Abstract
The present work is designed to evaluate the antioxidant activities of hydroalcoholic extract (HAE) and its fractions (viz., hexane (HF), chloroform (CF), ethyl acetate (AF), n-butanol (BF) and water (WF)) obtained from aerial part of Rumex vesicarius L. by using different in vitro antioxidant assays. The content in pigments (carotenoids and chlorophylls), total phenolics, flavonoids and tannins were determined using spectrophotometric methods. Qualitative analyses of major phenolics by TLC analysis were also evaluated. Experimental results obtained show that R. vesicarius is a rich source of β-carotene (116.83 ± 1.60 μg/g DW), lycopene (156.40 ± 1.59 μg/g DW) and chlorophyll a (271.45 ± 3.46 μg/g DW). The greatest antioxidant activity was found in AF (IC50.DPPH = 0.07 ± 0.00 mg/ml) followed by BF and CF (0.15 ± 0.00 and 0.16 ± 0.00 mg/ml, respectively). These fractions were also better in their effect on reducing the oxidation of β-carotene. Reducing power of crude methanol extract/fractions increased with increasing concentration of the extract. The amount of total phenolics varied from 0.37 ± 0.01 to 43.28 ± 0.28 mg GAE/g of dry weight, HAE had the higher content (43.28 ± 0.28 mg GAE/g of DW). A negative correlation was found between phenolic compounds and the antioxidant efficiencies of the crude extract/fractions, suggesting that phenolic compounds are not the only contributors to the antioxidant activities of Rumex vesicarius. The present findings suggest that Rumex vesicarius L. can be used as natural antioxidant source to prevent damage associated with free radicals.
Keywords: Rumex vesicarius, Pigments, Phenolics, Antioxidants, TLC analysis
Introduction
The use of medicinal plants for treating diseases is probably the oldest existing method that humanity has used to try to cope with illness. In fact plants represent an enormous reservoir of new, undiscovered and bioactive molecules. Over the last two decades there has been a resurgence of interest in the study and use of medicinal plants. The World Health Organization has confirmed the importance of traditional medicine to a majority of the world’s population and encourages all countries to preserve and to use the safe and positive elements of traditional medicine in their national health systems (Aquino et al. 1995).
The last decade is characterized by a growing interest in natural antioxidants. As a result, an overwhelming amount of scientific information is now available and promises to keep increasing. The use of synthetic antioxidants in the food industry, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG), tert-butyl hydroquinone (TBHQ), and ascorbyl palmitate (PA), is severely restricted by legislation concerning both the application and permitted concentrations (Regulation (EC) 2008). Moreover, there is some safety concerns related to the residual toxicity of chemical preservatives (EFSA 2012). Therefore, the recent worldwide tendency to avoid or at least decrease the use of synthetic additives has created a need for alternative cheap, renewable, natural and possibly safer sources of natural compounds with antioxidant activity to prevent oxidation in foods. Oxidation reactions are not only important to the food industry; antioxidants are also required to avoid deterioration of products found in the cosmetics, pharmaceutical and plastic industries (Moure et al. 2001).
A large number of scientific reports have described the properties of natural substances from plants. Of which, phenolics were one of the most notable groups. Rumex vesicarius L., locally called Hammeidda (Quézel and Santa 1962), is an edible green leafy plant that belongs to the family of Polygonaceae. This shrub, native to northern Africa and Asia (Pakistan and India) (Sankar et al. 2011), grows annually during the fall and spring rainy seasons. It was considered as a dietary complementary plant, since this plant is a rich source of ß-carotenes (Bélanger et al. 2010), vitamins (especially vitamin C), proteins, lipids and organic acids. It is also a good source of minerals such as; K, Na, Ca, Mg, Fe, Mn and Cu (Saleh et al. 1993; Al–Rumaih May et al. 2002; Alfawaz 2006; Filho et al. 2008). The whole plant is medicinally important and cures several diseases, the plant is stimulant, tonic, and acts as aphrodisiac agent (Gopal et al. 2008). It is also used in treatment of tumors, hepatic diseases, bad digestion, constipation, calcules, heart troubles, pains, diseases of the spleen, hiccough, flatulence, asthma, bronchitis, dyspepsia, piles, scabies, leucoderma, toothache and nausea. Finally, the plant can be used also to reduce biliary disorders and control cholesterol levels (Mostafa et al. 2011).
The previously mentioned bioactive phytochemicals, in addition to phenolic compounds and flavonoids, have a role as antioxidant and detoxifying agents. The intake of dietary antioxidant phytochemicals leads to protection against non-communicable diseases i.e. cancer, cardiovascular diseases and cataract (Rao 2003; Alberto et al. 2006; Matkowski 2008; Ghafar et al. 2010; Imran et al. 2011). The purpose of the present study was to prepare fractions from the crude extract of Rumex vesicarius L. that is rich in phenolic compounds and investigate their antioxidant activities, and as consequence to exploit its potential as a natural preservative.
Materials and methods
Plant materials
Aerial part of Rumex vesicarius L. used for this investigation was collected from Ghardaïa (South of Algeria) in March 2012, and its identity was verified by Dr. Mahboubi from Department of Ecology and Environment, University of Tlemcen, Algeria. Collected plant material was rinsed with distilled water, left at room temperature for 15 days in the dark, dried in a shaded and well-ventilated place, pulverized then kept refrigerated in glass containers before further processing.
Preparation of crude methanolic extract and fractions
Crude methanolic extract (Hydroalcoholic extract (HAE)) was obtained by refluxing of 10 g of powder in 70 % methanol (1:10, w/v) for 2 h at temperature no higher than 65 °C. The extraction was repeated three times. Extract was filtered through filter paper, evaporated under vacuum to dryness and kept at −20 °C before analysis.
The HAE was resuspended in warm water and then partitioned sequentially with hexane, chloroform, ethyl acetate, n-butanol and water. Hexane fraction (HF), chloroform fraction (CF), ethyl acetate fraction (AF), n-butanol fraction (BF) and water fraction (WF) were collected separately and concentrated using a vacuum evaporator to remove the solvent. Residues were dissolved in pure methanol and stored at −20 °C until analysis.
The yield (%) of evaporated dried extracts was calculated as 100 DWext/DWsamp, where DWext is the weight of extract after evaporation of solvent, and DWsamp is the dry weight of sample.
Determination of lipid-soluble pigments
A fine dried powder (150 mg) was vigorously shaken with 10 ml of acetone–hexane mixture (4:6) for 1 min and filtered through filter paper. The absorbance of the filtrate was measured at 453, 505, 645 and 663 nm (Barros et al. 2011). The content in pigments was calculated according to the following equations:
and further expressed in μg per g dry weight (DW).
Phenolic compounds analysis
Total phenolics content determination
Total phenolics were determined using Folin–Ciocalteu reagent, as described by Awah et al. (2012). Folin–Ciocalteu reagent (FCR) consists of a yellow acidic solution containing complex polymeric ions formed from phosphomolybdic and phosphotungstic heteropoly acids. Dissociation of a phenolic proton in a basic medium leads to a phenolate anion, which reduces FCR, forming a blue-coloured molybdenum oxide whose colour intensity is directly proportional to the phenolic contents (Huang et al. 2005). Briefly, crude extract and fractions (100 μl) dissolved in methanol were mixed with 750 μl of Folin–Ciocalteu reagent (diluted 10-fold in H2O) and allowed to stand at 22 °C for 5 min; 750 μl of Na2CO3 (60 g/l) solution were then added to the mixture. After 90 min, the absorbance was measured at 725 nm. The total phenolic content was determined using the standard gallic acid calibration curve and expressed as mg gallic acid equivalents (GAE) per g of dry weight (DW).
Total flavonoids and flavonols content determination
The flavonoids content was determined according to the method described by Barros et al. (2011). An aliquot of the extract solution (0.5 ml) was mixed with distilled water (2 ml) and subsequently with NaNO2 solution (5 %, 0.15 ml). After 6 min, AlCl3 solution (10 %, 0.15 ml) was added and allowed to stand for a further 6 min; thereafter, NaOH solution (4 %, 2 ml) was added to the mixture. Immediately, distilled water was added to bring the final volume to 5 ml. Then the mixture was properly mixed and allowed to stand for 15 min. The intensity of pink colour was measured at 510 nm. Catechin was used to calculate the standard curve and the results were expressed as mg catechin equivalents (CE) per g of dry weight (DW).
The content of flavonols was also determined by the aluminium chloride method described by Awah et al. (2012). Briefly, 1 ml of each extracts was mixed with 1 ml aluminium trichloride (20 mg/ml) and 3 ml of sodium acetate (50 mg/ml). The absorbance at 440 nm was read after 2.5 h. A standard curve was prepared using quercetin in methanol under the same conditions. Results were expressed as mg quercetin equivalents (QE) per g of dry weight (DW).
Condensed tannins (proanthocyanidins) content
Determination of condensed tannins by the vanillin assay was carried out using the procedure reported by Sun et al. (1998). The method is based on the ability of condensed tannins to react with vanillin in the presence of mineral acid to produce a red color. 500 μl of extracts solutions were mixed with 3 ml of 4 % vanillin-methanol solution and 1.5 ml hydrochloric acid. The mixture was allowed to stand for 15 min. The absorbance was measured at 500 nm, while the final result was expressed as mg catechin equivalent (CE)/g dry weight.
Hydrolysable tannins (gallotannins) content
The gallotannin content of the extracts was determined with the potassium iodate assay (Saad et al. 2012). 5 ml of KIO3 aqueous solution (2.5 % w/v) was heated for 7 min at 30 °C, and then 1 ml of the diluted sample was added. After additional 2 min of tempering at 30 °C, the absorbance was measured at 550 nm. A calibration curve was obtained using tannic acid solution (5000 mg/l) prepared by solubilization of 0.25 g of tannic acid in 50 ml of methanol (80 %). The analytical standard solutions of tannic acid were prepared by aqueous dilution. Results were expressed as mg tannic acid equivalent (TAE) per g of dry weight (DW).
In vitro evaluation of antioxidant activities
Total antioxidant capacity by phosphomolybdenum assay (P-Mo)
The assay is based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acid pH (Prieto et al. 1999). An aliquot (0.1 ml) of plant extracts was combined to 1 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were incubated in a thermal block at 95 °C for 90 min. Then, the samples were cooled to room temperature and the absorbance was measured at 695 nm against a blank. The antioxidant capacity was expressed as mg gallic acid equivalent per gram dry weight (mg GAE/g DW). All samples were analysed in triplicate.
DPPH radical-scavenging activity
The ability of the corresponding extracts to donate hydrogen atoms or electrons was measured from the bleaching of purple coloured methanol solution of 1,1-diphenyl-2-picrylhydrazyl (DPPH), according to the method described by Barros et al. (2011). The reaction mixture in each one of the 96 wells consisted of known concentrations of the extracts (30 μl) and aqueous methanolic solution (80:20 v/v, 270 μl) containing DPPH radicals (6 × 10−5 M). The mixture was left to stand for 60 min in the dark. The reduction of the DPPH radical was determined by measuring the absorption at 515 nm using Bio-Tek EL×800 microplate reader. The radical-scavenging activity (RSA) was calculated as a percentage of DPPH discolouration using the equation:
Where AS is the absorbance of the solution when the sample extract has been added at a particular level and ADPPH is the absorbance of the DPPH solution. The extract concentration providing 50 % of radical-scavenging activity (IC50) was calculated from the graph of RSA percentage against extract concentration. Vitamin E was used as standard.
Iron reducing power
The reducing power of R. vesicarius extracts was determined through the transformation of Fe3+ to Fe2+ induced by plant extracts, according to the method of Oyaizu (1986). Samples (1 ml) at different concentrations were mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of potassium ferricyanide (1 % w/v). The tubes were incubated at 50 °C for 20 min. Afterwards, 2.5 ml of 10 % TCA were added in each tube and the mixture was centrifuged for 10 min at 3.000 g. An aliquot of the supernatant (2.5 ml) was mixed with distilled water (2.5 ml) and 0.5 ml of ferric chloride (0.1 % w/v), and the absorbance was measured at 700 nm: higher absorbance indicates higher reducing power. Vitamin E was used as authentic standard and EC50 value (effective concentration of the extract which corresponds to 0.5 of absorbance) was obtained from linear regression analysis.
β-Carotene bleaching test
A slightly modified Koleva et al. (2002) method was employed to estimate R. vesicarius extracts capacity to inhibit the β-carotene bleaching. Two milligrams of β-carotene were dissolved in 20 ml chloroform and to 4 ml of this solution, linoleic acid (40 mg) and Tween 40 (400 mg) were added. Chloroform was evaporated under vacuum at 40 °C and 100 ml of oxygenated water was added, then the fresh emulsion was vigorously shaken. An aliquot (150 μl) of the β-carotene/linoleic acid emulsion was distributed in each of the wells of 96-well microtitre plates and methanolic solutions of the test samples or authentic standards (10 μl) were added. Three replicates were prepared for each concentration. The microtitre plates were incubated at 50 °C for 120 min, and the absorbance was measured using Bio-Tek EL×800 microplate reader at 490 nm. Readings of all samples were performed immediately (t = 0 min) and after 120 min of incubation. The antioxidant activity of the extracts was evaluated in terms of blanching inhibition of the β-carotene using the following formula:
Where C0 and C120 are the absorbance values of the control at 0 and 120 min, respectively, and S is the sample absorbance at 120 min. The results were expressed as IC50 values (mg ml−1).
TLC screening for phytochemical analysis
Phytochemical screening for the presence of secondary metabolites was performed using thin layer chromatography (TLC) analysis. Ten microlitres of each sample (10 mg/ml in methanol) was loaded on Merck TLC 60 F254 silica gel sheets. Development was carried out with different eluting systems viz.: Cyclohexan: AcOEt (10: 8 (v/v)), AcOEt: CHCl3: AcOH (8: 7: 0.5 (v/v/v)) and Toluene: AcOH: Formic acid (3: 5: 1 (v/v/v)) (Mamyrbekova-Bekro et al. 2013a). After drying, the plates were sprayed with (KOH, (CH3CO2)2Pb, NH3) to detect coumarin; AlCl3 and NH3 to detect flavonoids, Libermann-Burchard to detect sterols and polyterpens, Dragendorf’s reagent to detect alcaloïdes and ferric chloride to detect tannins (Lhuillier 2007; Guy et al. 2010; Kabran et al. 2011; Rathee et al. 2012). Detection was carried out visually in visible light and under UV light (λ = 366 nm).
The retention factor (Rf) was calculated using the following equation:
Statistical analysis
All results were carried out in triplicate and expressed as mean ± standard deviation (SD). The concentration needed for 50 % inhibition (IC50) was estimated graphically by linear regression analysis. The correlations between polyphenols and antioxidant activities were calculated using the Pearson coefficient (ρ) and linear regression analysis by Microsoft Excel program.
Results and discussion
Rumex vesicarius L. is a good source of bioactive compounds due to its content of various phytochemicals. In this study, total extracts yield, phenolic composition and antioxidant activities of hydroalcoholic extract/fractions from aerial part of R. vesicarius, collected from Algeria, were determined.
Yield of crude extract and fractions
The results of using different solvents for the extraction/fractionation of phenolic compounds are given in Table 1. From this table it was evident that R. vesicarius contained noticeable amounts of extractable compounds. It is clear that the different solvents used for the extraction and fractionation had different abilities to extract substances from this plant. In general, the amount of total extractable compounds decreased with decreasing polarity of the solvent. The extraction yield varied from 0.25 to 14.25 % (w/w). Among all the fractions, WF obtained the highest extraction yield (8.85 ± 4.46 %) while CF yielded the lowest (0.25 ± 0.16 %). The yield of all the fractions is presented in the following order: WF > BF > AF > HF > CF. The low extraction yield of CF is probably due to the low solubility of major components of R. vesicarius.
Table 1.
Extraction yields (%) and polyphenol contents in crude extract and fractions of Rumex vesicarius
| Extracts/Fractions | Extraction yield | Phenolicsa | Flavonoidsb | Flavonolsc | Condensed tanninsb | Hydrolysable tanninsd |
|---|---|---|---|---|---|---|
| HAE | 14.25 ± 4.35 | 43.28 ± 0.28 | 19.72 ± 0.37 | 2.68 ± 0.02 | 4.34 ± 0.19 | 6.21 ± 1.28 |
| HF | 0.54 ± 0.17 | 0.37 ± 0.01 | 1.98 ± 0.04 | 0.67 ± 0.01 | 0.02 ± 0.00 | 2.14 ± 0.20 |
| CF | 0.25 ± 0.16 | 2.45 ± 0.01 | 1.78 ± 0.03 | 0.42 ± 0.01 | 0.18 ± 0.00 | 2.69 ± 0.06 |
| AF | 0.54 ± 0.10 | 9.95 ± 0.01 | 4.65 ± 0.07 | 1.01 ± 0.02 | 0.09 ± 0.01 | 3.17 ± 0.30 |
| BF | 1.61 ± 0.30 | 12.40 ± 0.02 | 5.60 ± 0.04 | 1.09 ± 0.00 | 0.01 ± 0.00 | 0.41 ± 0.16 |
| WF | 8.85 ± 4.46 | 4.16 ± 0.32 | 3.17 ± 0.15 | 0.07 ± 0.00 | 0.34 ± 0.10 | 1.35 ± 0.11 |
aExpressed as mg gallic acid equivalents/g dry weight
bExpressed as mg catechin equivalents/g dry weight
cExpressed as mg quercetin equivalents/g dry weight
dExpressed as mg tannic acid equivalents/g dry weight
Values are expressed as means of three replicates ± SD
Lipo-soluble pigments composition
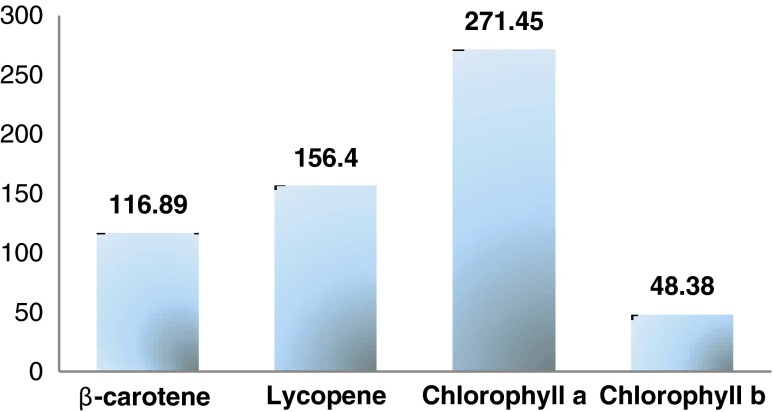
The content in pigments (carotenoids and chlorophylls) of the studied medicinal plant is given in Fig. 1. Experimental results obtained show that Rumex vesicarius contained a highest level of β-carotene (116.83 ± 1.60 μg/g DW), lycopene (156.40 ± 1.59 μg/g DW), chlorophyll a (271.45 ± 3.46 μg/g DW) and less level of Chlorophyll b (48.38 ± 3.96 μg/g DW).
Fig. 1.
Composition of R. vesicarius in pigments (μg/g DW)
Bhaskarachary et al. (1995) and Bélanger et al. (2010) reported the quantification of β-carotene in fresh weight (26 μg/g and 45 μg/g fresh weight, respectively), but nothing is reported about content of carotenoids or chlorophyll in dry weight of the studied plant. Carotenoids are amongst nature’s most widespread pigments and have also received substantial attention because of both their provitamin and antioxidant roles. Chlorophyll and its derivatives are also known to have antioxidant activity, being associated with reduced risks of diseases induced by free radicals, such as certain types of cancers (Carrapeiro et al. 2007; Barros et al. 2011). Javanmardi et al. (2003) reported that antioxidant activity of plant extracts is not limited to phenolics. The activity may also come from other antioxidant secondary metabolites, such as volatile oils, carotenoids and vitamins.
Phenolic compounds analysis
Table 1 summarizes the total phenolics, flavonoids, flavonols and tannins content of the aerial part of R. vesicarius. Principal results showed that R. vesicarius extracts exhibited an important amount of polyphenol content followed by flavonoid as major class and low tannin fraction. The amount of total phenolics varied from 0.37 ± 0.01 to 43.28 ± 0.28 mg GAE/g of dry weight. HAE had the higher content (43.28 ± 0.28 mg GAE/g of DW). This could be due to more interfering substances present in the crude extract as compared to those fractions. Moreover, total phenolic content increased in the fractions with increased the solvent polarity. The lower polarity solvents: hexane and chloroform showed much lower ability in extracting the phenolic compounds as compared to the polar solvents. Total phenolic content of fractions was found to be arranged in the following descending order: BF > AF > WF > CF > HF. This result suggests that BF fraction might be the part that is rich in phenolic compounds and that n-butanol is suitable to extract phenolic compounds from R. vesicarius. The same tendency was observed for flavonoid and flavonol contents (see Table 1). Considering tannin contents, it was concluded that hydrolysable tannins content was higher than condensed tannins content with 6.21 ± 1.28 mg TAE/g DW in HAE.
It is extremely important to point out that the most antioxidant activities from plant sources are correlated with phenolic compounds (Cai et al. 2004; Jain et al. 2008; Huang et al. 2010a, b). These compounds are known to act as antioxidants not only because of their ability to donate hydrogen or electrons but also to because they are stable radical intermediates. Our findings showed the richness of R. vesicarius on phenolic content. For that, the in vitro estimation of antioxidant activities was determined.
Antioxidant activity
The antioxidant capacities are influenced by many factors, which cannot be fully described with one single method. Therefore it is necessary to perform more than one type of antioxidant capacity measurement to take it in to account the various mechanisms of antioxidant action (Wong et al. 2006a; Erkan et al. 2008). In this study, the antioxidant activities of the HAE/fractions from R.vesicarius were evaluated by using in vitro antioxidant models, including total antioxidant activity, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, reducing power and β-Carotene bleaching test. The relation between the antioxidant activity and polyphenol contents was also studied.
Total antioxidant capacity (TAC)
This assay is a quantitative one since the antioxidant activity is expressed as numbers of equivalents of gallic acid. Table 2 showed that polar solvents have the higher antioxidant capacity, 32.97 ± 4.41 mg ascorbic acid/g dry weight was observed in HAE of R. vesicarius. BF and WF exhibited higher activity (6.92 ± 0.89 and 6.67 ± 1.84 mg/g DW, respectively) when compared to different other solvent fractions. Published reports on the total antioxidant activity of R. vesicarius extracts are not available. However, a total antioxidant activity of 245–376 mg ascorbic acid/g extract has been reported in higher plant extracts (Kumaran and Karunakaran 2007).
Table 2.
Antioxidant activities of hydroalcoholic extract/fractions from R. vesicarius
| Extract/Fractions/Standard antioxidant | TAC (mg GAE/g DW)a | IC50/DPPH a | EC50/Reducing power a | IC50/β-Carotene a |
|---|---|---|---|---|
| HAE | 32.97 ± 4.41 | 0.56 ± 0.00 | 1.38 ± 0.01 | 0.26 ± 0.01 |
| HF | 1.62 ± 0.00 | 1.01 ± 0.03 | 2.87 ± 0.03 | 2.57 ± 0.38 |
| CF | 0.56 ± 0.02 | 0.16 ± 0.00 | 0.80 ± 0.00 | 0.21 ± 0.00 |
| AF | 3.86 ± 0.16 | 0.07 ± 0.00 | 0.23 ± 0.00 | 0.24 ± 0.01 |
| BF | 6.92 ± 0.89 | 0.15 ± 0.00 | 0.72 ± 0.01 | 0.22 ± 0.01 |
| WF | 6.67 ± 1.84 | 2.90 ± 0.04 | 7.13 ± 0.07 | 0.63 ± 0.01 |
| Standard (Vit E) | – | 0.07 ± 0.01 | 0.24 ± 0.01 | 0.24 ± 0.01 |
aEach value is expressed as the mean ± standard deviation (n = 3); EC50 = effective concentration at which the absorbance was 0.5; IC50 = inhibition concentration 50 %; TAC total antioxidant capacity
DPPH radical scavenging activity
DPPH has been used extensively to test the ability of compounds and extracts from plants or food materials to act as free radical scavengers or hydrogen donors (Liyana-Pathirana and Shahidi 2006). As a kind of stable free radical, DPPH can accept an electron or hydrogen radical from antioxidant (A-H) to become a stable diamagnetic molecule as described in the following equation:
The degree of discoloration, quantified by measuring the absorbance at 515 nm, indicated the scavenging potential of the antioxidant compounds or extracts in the term of hydrogen donating ability (Mosquera et al. 2007).
Scavenging effects of R. vesicarius extracts on DPPH were examined at different concentrations, the IC50 values are given in Table 2. The ethyl acetate fraction shown the highest potent DPPH radical scavenger activity (0.07 ± 0.00 mg/ml) followed by n-butanol and chloroform fractions (0.15 ± 0.00 and 0.16 ± 0.00 mg/ml, respectively). In addition, the water fraction exhibited poor scavenging of DPPH• (2.90 ± 0.04 mg/ml). The free radical-scavenging activity of crude extract was eight times less important than those of ethyl acetate fraction, which may result from the active components through enrichment effects during the solvent–solvent partitioning processes. As it can be seen, vitamin E (α-tocopherol), well-known antioxidant compound used as the reference control in this study, had the same IC50 value as ethyle acetate fraction (0.07 ± 0.01 mg/ml), this indicates potent free radical scavenging activity of AF of R. vesicarius. From the result, it revealed that R. vesicarius was a high potential natural antioxidant.
Reducing power
For the determination of the reductive ability, the Fe3+ - Fe2+ transformation was investigated in the presence of R. vesicarius extracts. Samples with different concentration were used for this assay and all of them exhibited the dose-dependent activity. As shown in Table 2, the AF revealed a good reducing power (0.23 ± 0.00 mg/ml), while WF yielded the lowest (7.13 ± 0.07 mg/ml). The reducing power of all fractions is presented in the following order: AF > BF > CF > HAE > HF > WF. The data of the positive control (vit E) was 0.24 ± 0.01 mg/ml. Ethyl acetate fraction has a great ability to terminate radical chain reaction by converting free radicals and reactive oxygen species to more stable non-reactive products (Siddhuraju and Becker 2003). Our results were in accordance with those found by Yao et al. (2013).
β-Carotene/linoleic acid method
The assay measures the ability of a plant extract to prevent or minimize the coupled oxidation of β-carotene and linoleic acid in an emulsified aqueous system (Parejo et al. 2002). The IC50 of crude extract and its different fractions from R. vesicarius was revealed in Table 2. CF, BF, AF and HAE were better in their effect on reducing the oxidation of β-carotene than WF and HF. The IC50 values of Chloroform and butanol fractions (0.21 ± 0.00 and 0.22 ± 0.01 mg/ml, respectively) were higher to that of positive control vit E (0.24 ± 0.01 mg/ml). Meanwhile, the IC50 values of ethyl acetate fraction (0.24 ± 0.01 mg/ml) and crude extract (0.26 ± 0.01 mg/ml) were close to that of positive control. According to Liyana-Pathirana and Shahidi (2006) an extract capable of retarding or inhibiting the oxidation of β-carotene may be described as a free radical scavenger and primary antioxidant.
Relationship between content in phenolic compounds and antioxidant activities
For better understanding the relationship between polyphenols and antioxidant capacities of R. vesicarius, all the prepared extracts were used in an analysis of the correlation using the Pearson coefficient (ρ) and linear regression analysis, the results are shown in Table 3. It is important to note that as the correlations between polyphenols and free radical scavenging ability were determined using IC50, a negative ρ value (−1) is considered as perfect positive correlation (da Silva et al. 2011).
Table 3.
Pearson’s correlation coefficients (ρ) of phenolic compounds and antioxidant capacity
| ρ | TPC | TFC | CTC | HTC | CTAC | CDPPH | CR | CAnti-β-carotene bleaching |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.996 | 0.949 | 0.787 | 0.976 | −0.221 | −0.268 | −0.396 |
| TFC | 1 | 0.968 | 0.803 | 0.989 | −0.166 | −0.213 | −0.328 | |
| CTC | 1 | 0.870 | 0.978 | −0.059 | −0.101 | −0.245 | ||
| HTC | 1 | 0.775 | −0.244 | −0.288 | −0.173 | |||
| CTAC | 1 | −0.026 | −0.071 | −0.294 | ||||
| CDPPH | 1 | 0.998 | 0.259 | |||||
| CR | 1 | 0.295 | ||||||
| CAnti-β-carotene bleaching | 1 |
TPC Total phenolic content, TFC Total flavonoid content, CTC Condensed tannin content, HTC Hydrolysable tannin content, C TAC Coefficient of total antioxidant capacity, C DPPH Coefficient of DPPH radical scavenging activity, C R Coefficient of reducing power, C Anti - β-carotene bleaching Coefficient of antioxidant activity determined with β-carotene bleaching test
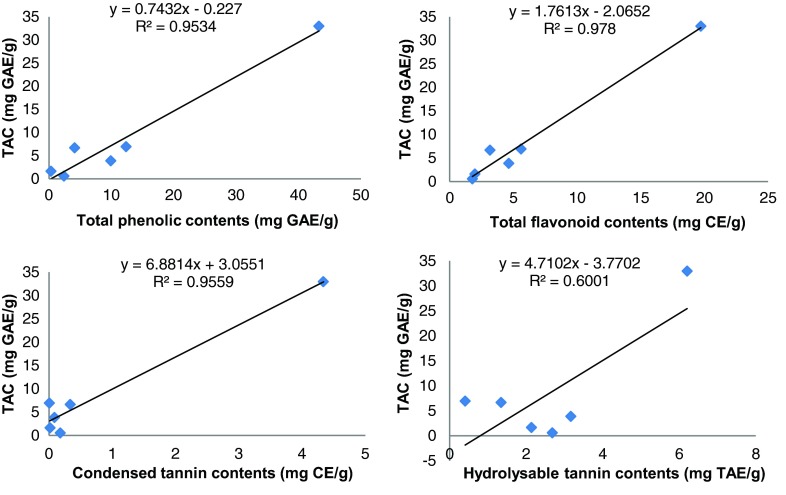
With regard to the results, a linear correlation appeared between the total antioxidant capacity and the total phenolic, flavonoid and condensed tannin contents with excellent ρ correlation coefficients (ρ = 0.976, 0.989 and 0.978, respectively). Also, good ρ correlation coefficient (ρ = 0.775) appeared between the total antioxidant capacity and hydrolysable tannin contents (Table 3, Fig. 2).
Fig. 2.
Correlation coefficients (R2) between total antioxidant capacity and phenolic compounds of R. vesicarius extracts
These results are in good accordance with previous studies which showed that high total phenolic content increases the antioxidant activity (Wong et al. 2006b; Kumaran and Karunakaran 2007). However, for other activities weak correlation was found, suggesting the involvement of non phenolic compounds such as ascorbic acid, tocopherols, saponins, carotenoids and polysaccharide (Nsimba et al. 2008; Erkan et al. 2008; Ananthi et al. 2010). Several studies established a linear correlation between the total content of phenolics and the antioxidant capacity (Cai et al. 2004; Kumaran and Karunakaran 2007) whereas, some studies reported that there is no correlation (Kähkönen et al. 1999; Yu et al. 2002; Tongpoothorn et al. 2012; Yao et al. 2013).
As shown in Table 3, the mutual correlations among the four methods were determined by Pearson correlation analysis. High correlation among DPPH and reducing power was found (ρ = 0.998) whereas DPPH/β-carotene-linoleic acid bleaching and reducing power/β-carotene-linoleic acid bleaching exhibited weaker correlations. Furthermore, no correlations were obtained with TAC and other methods.
Phytochemical analysis
Due to the high activity of the ethyl acetate, n-butanol and chloroform fractions of R. vesicarius, they were subjected to some phytochemical and TLC analysis. The tested plant showed positive results for variable amounts of sterols/triterpenes, polyphenols, anthraquinones, coumarins, tannins and flavonoids. Alkaloids were totally absent in all the three fractions (see Tables 4, 5 and 6).
Table 4.
Rf-values and color of spots of the chloroform fraction
| Extracts | Without revelation a | With revelation | Identified compounds | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NH3 A | Liebermann-Burchard B | FeCl3 C | KOH D | (CH3CO2)2Pb E | ||||||||||||||
| Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | Rf | Visible | Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | ||
| CF | 0.00 | Yellow | Yellow | 0.00 | Yellow | Yellow | 0.00 | Green | Yellowg | – | – | 0.00 | Brown | Yellow | 0.00 | Yellow | Yellow | Steroids B (Mamyrbekova-Bekro et al. 2013b; Kabran et al. 2011; Lagnika 2005) |
| 0.07 | – | Redl | 0.04 | – | Violet | – | – | – | – | – | – | – | – | 0.06 | – | Violet | Anthraquinone a (Mamyrbekova-Bekro et al. 2013b) | |
| – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0.12 | – | Violetd | Unidentified | |
| 0.70 | – | Violetd | – | – | – | 0.89 | Violet | Violet | – | – | – | – | – | 0.75 | – | Violetd | Steroidal saponin B (Mamyrbekova-Bekro et al. 2013a; Guy et al. 2010) | |
Mobile phase : Cyclohexan: AcOEt (10: 8 (v/v))
l Light, d Dark, g Greenish
Table 5.
Rf-values and color of spots of the ethyl acetate fraction
| Extracts | Without revelation a | With revelation | Identified compounds | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NH3 A | KOH B | AlCl3 C | FeCl3 D | ||||||||||||
| Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | Rf | Visible | ||
| AF | 0.00 | Brown | Green | 0.00 | Brown | Yellow | 0.00 | Brown | Green | 0.00 | Yellow | Brown | 0.00 | Grey | Coumarins A (Guy et al. 2010), B (Mamyrbekova-Bekro et al. 2013a, b), Flavonoids C (Kabran et al. 2011; Guy et al. 2010); Tannins D (Mamyrbekova-Bekro et al. 2013a; Kabran et al. 2011) |
| 0.04 | – | Purple | – | – | – | 0.03 | Yellow | Violet | – | – | – | 0.04 | Grey | Tannins D (Mamyrbekova-Bekro et al. 2013a; Kabran et al. 2011) | |
| 0.12 | Yellow | Orange | 0.12 | – | Orange | 0.14 | Yellow | Orange | – | – | – | – | – | Coumarins B (Mamyrbekova-Bekro et al. 2013a; Kabran et al. 2011), Xanthone a (Ladyguina et al. 1983), | |
| 0.16 | – | Blue | 0.17 | Yellow | Green | – | – | – | 0.16 | Yellow | Blue | – | – | Phenolic acids or catechols a, C (Guy et al. 2010; Dawson et al. 1991) | |
| 0.91 | – | Purple | – | – | – | 0.98 | – | Purple | 0.91 | – | Violet | – | – | Hydroxyl flavonols a (Dawson et al. 1991) | |
Mobile phase : AcOEt: CHCl3: AcOH (8: 7: 0.5 (v/v/v))
l Light, d Dark, g Greenish, f Fluorescent
Table 6.
Rf-values and color of spots of the n-butanol fraction
| Extracts | Without revelation a | With revelation | Identified compounds | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AlCl3 A | FeCl3 B | ||||||||
| Rf | Visible | UV/366 nm | Rf | Visible | UV/366 nm | Rf | Visible | ||
| BF | 0.00 | Brown | Yellow | – | – | – | – | – | Unidentified |
| 0.16 | Yellow | Green | – | – | – | – | – | Unidentified | |
| 0.19 | Yellow | Yellow | 0.18 | Yellow | Yellow | – | Flavonols a, A (Guy et al. 2010; N’gaman Kohué et al. 2009) | ||
| 0.39 | Yellow | Green | 0.42 | Yellow | Yellow | 0.39 | Grey | Flavonoids A (N’gaman Kohué et al. 2009), Tannins B (Mamyrbekova-Bekro et al. 2013a; Kabran et al. 2011) | |
| 0.47 | Yellow | Green | 0.51 | Yellow | Yellow | – | – | Flavonoids A (N’gaman Kohué et al. 2009) | |
| 0.6 | Yellow | Yellow | 0.63 | Yellow | Yellow | 0.6 | Brown | Flavonols a, A (Guy et al. 2010; N’gaman Kohué et al. 2009), Tannins B (Kabran et al. 2011) | |
| 0.85 | – | Green | 0.89 | – | Pink | – | – | Unidentified | |
| 0.87 | – | Orange | 0.91 | – | Pink | – | – | Xanthone a (Mamyrbekova-Bekro et al. 2013a; Ladyguina et al. 1983) | |
Mobile phase: Toluene: AcOH: Formic acid (3: 5: 1 (v/v/v))
y Yellowish, l Light, f Fluorescent
Table 4 shows the groups of secondary metabolites which are contained in chloroform fraction. The presence of steroids was confirmed by Liebermann-Burchard reagent under UV/366 nm. Two spots appear on the plate (Yellow greenish at Rf = 0.00 and violet at Rf = 0.89). Anthraquinones were also found in CF (red spot at Rf = 0.07), while all other phytoconstituents were absent. These observations were made by Lagnika (2005), Guy et al. (2010), Kabran et al. (2011) and Mamyrbekova-Bekro et al. (2013a, b) in their work on other plants. TLC studies on ethyl acetate fraction showed the presence of flavonoids, tannins and coumarins (Table 5). Presence of coumarins was confirmed after spraying of KOH reagent (Rf = 0.14, yellow in the visible and orange fluorescent under UV) (Mamyrbekova-Bekro et al. 2013a; Kabran et al. 2011). Flavonoids have variable coloring spots under UV. Indeed, xanthones (Rf = 0.12) appear in orange (Ladyguina et al. 1983), methylated flavones and hydroxyl flavonols are blue and purple (Dawson et al. 1991). This is the case of the Rf = 0.16 and 0.91. Presence of flavonoids was confirmed after spraying of AlCl3 and NH3 reagents. AlCl3 reveals yellow spots in visible and in UV colorings from blue to brown (Lagnika 2005) while NH3 reveals them as fluorescent yellow, green and blue spots under UV (Dawson et al. 1991; Ladyguina et al. 1983). The presence of tannins was further confirmed by FeCl3 reagent. The grey color was obtained after spraying of the reagent (Rf = 0.00 and Rf = 0.04) (Mamyrbekova-Bekro et al. 2013a; Kabran et al. 2011).
In butanolic fraction, we have tried to identify polyphenols and saponins. For this purpose, specific revelators were chosen. Table 6 summarizes the groups of phenolic compounds which are contained in BF.
Using the methodological approach (color of spot before and after spraying of reagents) used previously for the detection and identification of phenolic compounds; we showed the richness of BF in flavonoids. Thus, traces of tannins were detected.
Previous studies of the genus have led to the isolation of anthraquinone derivatives (Midiwo and Rukunga 1985; Demirezer 1994), particularly in the roots, which showed various pharmacological properties, such as antitumor, antimutagenicity and antioxidant activities (Demirezer et al. 2001). Flavonoids reported in Rumex species were either flavonols or C-glycosides. Thus R. vesicarius contained vitexin, isovitexin, orientin and isoorientin (Halim et al. 1989). The presence of 8-C-glucosyl-apigenin, 8-C-glucosyl-luteolin, 6-C-hexosyl-quercetin, 3-O-rutinosyl-quercetin, 7-O-rhamno-hexosyl-diosmetin, 7-O-rhamno-acetylhexosyl-diosmetin, catechin, epicatechin, ferulohexoside, 6-C-glucosyl-naringenin, epicatechin gallate, 6-C-glucosyl-catechin, and epigallocatechin gallate has also been reported in R. vesicarius (El-hawary et al. 2011).
Conclusion
This study was designed to evaluate the antioxidant activities of hydroalcoholic extract and its fractions obtained from aerial part of Rumex vesicarius (Polygonaceae) by using different in vitro antioxidant assays. Results indicate that ethyl acetate fraction (AF) possessed significant antioxidant activities approaching the activity of α-tocopherol examined by the same tests. Interestingly, a negative correlation was found between phenolic compounds and the antioxidant efficiencies of the crude extract/fractions, suggesting that phenolic compounds are not the only contributors to the antioxidant activities of Rumex vesicarius. In contrast, DPPH radical-scavenging activity and reducing power are compatible due to their similar mechanism of radical scavenging. Qualitative TLC analysis showed the presence of some phenolic compounds such as flavonoids, tannins, coumarins and steroids that could provide scientific evidence for some folk uses in the treatment of diseases related to the production of reactive oxygen species and oxidative stress. Further work is still needed to identify and characterize the inherent phytocompounds from AF and other fractions and to investigate the in vivo antioxidant efficacy of R. vesicarius.
Acknowledgments
We thank the laboratory staff of Extremophile Plants (Biotechnologic Center in Borj-Cedria Technopol, Tunisia) for their help and suggestions in antioxidant assays.
References
- Alberto MR, Canavosio MAR, Nadra MCM (2006) Antimicrobial effect of polyphenols from apple skins on human bacterial pathogens. Electron J Biotechnol 9(3). doi:10.2225/vol9-issue3-fulltext-1
- Alfawaz MA. Chemical composition of hummayd (Rumex vesicarius) grown in Saudi Arabia. J Food Compos Anal. 2006;19:552–555. doi: 10.1016/j.jfca.2004.09.004. [DOI] [Google Scholar]
- Al–Rumaih May M, Al-Saad FA, Warsy AS (2002) Seasonal variation in mineral content of different organs development of Rumex vesicarius L. Saudi J Biol Sci 9(1):69–79. http://ipac.kacst.edu.sa/edoc/2009/174033_1.pdf
- Ananthi S, Raghavendran HRB, Sunil AG, et al. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga) Food Chem Toxicol. 2010;48:187–192. doi: 10.1016/j.fct.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Aquino R, De Simone F, De Tommasi N, Pizza C. Structure and biological activity of triterpenoids and aromatic compounds from medicinal plants. Stud Nat Prod Chem. 1995;17:113–152. [Google Scholar]
- Awah FM, Uzoegwu PN, Ifeonu P, et al. Free radical scavenging activity, phenolic contents and cytotoxicity of selected Nigerian medicinal plants. Food Chem. 2012;131:1279–1286. doi: 10.1016/j.foodchem.2011.09.118. [DOI] [Google Scholar]
- Barros L, Cabrita L, Boas MV, et al. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011;127:1600–1608. doi: 10.1016/j.foodchem.2011.02.024. [DOI] [Google Scholar]
- Bélanger J, Balakrishna M, Latha P, Katumalla S. Contribution of selected wild and cultivated leafy vegetables from South India to lutein and β-carotene intake. Asia Pac J Clin Nutr. 2010;19(3):417–424. [PubMed] [Google Scholar]
- Bhaskarachary K, Sankar Rao DS, Deosthale YG, Reddy V. Carotene content of some common and less familiar foods of plant origin. Food Chem. 1995;54:189–193. doi: 10.1016/0308-8146(95)00029-I. [DOI] [Google Scholar]
- Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrapeiro MM, Donato J, Goncalves RC, et al. Effect of lycopene on biomarkers of oxidative stress in rats supplemented with [omega]-3 polyunsaturated fatty acids. Food Res Int. 2007;40:939–946. doi: 10.1016/j.foodres.2007.04.004. [DOI] [Google Scholar]
- da Silva LCN, da Silva Júnior CA, de Souza RM, et al. Comparative analysis of the antioxidant and DNA protection capacities of Anadenanthera colubrina, Libidibia ferrea and Pityrocarpa moniliformis fruits. Food Chem Toxicol. 2011;49:2222–2228. doi: 10.1016/j.fct.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Dawson R, Elliott D, Elliott W, Jones K (1991) Dictionnaire de biochimiste. Mir, Moscou. In: N’gaman Kohué CC, Békro Y-A, Mamyrbékova-Békro JA, Bénié A, Gooré BS (2009) Sur la Composition en Métabolites Secondaires et L’activité Anti-Oxydante D’extraits Bruts de Gmelina Arborea Roxb. (Verbanaceae) de Côte d’Ivoire, Afrique de l’Ouest : Analyse par Chromatographie en Couche Mince. Eur J Sci Res 36(2):161–171. http://www.csrs.ch/Pasres/documents/publication/Ngaman-chrsitelle.pdf
- Demirezer LO (1994) Quantitative determination of some Rumex species with regards of anthraquinone derivatives. Pharmazie 49(12):936–937. ISSN: 0031–7144. Accession number: WOS:A1994QB93800024
- Demirezer LO, Kuruüzüm-Uz A, Bergere I, Schiewe HJ, Zeeck A. The structures of antioxidant and cytotoxic agents from natural source: anthraquinones and tannins from roots of Rumex patientia. Phytochemistry. 2001;58:1213–1217. doi: 10.1016/S0031-9422(01)00337-5. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific opinion on the reevaluation of butylated hydroxytoluene BHT (E 321) as a food additive. EFSA J. 2012;10(3):2588. [Google Scholar]
- El-Hawary SA, Sokkar NM, Ali ZY, Yehia MM (2011) A Profile of Bioactive Compounds of Rumex vesicarius L. J Food Sci 76(8) [DOI] [PubMed]
- Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008;110:76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Filho JMB, Alencar AA, Nunes XP, et al. Source of alpha-, beta-, gamma-, delta-, and epsilon carotenes: A twenties century review. Rev Bras Farmacogn. 2008;18(1):135–154. doi: 10.1590/S0102-695X2008000100023. [DOI] [Google Scholar]
- Ghafar MF, Prasad KN, Weng KK, Ismail A. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr J Biotechnol. 2010;9(3):326–330. [Google Scholar]
- Gopal R, Vijayakumaran M, Venkatesan R, Kathiroli S (2008) Marine organisms in Indian medicine and their future prospects. Nat Prod Radiance 7(2):139–145. http://nopr.niscair.res.in/bitstream/123456789/5658/1/NPR%207(2)%20139-145.pdf
- Guy BK, Akhanovna M-BJ, Odette DD, Jonathan GS, Yves-Alain B. Sur la Composition Phytochimique Qualitative des Extraits bruts Hydrométhanoliques des Feuilles de 6 Cultivars de Manihot Esculenta Crantz de Côte d’Ivoire. Eur J Sci Res. 2010;45(2):200–211. [Google Scholar]
- Halim AF, Fattah HA, EI-Gamal AA (1989) Anthraquinones and flavonoids of Rumex vesicarius. Annual Conference of the Arab Society of Medicinal Plants Research, p. 4. National Research Centre, Cairo
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Huang B, Ban X, He JS, et al. Heteroprotrective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaern.) leaves. Food Chem. 2010;120:873–878. doi: 10.1016/j.foodchem.2009.11.020. [DOI] [Google Scholar]
- Huang B, Ban X, He JS, et al. Heteroprotective and antioxidant effects of the methanolic extract from Halenia elliptica. J Ethnopharmacol. 2010;131:276–281. doi: 10.1016/j.jep.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Imran MM, Raja MM, Abdul Basith J, Asarudeen A. Determination of total phenol, flavonoid and antioxidant activity of edible mushrooms Pleurotus florida and Pleurotus eous. Int Food Res J. 2011;18(2):574–577. [Google Scholar]
- Jain A, Soni M, Deb L, et al. Antioxidant and heteroprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leaves. J Ethnopharmacol. 2008;115:61–66. doi: 10.1016/j.jep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. doi: 10.1016/S0308-8146(03)00151-1. [DOI] [Google Scholar]
- Kabran GR, Ambeu NC, Mamyrbékova-Békro JA, Békro Y-A. CCM D’extraits Sélectifs de 10 Plantes Utilisées Dans le Traitement Traditionnel du Cancer du Sein en Côte d’Ivoire. Eur J Sci Res. 2011;63(4):592–603. [Google Scholar]
- Kähkönen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Koleva II, Teris AB, Jozef PH, Linssen AG, Lyuba NE. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Kumaran A, Karunakaran RJ (2007) In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT 40: 736 344–352
- Ladyguina EY, Safronitch LN, Otriachenkova VE, Bolandina IA, Grinkevitch NI (1983) Analyse chimique des plantes médicinales. Edition Moskva, Vischaya Chkola
- Lagnika L (2005) Etude phytochimique et activité biologique de substances naturelles isolées de plantes béninoises. Dissertation, Université Louis Pasteur (Strasbourg/France)
- Lhuillier A (2007) Contribution à l’étude phytochimique de quatre plantes malgaches : Agauria salicifolia hook.f ex Oliver, Agauria polyphylla baker (Ericaceae), Tambourissa trichophylla baker (Monimiaceae) et Embelia concinna baker (Myrsinaceae). Dissertation, Université Paul Sabatier (Toulouse III/France)
- Liyana-Pathirana CM, Shahidi F. Antioxidant properties of commercial soft and hard winter wheats (Triticum aestivum L.) and their milling fractions. J Sci Food Agric. 2006;86:477–485. doi: 10.1002/jsfa.2374. [DOI] [Google Scholar]
- Mamyrbekova-Bekro JA, Boua BB, Kouassi KC, Békro Y-A. Sur l’analyse qualitative et pharmacologique de 2 plantes antihypertensives utilisées à N’gramanssabo en Côte d’Ivoire. Revue « Nature & Technologie ». B Sci Agronom Biol. 2013;8:2–12. [Google Scholar]
- Mamyrbekova-Bekro JA, Boua BB, Diaby A, Békro Y-A. Screening phytochimique bio guidé et évaluation in vitro des propriétés purgatives de Anchomanes difformis (Blume) Engl., une plante utilisée en Côte d’Ivoire dans le traitement folklorique de la constipation. Revue « Nature & Technologie ». B Sci Agronom Biol. 2013;9:20–26. [Google Scholar]
- Matkowski A. Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv. 2008;26:548–560. doi: 10.1016/j.biotechadv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Midiwo JO, Rukunga GM. Distribution of anthraquinone pigments in Rumex species of Kenya. Phytochemistry. 1985;24:1390–1391. doi: 10.1016/S0031-9422(00)81145-0. [DOI] [Google Scholar]
- Mosquera OM, Correa YM, Buitrago DC, Niö J. Antioxidant activity of twenty five plants from Colombian biodiversity. Mem Inst Oswaldo Cruz. 2007;102:631–634. doi: 10.1590/S0074-02762007005000066. [DOI] [PubMed] [Google Scholar]
- Mostafa HAM, ElBakry AA, Alam EA. Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L. (Polygonaceae) Int J Pharm Pharm Sci. 2011;3(2):109–118. [Google Scholar]
- Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, et al. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- N’gaman Kohué CC, Békro Y-A, Mamyrbékova-Békro JA, Bénié A, Gooré BS (2009) Sur la Composition en Métabolites Secondaires et L’activité Anti-Oxydante D’extraits Bruts de Gmelina Arborea Roxb. (Verbanaceae) de Côte d’Ivoire, Afrique de l’Ouest : analyse par Chromatographie en Couche Mince. Eur J Sci Res 36 (2):161–171. http://www.csrs.ch/Pasres/documents/publication/Ngaman-chrsitelle.pdf
- Nsimba RY, Kikuzaki H, Konishi Y. Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp. Seeds. Food Chem. 2008;106:760–766. doi: 10.1016/j.foodchem.2007.06.004. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of the browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Parejo I, Viladomat F, Bastida J, et al. Comparison between the radical scavenging activity and antioxidant activity of six distilled and non distilled Mediterranean herbs and aromatic plants. J Agric Food Chem. 2002;50:6882–6890. doi: 10.1021/jf020540a. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Quézel P, Santa S (1962) Nouvelle flore d’Algérie et des régions désertiques méridionales. Éditions du Centre national de la Recherche scientifique, Paris 7e
- Rao BN. Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr. 2003;12(1):9–22. [PubMed] [Google Scholar]
- Rathee D, Rathee P, Rathee S, Rathee D. Phytochemical screening and antimicrobial activity of Picrorrhiza kurroa, an Indian traditional plant used to treat chronic diarrhea. Arab J Chem. 2012 [Google Scholar]
- Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 On food additives. Off J Eur Union. 2008;L 354:16–33. [Google Scholar]
- Saad H, Charrier-El Bouhtouryb F, Pizzi A. Characterization of pomegranate peels tannin extractives. Ind Crop Prod. 2012;40:239–246. doi: 10.1016/j.indcrop.2012.02.038. [DOI] [Google Scholar]
- Saleh NAM, El- Hadidi MN, Raafat A. Flavonoids and anthraquinones of some Egyptian Rumex species (Polygonaceae) Biochem Syst Ecol. 1993;21(2):301–303. doi: 10.1016/0305-1978(93)90049-W. [DOI] [Google Scholar]
- Sankar NR, Devamma MN, Giridhar D. First report of Alternaria alternata causing leaf spot on Rumex vesicarius in India. Australas Plant Dis Notes. 2011;7(1):17–18. doi: 10.1007/s13314-011-0036-4. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drum-stick tree (Moringa oleifera Lam) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Sun BS, Ricardo-Da-Silva JM, Spranger MI. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–4274. doi: 10.1021/jf980366j. [DOI] [Google Scholar]
- Tongpoothorn W, Chanthai S, Sriuttha M, Saosang K, Ruangviriyachai C. Bioactive properties and chemical constituents of methanolic extract and its fractions from Jatropha curcas oil. Ind Crop Prod. 2012;36:437–444. doi: 10.1016/j.indcrop.2011.10.011. [DOI] [Google Scholar]
- Wong SP, Leong LP, Koh JHW. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006;99:775–783. doi: 10.1016/j.foodchem.2005.07.058. [DOI] [Google Scholar]
- Wong C, Li H, Cheng K, Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. doi: 10.1016/j.foodchem.2005.05.049. [DOI] [Google Scholar]
- Yao XH, Zhang DY, Zu YG et al (2013) Free radical scavenging capability, antioxidant activity and chemical constituents of Pyrola incarnata Fisch. leaves. Industrial Crops and Products 49: 247--255
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]