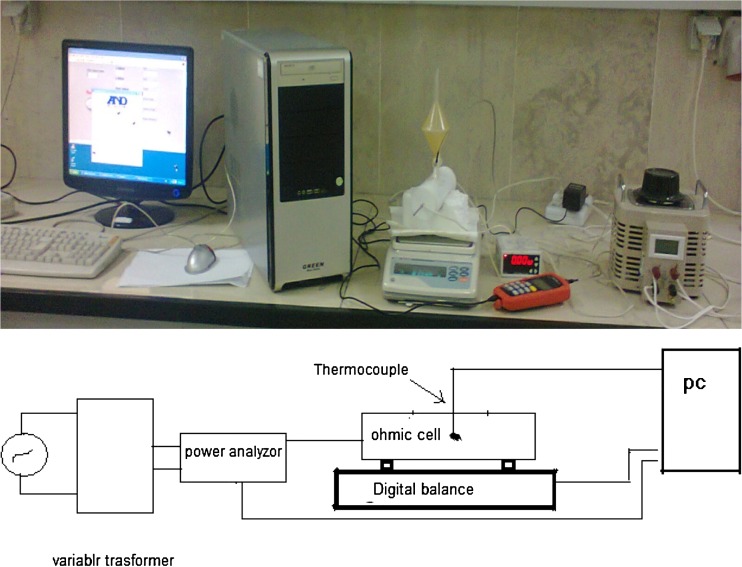
Ohmic cooking experiments were conducted in laboratory scale ohmic heating system consists of a power supply, a variable transformer, power analyzer (Lutron DW-6090) and a microcomputer (Fig. 2). The ohmic cell has a PTF cylinder with an inner diameter of 0.05 m, a length of 0.05 m and two removable stainless steel electrodes (thickness of electrode is 2 mm and its diameter is 49 mm). A hole with diameter 1 and 0.3 cm was created on surface of cell for insert of thermocouple, and two hole with diameter 1 and 0.5 cm was created on surface of cell for exit of vapor on cell.
Fig. 2.
Picture and schematic diagram of the experimental ohmic heating system
Temperature uniformity was checked during previous heating experiments by measuring the temperatures at different locations in the test cell. Since the temperature variation at different points inside the test cell was ±1.5 °C during heating, the ohmic heating process was assumed as uniform. Therefore, only the temperature in the center of the test cell was measured. Temperature at the geometric center of the sample was continuously measured with a CHY-802U thermometer. The accuracy of ohmic system was compared and calibrated with the standard conductivity. The calibration results for the accuracy of electrical conductivity of 0.1 M NaCl solution revealed that there was no significant difference between standard electrical conductivity of 0.1 M NaCl solution and the experiment data (maximum 4.3 %). The electrodes were thoroughly rinsed using a brush and dematerialized with twice-distilled water after each run.
The samples were placed in the test cell; the thermocouples were inserted and fitted into geometric center of the sample. The ohmic system was operated at five voltage inputs: 30, 40, 50, 60, and 70 V at 60Hz frequency or voltage gradients: 6, 8, 10, 12 and 14 V/cm. A digital balance (A&D GF6000, Japan) with accuracy of ±0.01 g was positioned on the down of the cell for mass determination. Moisture loss was recorded at 1 s intervals during the cooking process in order to determine the energy consumption, evaporation rate and contact area of sample and electrodes.
Electrical conductivity
Electrical conductivity was determined from the resistance of the sample and the geometry of the cell using the following equation (Darvishi et al., 2012a; Icier and Ilicali, 2005b):
| 1 |
where L is the gap between two electrodes (m); A is the cross-section area of the sample in the heating cell (m2); I is the current (A); and V is the voltage (V). During cooking, the contact area between tomato sample and electrodes duo to reduce of water content of tomato was reduced. The contact area can be calculated as follow:
| 2 |
where ρ is the tomato density (kg/m3) and mt is the mass of sample at any time (kg). Therefore, the electrical conductivity of sample is calculated using equation:
| 3 |
Samples density was determined by applying the pycnometric method. The sample kept in a 50 ml standard volumetric pycnometer was weighed using a digital balance (A&D GF 600, Japan) with an accuracy of ±0.001 g.
Colour
The colour of cooked tomato samples were quantified by using a Hunter-Lab ColorFlex, A60-1010-615 model color-meter (HunterLab, Reston, VA). The colour values were expressed as L (whiteness or brightness/darkness), a (redness/greenness) and b (yellowness/blueness). And also, chroma (C) and Hue angle (h) were calculated from the Hunter L-, a-, b-values and used to describe the colour change of samples:
| 4 |
| 5 |
pH
pH was determined using a membrane pH meter (HI 8314, Hanna Instrument, USA). The percentage of the pH change was calculated according to the following equation:
| 6 |
Energy consumption
The specific energy for the evaporation process was estimated according to Eq. (7) (Assiry, 2011).
| 7 |
where, Es is the specific energy consumption (J/kg water) and mw is mass of evaporated water (kg).
The energy loss is the sum of the heat required to heat up the test cell and electrodes, the heat loss to the surroundings by natural convection, the heat loss for physical, chemical and electrochemical changes of tomato samples, and the electrical energy which has not been converted into heat. The specific energy loss was calculated using Eq. (8) below:
| 8 |
Where ms is the mass of sample (kg), Cps is the specific heat capacity of tomato (J/kg K), t is the heating time (s), mw is the mass of evaporated water (kg), and λ is the latent heat of vaporization of water (J/kg).
Latent heat of tomato samples was calculated according to the following equation (Abdelmotaleb et al., 2009):
| 9 |
where λw is the latent heat of free water (J/kg), and M is the moisture content (%, d.b.).
Statistical analysis
Non-linear regression, linear regression, One-way ANOVA and post hoc comparison (at significance level a = 0.05) statistical analyses were performed by using SPSS 21. The results were reported as an average of five replicates.
Results and discussion
Water evaporation rate
Figure 3 illustrates the moisture content during the ohmic heating time at different voltage gradients. It can be seen from Fig. 3 that, as the voltage gradients increases, the tomato samples moisture content significantly decreases, in other words, this was significant increases in the evaporation rate by increasing the voltage gradient. Similar results were reported by Zhong and Lima (2003) for sweet potato; Salengke and Sastry (2005) for grape and raisins. The total cooking times to reach the final moisture content for the tomato samples were 1622, 801, 520, 333 and 257 s, at 6, 8, 10, 12, and 14 V/cm, respectively. As voltage gradient was increased from 6 to 14 V/cm, the cooking time was reduced by about 83.2 %. Similarly, several researchers (Bozkurt and Icier (2010)); Darvishi et al. (2012a)); de Halleux et al. (2005); Assiry et al. (2010a); Assiry (2011)) have already concluded that the increasing in the voltage gradient provided the decrease in the ohmic heating time of bioproducts. Variation of moisture content during ohmic cooking can be present by the following equation:
| 10 |
where, M is moisture content of tomato samples during ohmic cooking (d.b), ∇V is the voltage gradient (V/cm), and t is time (min).
Fig. 3.
The moisture content during the ohmic heating time at different voltage gradients
Electrical conductivity
Figures 4 and 5 show the changes in electrical conductivity as a function of moisture content and temperature at different voltage gradients. The moisture content had significant effect on the electrical conductivity of tomato samples during ohmic treatment (p < 0.05). For all samples, electrical conductivity increased with temperature, as is expected and consistent with literature data (Icier et al. (2008); Darvishi et al. (2011); Sarang et al. (2008); Icier and Ilicali (2004); Amiali et al. (2006); Castro et al. (2004)). When biological tissue is heated, its electrical conductivity increases due to increase in the ionic mobility. This phenomenon occurs because of structural changes in the tissue like cell wall protopectin breakdown, expulsion of non conductive gas bubbles, softening, and lowering in aqueous phase viscosity. Bubbling was observed above 84 ºC at especially high voltage gradients. It was observed that electrical conductivities decreased with temperature rise after bubbling started till temperature of 95 ºC, then electrical conductivity increase with increasing evaporation water. Icier and Ilicali (2005a) reported that the increase in the electrical conductivity values with temperature has been explained by reduced drag for the movement of ions. It has been reported that when water is boiling, gas bubbles are formed. This phenomenon appears due to localized high current densities of various oxidation/reduction reactions (e.g. H2 or O2 gas) (Zhao et al. (1999)).
Fig. 4.
Variations of electrical conductivity and moisture content of tomato samples at different voltage gradients
Fig. 5.
The electrical conductivity profile of the tomato samples during ohmic heating different voltage gradients
As seen in Fig. 4, all curves have four stages. The electrical conductivity rapidly increases during warming up period, when the samples approached the boiling point, the electrical conductivity reduced due to the formation of vapor which worked as an electrical insulation. As more water evaporated the concentration of the tomato samples increased causing increase in the electrical conductivity till the moisture content become 2.2 (dry basis). Because of the boiling action and the relatively high formation of vapor bubbles, the electrodes were gradually not in full contact with the sample, therefore the electrical conductivity dropped again and the heating was stopped. It can be seen from Fig. 4 that increasing the voltage gradient from 6 to 14 V/cm led to a decrease in the electrical conductivity which could be due to the increasing rates of vapor formation at higher voltage gradient (14 V/cm). It was observed during the experiments that at low voltage gradient (6 V/cm) a lot of electrodes scales occurred. The electrical conductivity increased as the tomato samples became more concentrated (reduce of moisture content), and then a sudden drop occurred in the electrical conductivity at about 2.2 (d.b) of moisture content. The electrical conductivity values of the tomato samples were within the general ranges of 0.01 to 10 S/m for biological materials (Zell et al. (2009); De Alwis et al. (1989)). The values of electrical conductivity of tomato samples are comparable with the reported values of 5.5–39.9 S/m mentioned for seawater (range 38.9–106.1 PPT) at 6.35–11.04 V/cm and 20–95 °C (Assiry et al. 2010a); 0.1–1.6 S/m for apple and sour cherry juices at 20–60 V/cm and 30–75 °C (Icier and Ilicali. 2004), 1–5.2 S/m for ohmic evaporation process of seawater at 24–87 V/cm and 25–100 ºC (Assiry. 2011);0.4–1.0 S/m for lemon juice at 30–55 V/cm and 20–74 °C (Darvishi et al. (2011)), 0.38–0.78 S/m for grape juice at 20–40 V/cm and 20–80 °C (Icier et al. 2008), 0.15–1.15 S/m for orange juice at 20–60 V/cm and 30–60 °C (Icier and Ilicali. 2005a), 0.51–0.91 S/m for peach puree and 0.61–1.2 S/m for apricot puree at 20–70 V/cm and 20–60 °C (Icier and Ilicali. 2005c). Higher electrical conductivity of tomato sample may be attributed to the softer tissues and hence higher ionic mobility in comparison to the harder tissues of potato, carrot, apple, pineapple, pear, meat, apricot and peach puree. Also, as mentioned earlier, the presence of air can lower the electrical conductivity of material. The other most important factor influencing the conductivity is the total ionic (salt) content of tomato samples into juices and fruits.
The relationship between electrical conductivity and temperature can be represented as:
| 12 |
Where a, b and c are the constants of regression, and T is temperature of sample during ohmic heating (ºC).
Regression constants for electrical conductivity of tomato samples under different voltage gradients are presented in Table 1. The high values of R2 are indicative of good fitness of empirical relationship to represent the variation in electrical conductivity with temperature of tomato samples. In order to take into account the effect of voltage gradient on the constants and coefficients of Eq. (3), the regression analysis was used to set up the relations between these parameters and the voltage gradient. Thus, the regression equations of these parameters against voltage gradient are as follows:
| 13 |
| 14 |
Table 1.
Results of statistical analysis on the modeling of electrical conductivity with temperature for tomato
| ΔV (V/cm) | 25 ≤ T ≤ 95 °C | T ≥ 95 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | R2 | a | B | c | R2 | ||
| 14 | −0.0012 | 0.1927 | −1.5278 | 0.9813 | −0.6718 | 131.98 | −6473.9 | 0.9745 | |
| 12 | −0.0009 | 0.1604 | −0.6830 | 0.9841 | −1.1421 | 223.58 | −10933 | 0.9712 | |
| 10 | −0.0007 | 0.1404 | −0.1045 | 0.9976 | −0.6367 | 125.23 | −6148.5 | 0.9611 | |
| 8 | −0.0006 | 0.1305 | 0.4810 | 0.9981 | −0.0862 | 18.01 | −928.1 | 0.9745 | |
| 6 | −0.0001 | 0.0828 | 1.7454 | 0.9988 | −1.5203 | 291.35 | −13950 | 0.9218 | |
Heating rate
Figure 6 shows how the heating rate of tomato samples was increased with increased voltage gradient. Due to the passing electrical current through the sample, heat is generated and the sample temperature linearly increased from the initial room temperature (24 °C) to the boiling point temperature (96-98 °C), then the sample temperature was constant at maximum value during the evaporation water process. The rate of heat generation within the tomato samples depended on voltage gradient and electrical conductivity which varied during the heating and evaporation processes of the sample. This observation of voltage gradient and electrical conductivity effect on the heating rate of tomato samples is in good agreement with the findings of Assiry et al. (2010b) and Jakob et al. (2010).
Fig. 6.
Variation of temperature of tomato samples at different voltage gradients
Colour assessment
The voltage gradient had a significant effect on the colour parameters of tomato samples during ohmic cooking (P < 0.05). Figure 7 shows the colour data in terms of L*, a*, b*, h and C values of cooked tomato samples as a function of voltage gradient during ohmic cooking. The range of values recorded were 38.17–53.77, 14.99–18.63, −7.82– -3.88; −27.53– -12.01 and 16.91–18.83 for the L*, a*, b*, h and C, respectively. It can be seen from Fig. 4 that the values of L* parameter for tomato samples increases with voltage gradient, thus the luminance of the samples is improved. Also the values of a* parameter decreased slightly with voltage gradient which means that heating process preserves or enhances slightly the green colour of the tomato samples.
Fig. 7.
Kinetics of change of the colour parameters as a function of voltage gradient
It can be seen that b* and h values decreased with the increasing voltage gradient. The b* values merely showed that the tomato samples were more yellow in colour, thus less browning. This is clearly indicated by the values of hue angels (h). A larger value of hue angle indicates a more shift from red to yellow. The a*/b* value is the ratio of red-green component of colour and relates better to the colour variation in tomato (Kerkhofs (2003); Arias et al. (2000); Toor and Savage (2006). The values of a*/b* found 1.92, 2.80, 2.87, 7.08 and 4.70 for voltage gradient of 6, 8, 10, 12 and 14 V/cm, respectively. Lower values indicated yellowness or lightness or in other word, less of darkness of the product (Toor and Savage 2006).
pH
Table 2 show the pH as a function of voltage gradient for tomato samples. It is clear that the pH was decrease with increased the voltage gradient. The maximum increase in the pH was 8.39 % at 6 V/cm. This behavior was probably due to the residence time of different reactions such as hydrolysis of the tomato samples and corrosion of electrodes that might occur during the ohmic heating. For example, at high voltage gradient, 14 V/cm, the heating rate (14.04 °C/min) and water evaporation rate (1.478 kg water/kg dry mater. Min) were high, therefore the residence time for the sample to heat up from 26 to 96 °C and remove moisture content from 9.2 to 2.2 (dry basis) were short, thus the change of the pH was limited (1.2 %) because the reaction time was short. In comparison, at low voltage gradient, 6 V/cm, the heating rate and water evaporation rate were 2.88 (°C/min) and 0.262 (kg water/kg dry matter. min), respectively, which means high residence time at which the change in the pH was maximum (8. 39 %) because of the longer reaction time. It has been reported that during ohmic heating, hydrolysis and corrosion reactions between the electrodes and the electrolyte solution may occur, where at high electrical power and salt content, a significant loss of buffering capacity was noted (Assiry et al. 2010a; Darvishi et al. 2012a).
Table 2.
The average pH of tomato samples at room temperature as affected by the voltage gradient
| ∇V (V/cm) | pH | Standard deviation | ΔpH (%) |
|---|---|---|---|
| 6 | 4.53 | 0.07 | 8.39 |
| 8 | 4.43 | 0.021 | 6.32 |
| 10 | 4.32 | 0.021 | 3.94 |
| 12 | 4.25 | 0.045 | 2.35 |
| 14 | 4.20 | 0.068 | 1.20 |
| Fresh | 4.15 | 0.017 | - |
Energy consumption
Variation in specific energy requirement of tomato samples with moisture content at different voltage gradients is shown in Fig. 8. The specific energy requirement decreased with decrease in moisture content. However, the energy requirement further was lower at any level of moisture content at higher voltage gradient level. This may indicate that as moisture content decreased, the concentration of the tomato samples increased causing increase in the electrical conductivity. Therefore, the rate of heat generation increased and resulting of decreased of energy requirement. Table 3 shows the average specific energy requirement for processing of tomato samples as function of voltage gradient. The best result with regard to energy consumption was obtained for 14 V/cm with 3.05 MJ/kg water. The Es values decreased with the increase in voltage gradient. The specific energy consumption obtained in the heating process using 14 V/cm voltage gradient level was 1.52-fold lower than 6 V/cm voltage gradient level. One of many reasons might be that the heating time is longer under lower voltage gradient and results in the increase in energy consumption.
Fig. 8.
Variation in specific energy requirement with moisture content at various voltage gradients
Table 3.
The average values of specific energy requirement and energy loss as affected by the voltage gradient
| ΔV (V/cm) | E (MJ/kg) | Eloss (MJ/kg) |
|---|---|---|
| 6 | 4.63 (±0.40) | 1.49 (±0.23) |
| 8 | 3.45 (±0.29) | 0.98 (±0.14) |
| 10 | 3.27 (±0.31) | 0.84 (±0.16) |
| 12 | 3.11 (±0.19) | 0.62 (±0.09) |
| 14 | 3.05 (±0.27) | 0.67 (±0.11) |
The relationship between specific energy requirement and voltage gradient can be represented as:
| 15 |
The difference between the energies given and taken was called energy loss in this study. The increase in the voltage gradient applied was statistically significant in the specific energy losses during ohmic heating (p <0.05, Table 3). As the voltage gradient increased, specific energy losses decreased from 1.49 to 0.62 MJ/kg, which indicated that 32.18–19.94 % of the electrical energy given to the system was not used in heating up the test sample. At low voltage gradients, the difference between the energy given to the system and the energy taken by the tomato samples can be explained partly by these losses. However, at higher voltage gradients the energy losses mentioned above is only a small portion of the total energy losses. The energy losses can be mostly explained by the energies used for the purposes of physical, chemical and electrochemical changes during heating.
Conclusions
The tomato samples were heated on a laboratory scale static ohmic heater by applying voltage gradients in the range of 6–14 V/cm. Processing was carried out until the final moisture content reaches to a level less than 2.2 (dry basis). The voltage gradient was statistically significant on the ohmic heating time, heating rate, water evaporation rate, pH, colour parameters, and energy consumption (P < 0.05). The range of conductivity during ohmic heating was 3.19–8.95 (S/m) and strongly dependent on moisture content and temperature. The non linear temperature dependent electrical conductivity relations were obtained. Bubbling was observed above 80 °C especially at low voltage gradients. As the voltage gradient increased, time, pH and specific energy consumption decreased. The rate of heat generation will increase as the electrical conductivity increases due to the increasing concentration as the water evaporate. The best values of colour criteria, heating time, pH and energy consumption were obtained at 14 V/cm voltage gradient levels.
References
- Abdelmotaleb A, El-Kholy MM, Abou-El-Hana NH, Younis MA. Thin layer drying of garlic slices using convection and combined (convection-infrared) heating modes. Misr J. Ag. Eng. 2009;26(1):251–281. [Google Scholar]
- Al-Harahsheh M, Al-Muhtaseb AH, Magee TRA. Microwave drying kinetics of tomato pomace: Effect of osmotic dehydration. Chem Eng Process. 2009;48:524–531. doi: 10.1016/j.cep.2008.06.010. [DOI] [Google Scholar]
- Allali H, Marchal L, Vorobiev E. Blanching of strawberries by ohmic heating: effects on the kinetics of mass transfer during osmotic dehydration. Food and Bioprocess Technology. 2008 [Google Scholar]
- Amiali M, Ngadi M, Raghavan VGS, Nguyen DH. Electrical conductivities of liquid egg product and fruit juices exposed to high pulsed electric fields. International Journal of Food Properties. 2006;9:533–540. doi: 10.1080/10942910600596456. [DOI] [Google Scholar]
- Arias R, Lee TC, Logendra L, Janes H. Correlation of lycopene measured by HPLC with the L, a, b colour readings of a hydroponic tomato and the relationship of maturity with colour and lycopene content. Journal of Agricultural and Food Chemistry. 2000;48:1697–1702. doi: 10.1021/jf990974e. [DOI] [PubMed] [Google Scholar]
- Assiry AM. Application of ohmic heating technique to approach near-ZLD during the evaporation process of seawater. Desalination. 2011;280:217–223. doi: 10.1016/j.desal.2011.07.010. [DOI] [Google Scholar]
- Assiry AM, Gaily MH, Alsamee M, Sarfudin A. Electrical conductivity of seawater during ohmic heating. Desalination. 2010;260:9–17. doi: 10.1016/j.desal.2010.05.015. [DOI] [Google Scholar]
- Assiry AM, Gaily MH, Alsamee M, Sarifudin A. Electrical conductivity of seawater during ohmic heating. Desalination, 260: 9–17 Lima, M.., Heskitt, B., Sastry, S., 2001. Diffusion of beet dye during electrical and conventional heating at steady-state temperature. J. Food Process Eng. 2010;24(5):331–340. [Google Scholar]
- Bansal B, Chen XD. Effect of temperature and power frequency on milk fouling in an ohmic heater. Food and Bioprod Process. 2006;84:286–291. doi: 10.1205/fbp.06029. [DOI] [Google Scholar]
- Bozkurt H, Icier F. Electrical conductivity changes of minced beef–fat blends during ohmic cooking. J Food Eng. 2010;96:86–92. doi: 10.1016/j.jfoodeng.2009.06.048. [DOI] [Google Scholar]
- Castro I, Teixeira JA, Salengke S, Sastry SK, Vicente AA. Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics. Innovative Food Sci Emerg Technol. 2004;5:27–36. doi: 10.1016/j.ifset.2003.11.001. [DOI] [Google Scholar]
- Chen C, Abdelrahim K, Beckerich I. Sensitivity analysis of continuous ohmic heating process for multiphase foods. J Food Eng. 2010;98:257–265. doi: 10.1016/j.jfoodeng.2010.01.005. [DOI] [Google Scholar]
- Darvishi H, Hosainpour A, Nargesi F. Ohmic heating behaviour and electrical conductivity of tomato paste. Journal of Nutrition & Food Sciences. 2012;2(167):1–5. [Google Scholar]
- Darvishi H, Hosainpour A, Nargesi F, Khoshtaghza MH, Torang H. Ohmic processing: temperature dependent electrical conductivities of lemon juice. Modern Applied Science. 2011;5(1):210–216. doi: 10.5539/mas.v5n1p209. [DOI] [Google Scholar]
- Darvishi, H., Khoshtaghaza, M.H., & Najafi, G. (2012a) Ohmic heating of pomegranate juice: Electrical conductivity and pH change. JSSAS, http://dx.doi.org/10.1016/j.jssas.2012.08.003
- De Alwis AAP, Halden K, Fryer PJ. Shape and conductivity effects in the ohmic heating of foods. Chemical Engineering Research and Design. 1989;67:159–168. [Google Scholar]
- de Halleux D, Piette G, Buteau ML, Dostie M. Ohmic cooking of processed meats: Energy evaluation and food safety considerations. Canadian Biosystems Engineering. 2005;47:341–347. [Google Scholar]
- Icier F, Ilicali C. Electrical conductivity of apple and sour cherry juice concentrates during ohmic heating. J Food Process Eng. 2004;27:159–180. doi: 10.1111/j.1745-4530.2004.tb00628.x. [DOI] [Google Scholar]
- Icier F, Bozkurt H. Ohmic heating of liquid whole egg: rheological behavior and fluid dynamics. Food Bioprocess Technol. 2009 [Google Scholar]
- Icier F, Ilicali C. The Effects of concentration on electrical conductivity of orange juice concentrates during ohmic heating. European Food Research and Technology. 2005;220:406–414. doi: 10.1007/s00217-004-1043-x. [DOI] [Google Scholar]
- Icier F, Ilicali C. The Use of tylose as a food analog in ohmic heating studies. J Food Eng. 2005;69:67–77. doi: 10.1016/j.jfoodeng.2004.07.011. [DOI] [Google Scholar]
- Icier F, Ilicali C. Temperature dependent electrical conductivities of fruit purees during ohmic heating. Food Research International. 2005;38:1135–1142. doi: 10.1016/j.foodres.2005.04.003. [DOI] [Google Scholar]
- Icier F, Yildiz H, Baysal T. Polyphenoloxidase deactivation kinetics during ohmic heating of grape juice. J Food Eng. 2008;85:410–417. doi: 10.1016/j.jfoodeng.2007.08.002. [DOI] [Google Scholar]
- Jakob A, Bryjak J, Wojtowicz H, Illeova V, Annus J, Polakovic M. Inactivation kinetics of food enzymes during ohmic heating. Food Chemistry. 2010;123:369–376. doi: 10.1016/j.foodchem.2010.04.047. [DOI] [Google Scholar]
- Jun S, Sastry S. Modelling and optimization of ohmic heating of foods inside a flexible package. Journal of Food Process Engineering. 2005;28(4):417–436. doi: 10.1111/j.1745-4530.2005.00032.x. [DOI] [Google Scholar]
- Kerkhofs, N. S. (2003) An investigation of the influence of air-drying on the antioxidant components and antioxidant activity of New Zealand grown tomatoes. B.Sc. (Honours), Lincoln University
- Lima M, Heskitt B, Sastry S. Diffusion of beet dye during electrical and conventional heating at steady-state temperature. J Food Process Eng. 2001;24(5):331–340. doi: 10.1111/j.1745-4530.2001.tb00547.x. [DOI] [Google Scholar]
- Marra F, Zell M, Lyng JG, Morgan DJ, Cronin DA. Analysis of heat transfer during ohmic processing of a solid food. J Food Eng. 2009;91:56–63. doi: 10.1016/j.jfoodeng.2008.08.015. [DOI] [Google Scholar]
- Salengke S, Sastry SK. Effect of ohmic pretreatment on the drying rate of grapes and adsorption isotherm of raisins. Drying Tech. 2005;23:551–564. doi: 10.1081/DRT-200054131. [DOI] [Google Scholar]
- Sarang S, Sastry SK, Knipe L. Electrical conductivity of fruits and meats during ohmic heating. J Food Eng. 2008;87:351–356. doi: 10.1016/j.jfoodeng.2007.12.012. [DOI] [Google Scholar]
- Sarimeseli A. Microwave drying characteristics of coriander (Coriandrum sativum L.) leaves. Energ Convers Manage. 2011;52:1449–1453. doi: 10.1016/j.enconman.2010.10.007. [DOI] [Google Scholar]
- Shirsat N, Lyng JG, Brunton NP, Mchenna B. Ohmic processing: Electrical conductivities of pork cuts. Meat Science. 2008;67:507–514. doi: 10.1016/j.meatsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Stancl J, Zitny R. Milk fouling at direct ohmic heating. J Food Eng. 2010;99:437–444. doi: 10.1016/j.jfoodeng.2009.11.019. [DOI] [Google Scholar]
- Toor RK, Savage GP. Effect of semi-drying on the antioxidant components of tomatoes. Food Chemistry. 2006;94:90–97. doi: 10.1016/j.foodchem.2004.10.054. [DOI] [Google Scholar]
- Tulsiyan P, Sarang S, Sastry SK. Electrical conductivity of multi-component systems during ohmic heating. International Journal of Food Properties. 2008;11(1):233–241. doi: 10.1080/10942910701302580. [DOI] [Google Scholar]
- Ye XF, Ruan R, Chen P, Christopher D. Simulation and verification of Ohmic heating in static heater using MRI temperature mapping. LWT Food Sci. Technol. 2004;37:49–58. doi: 10.1016/S0023-6438(03)00133-6. [DOI] [Google Scholar]
- Zareifard MR, Ramaswamy HS, Trigui M, Marcotte M. Ohmic heating behaviour and electrical conductivity of two-phase food systems. Innovative Food Sci. Emerg. Technol. 2003;4:45–55. doi: 10.1016/S1466-8564(02)00088-7. [DOI] [Google Scholar]
- Zell M, Lyng JG, Morgan DJ, Cronin DA. Minimising heat losses during batch ohmic heating of solid food. Food Bioprod Process. 2010 [Google Scholar]
- Zell M, Lyng JG, Morgan DJ, Cronin DA, Morgan DJ. Ohmic heating of meats: Electrical conductivities of whole meats and processed meat ingredients. Meat Science. 2009;83:563–570. doi: 10.1016/j.meatsci.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kolbe E, Flugstad B. A method to characterize electrode corrosion during ohmic heating. J Food Process Eng. 1999;22:81–89. doi: 10.1111/j.1745-4530.1999.tb00472.x. [DOI] [Google Scholar]
- Zhong T, Lima M (2003). The effect of ohmic heating on vacuum drying rate of sweep potato tissue. Bioresour Technol., 87(3):215--20 [DOI] [PubMed]
- Zhu SM, Zareifard MR, Chen CR, Marcotte M, Grabowski S. Electrical conductivity of particle–fluid mixtures in ohmic heating: Measurement and simulation. Food Res Int. 2010;43:1666–167. doi: 10.1016/j.foodres.2010.05.009. [DOI] [Google Scholar]