Abstract
Optimization of substrate concentration, time of incubation and temperature for crude pectinase production from A. niger was carried out using Bhimkol banana (Musa balbisiana) peel as substrate. The crude pectinase produced was partially purified using ethanol and effectiveness of crude and partially purified pectinase was studied for banana juice clarification. The optimum substrate concentration, incubation time and temperature of incubation were 8.07 %, 65.82 h and 32.37 °C respectively, and the polygalacturonase (PG) activity achieved was 6.6 U/ml for crude pectinase. The partially purified enzyme showed more than 3 times of polygalacturonase activity as compared to the crude enzyme. The SDS-PAGE profile showed that the molecular weight of proteins present in the different pectinases varied from 34 to 42 kDa. The study further revealed that highest clarification was achieved when raw banana juice was incubated for 60 min with 2 % concentration of partially purified pectinase and the absorbance obtained was 0.10.
Keywords: Pectinase, Banana peel, Aspergillus niger, Banana juice, Clarification
Introduction
Pectinase is a general term for enzymes, such as pectin lyase, pectin methylesterase and polygalacturonase, commonly referred to as pectic enzymes. These break down pectin, a polysaccharide substrate that is found in the cell walls of plants.
Most pectic enzyme preparations are used in the fruit processing industry and pectic enzymes alone account for about one quarter of the world’s food enzyme production (Patil and Dayanand 2006). Bacteria, fungi and yeast produce and secrete pectinases (Rombouts and Pilnik 1980). Enzymes produced from the fungi Aspergillus, Rhizopus and Penicillium are generally regarded as safe (GRAS) and produces extracellular enzymes which can be easily recovered (Mrudula and Anitharaj 2011). The increasing energy demand has attracted worldwide attention on the renewable agricultural and industrial wastes (Martin et al. 2004), as their disposal pose environmental problems. Various fruit and vegetable processing waste have been used for the production of pectinases (Taskin and Eltem 2008; Bari et al. 2010; Kumar et al. 2012).
After citrus, banana is the second largest fruit produced worldwide (Mohapatra et al. 2010). Banana is mainly eaten fresh but it is also suitable for processing into products (Lee et al. 2006). They are mostly grown by small-scale farmers and play an important socioeconomic role in many developing countries of the tropics and subtropics (Panis and Thinh 2001).
Banana peel is an abundant and low cost agricultural waste residue. It is easily available in large quantities. It accounts for about 40 % of the weight of the raw fruit and is rich in carbohydrates, protein and various vitamins and mineral elements (Ramli et al. 2009; Dhabekar and Chandak 2010). However, banana peel does not find any significant commercial application till now and is generally disposed of in open areas, leading to potentially serious environmental problems. It is necessary to explore its industrial reutilization.
Banana are usually too pulpy and pectinaceous to yield juice by normal hydraulic pressing or centrifugation (Lee et al. 2006). Traditional mechanical method of pressing, folding and turning the pulp mash repeatedly till appearance of juice (Kasozi and Kasisira 2005) or hot water extraction method (Lee et al. 2006) can be used for extraction of banana juice. The banana juice obtained by these methods is turbid, viscous and grey in colour and tends to settle during storage. Further processing such as enzyme treatment is necessary to produce a ready to drink clarified banana juice.
Application of enzyme in fruit juice clarification and extraction has been known widely in fruit processing industries. Enzyme is an essential tool in juice processes, both in terms of quality improvement and cost saving (Ramadan and Moersel 2007). The cloudiness in the juices is mainly caused by the presence of polysaccharides such as pectin and starch. Therefore, enzymatic treatment by using pectinase is an effective way to reduce the pectin in the fruit juices because pectinase has the ability to hydrolyze pectin and cause pectin–protein complexes to flocculate (Liew Abdullah et al. 2006; Sun et al. 2006; Sin et al. 2006) which could be easily removed by filtration or centrifugation. A complete enzymatic breakdown of pectin is the key for producing clear and stable fruit juices. Many studies have shown that pectinases can be used for clarification of juices and increase the storage stability (Mieszczakowska-Frąc et al. 2012; Sandri et al. 2011; Pinelo et al. 2010; Wang et al. 2009; Mantovani et al. 2005).
Since pectinases are widely used enzyme for different industrial application, it is necessary to use inexpensive and readily available raw material for its production. Thus, the objectives of the present study were (1) to optimize crude pectinase production using Bhimkol banana peel as substrate for higher polygalacturonase activity, (2) to partially purify crude pectinase using ethanol and characterize the crude and partially purified pectnases, and (3) to study the effect of crude and partially purified pectinase on quality of clarified banana juice.
Materials and methods
Microorgamism
Pure culture of Aspergillus niger MTCC 281 was purchased from Institute of Microbial Technology, Chandigarh, India in lyophilized form. The pure culture was cultivated and allowed to grow on Czapek Yeast Extract Agar medium at a temperature of 30 ° C and incubation time of 7 days. The culture was then maintained on potato dextrose agar (PDA) slants and sub cultured after every 30 days.
Substrate preparation for enzyme production
Bananas (Musa balbisiana, BBB genotype) locally known as bhimkol were obtained from Tezpur market, Assam, India. The bananas were washed under tab water and peeled with hand. The peels were then dried at 45 °C for 24 h and then grinded using commercial grinder and passed through 200 mesh sieve to obtain banana peel powder. The powder thus produced was then used as substrate for the production of Pectinase enzyme. Moisture content of the dried banana peel powder was determined by gravimetric method (AOAC 1990) for three replications by direct heating in a drying oven at 105 °C for 48 h and was found to be 7.8 ± 0.21 % (n = 3). Pectin content of the dried banana peel powder was estimated as per the method of Ranganna (1977) and was found to be 13.05 ± 0.35 % (n = 3).
Pectinase production
Experiments were carried out in 250 ml Erlenmeyer flasks containing KH2PO4 (0.02 % w/v), MgSO4 (0.01 % w/v), (NH4)2 SO4 (0.04 % w/v), FeSO4 (0.01 % w/v), MnSO4 (0.001 % w/v) in 100 ml distilled water. The concentration of the pectic substrate (Dried banana peel powder) was varied as per the experimental design for optimization. The medium was then sterilized and cooled to room temperature. The pH of the medium was adjusted to 5.8 using citrate buffer. The sterilized flasks were then inoculated with approximately 2 × 108 spores of A. niger and kept for incubation in an incubator shaker with a shaking speed of 150 rpm for pre determined time and temperature as per experimental design. After incubation the culture filtrate was cooled to 4 °C and centrifuged at 10,000 rpm for 10 min in refrigerated centrifuge. Supernatants were collected which were then used for further investigation. Further bulk production of pectinase was carried out at the optimum condition for partial purification and clarification of banana juice.
Partial purification
Partial purification of the crude enzyme was done using ethanol by the method described by Khairnar et al. (2009) with slight modifications 50 ml of the supernatant was treated with three volumes of chilled ethanol and allowed to stand for 15 min at 4 °C as lower temperature will facilitate separation of proteins by reducing the solubility without any damage to the enzyme protein, and centrifuged at 10,000 rpm for 10 min. The precipitate thus obtained was dissolved in 15 ml distilled water and used for further investigation.
Polygalacturonase (PG) activity
Polygalacturonase activity was determined by incubating 0.5 % polygalacturonic acid (Sigma-Aldrich, USA) in 0.2 M citrate buffer (pH 5.8) with the enzyme extract. Reaction was carried out at 37 °C for 60 min. The release of reducing groups was determined by the Nelson-Somogyi method (Somogyi 1952). A calibration curve was made using galacturonic acid (Sigma-Aldrich, USA) as standard. One unit of polygalacturonase activity was defined as the amount of enzyme that released 1 μmol of galacturonic acid per minute.
Protein content
Protein content of the crude and partially purified enzymes was determined by Lowry’s method (Lowry et al. 1951) using bovine serum albumin as protein standard.
Molecular weight determination
Molecular weight of the crude and partially purified enzymes was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10 % acrylamide-bis-acrylamide separating gel and 4 % stacking gel. Staining was done using Commassie Brilliant Blue R-250. Molecular weight of the enzymes was identified by standard protein molecular weight markers (Merck, Germany) and comparing with pure pectinase (Himedia, India).
Juice extraction and clarification
Ripe bananas (Musa acuminata, AAA genotype) locally known as Jahaji Kol of same maturity were obtained from Local market of Tezpur, Assam, India for juice extraction. The bananas were washed, hand peeled and cut into pieces. Banana pieces were mixed with distilled water in the ratio of 1:2 (w/v) and blended in a commercial blender (Philips, HL1632). The juice was then separated using double fold cheese cloth. The extracted juice (15 ml) was incubated at 35 °C with different concentrations of crude and partially purified pectinase for 30 and 60 min for clarification.
Properties of clarified juice
After incubation the juice was centrifuged at 5,000 rpm for 5 min to remove impurities and the clarity of the juices was determined spectrometrically by measuring the absorbance at 660 nm using UV–VIS spectrophotometer (CECIL CE 7400). The titratable acidity of the clarified juices was determined as per Ranganna (1977) using 0.1 N NaOH and phenolphthalein as indicator. The reducing sugar content of the juices was measured colourimetrically by Nelson-Somogyi method (Somogyi 1952).
Experimental design and analysis
Optimization of substrate concentration (S), time (t) and temperature of incubation (T) for pectinase production with highest polygalacturonase activity was done numerically by desirability function using central composite rotatable design (CCRD). The substrate concentration was varied from 5 to 10 %, time from 50 to 80 h and temperature ranged from 25 to 40 °C. A total of 20 experiments were performed with six replications at the centre point (Table 1).
Table 1.
Polygalacturonase (PG) activity for different combinations of experimental conditions according to CCRD
| Experiment number | Concentration of substrate (S), % | Time (t), h | Temperature of incubation (T), °C | Polygalacturonase (PG) activity, U/ml |
|---|---|---|---|---|
| 1 | 5.00 | 65.00 | 32.50 | 2.9 |
| 2 | 6.01 | 56.08 | 28.04 | 1.7 |
| 3 | 6.01 | 73.92 | 28.04 | 1.5 |
| 4 | 6.01 | 56.08 | 36.96 | 1.9 |
| 5 | 6.01 | 73.92 | 36.96 | 1.5 |
| 6 | 7.50 | 65.00 | 25.00 | 2.7 |
| 7 | 7.50 | 50.00 | 32.50 | 1.2 |
| 8 | 7.50 | 65.00 | 32.50 | 6.2 |
| 9 | 7.50 | 65.00 | 32.50 | 6.9 |
| 10 | 7.50 | 65.00 | 32.50 | 5.3 |
| 11 | 7.50 | 65.00 | 32.50 | 7.3 |
| 12 | 7.50 | 65.00 | 32.50 | 6.7 |
| 13 | 7.50 | 65.00 | 32.50 | 6.1 |
| 14 | 7.50 | 80.00 | 32.50 | 3.8 |
| 15 | 7.50 | 65.00 | 40.00 | 1.7 |
| 16 | 8.99 | 56.08 | 28.04 | 2.7 |
| 17 | 8.99 | 73.92 | 28.04 | 1.3 |
| 18 | 8.99 | 56.08 | 36.96 | 1.2 |
| 19 | 8.99 | 73.92 | 36.96 | 2.7 |
| 20 | 10.00 | 65.00 | 32.50 | 6.9 |
A three factor two level full factorial experiment was designed to study the effect of pectinase treatment on clarification and quality of clarified banana juice. The factors were type of enzyme (E) i.e., crude and partially purified pectinase, time of incubation (I) i.e., 30 and 60 min; and concentration of enzyme (C) i.e., 1 and 2 % v/v. Amount of juice used for clarification was 50 ml for each experiment. All the experiments were carried out in triplicates at 30 °C as the optimum temperature for pectinase production obtained was near 30 °C.
Analysis of variance (ANOVA) using Design Expert Software was conducted to see the significance of the main effects as well as their interactions on the polygalacturonase activity for the optimization study and to study the effect of various factors on the responses for the banana juice clarification study. Fisher’s Least Significant Difference (LSD) test was used to determine the statistical difference between the treatment results for the responses. Further, the effect of two factor interaction was investigated graphically to get better insight of the clarification process and to know how the interaction of the various processing parameters affect the clarity, reducing sugar content and acidity of the clarified juice.
Results and discussion
Effect of processing parameters on polygalacturonase activity and optimization for maximum activity
The values of polygalacturonase activity for different experimental combinations are shown in Table 1. Analysis of variance (ANOVA) was performed to determine the significant effects of the process variables on the response (Table 2). From Table 2 it was observed that the model was significant while lack of fit was insignificant, which is desirable. Further, only the quadratic terms were found to be significant while the linear terms and their interactions were insignificant, implying that polygalacturonase activity increases initially with increase in substrate concentration, time of incubation and temperature of incubation, and then decreases with further increase in the processing parameters within the experimental range. Similar results were obtained by Mukesh Kumar et al. (2012) for pectinase produced from Bacillus sp. MFW7 using cassava waste.
Table 2.
Analysis of variance (ANOVA) for quadratic model of polygalacturonase (PG) activity
| SS | DF | F-value | p-value | |
|---|---|---|---|---|
| Model | 0.85 | 9 | 6.65 | 0.0033* |
| S | 0.05 | 1 | 3.34 | 0.0975 |
| t | 0.01 | 1 | 0.79 | 0.3958 |
| T | 1.84 × 10−3 | 1 | 0.13 | 0.7260 |
| S2 | 0.09 | 1 | 6.23 | 0.0317* |
| t2 | 0.39 | 1 | 27.27 | 0.0004* |
| T2 | 0.43 | 1 | 30.39 | 0.0003* |
| S × t | 4.53 × 10−4 | 1 | 0.03 | 0.8617 |
| S × T | 1.28 × 10−4 | 1 | 9.03 × 10−3 | 0.9262 |
| t × T | 0.01 | 1 | 0.71 | 0.4197 |
| Residual | 0.14 | 10 | ||
| Lack of Fit | 0.12 | 5 | 4.87 | 0.0536 |
*Significant terms (p < 0.05)
S concentration of substrate; t time of incubation; T temperature of incubation; SS sum of squares; DF degrees of freedom
It was observed that polygalacturonase activity of crude pectinase initially increased when incubation time of A. niger was increased up to a certain extent, but when the incubation time was further increased there was a decrease in the polygalacturonase activity of the crude pectinase produced (Fig. 1a). This might be to due to inactivation of some amount of enzyme as enzymes starts losing their activity after certain time due to denaturation of the protein 3-D structure (Bailley and Ollis 1986). Furthermore, substrate might become exhausted with time thereby decreasing the activity of the enzyme. The polygalacturonase activity of crude pectinase was found to increase with increase in substrate concentration at the initial stages, but in the later stages polygalacturonase activity was not affected by increase in substrate concentration and was found to be constant. The reason for this might be that the enzyme production reached its maximum when certain amount of substrate was present in the medium and further increase in concentration of substrate could not increase the capacity of the microorganism to produce pectinase. The incubation time is generally dictated by the composition of the substrate and properties of the strain, such as its growth rate, enzyme production profile, initial inocula, and others (Pilnik and Voragen 1993).
Fig. 1.
Variation in polygalacturonase activity using banana peel as substrate with (a) time and concentration of substrate, (b) temperature and concentration of substrate, and (c) temperature and time
Temperature is directly related to the metabolic activities of the microorganism, and it affects the proper growth and product formation of the organism (Mankarios and Friend 1980). An increase in polygalacturonase activity was noticed initially with increase in temperature (Fig. 1b). When temperature was further increased beyond a certain temperature, polygalacturonase activity was found to decrease. The reason for such behaviour might be attributed to the doubling of growth rate of microorganism with every 10 °C rise in temperature till it reaches its optimum temperature of growth. A further increase in temperature results in decreased growth rate, as microorganisms are not able to survive at higher temperatures. In this case higher growth rate corresponds to higher amount of pectinase production, thereby increasing the concentration and activity. Increasing the substrate concentration resulted in an increase in polygalacturonase activity initially and then became constant. This trend was observed at all temperatures.
The polygalacturonase activity of crude pectinase increased with increase in temperature and time reached an optimum value and then decreased with further increase in temperature and time of incubation (Fig. 1c). It can be concluded that growth of A. niger and thus production of pectinase could be achieved best at some optimum condition of temperature and time and any deviation from the optimum value will result in decreased production of pectinase.
Optimization of the processing parameters to achieve maximum polygalacturonase activity was done using desirability function. One solution was obtained with desirability of 0.88 (Table 3). The optimum processing conditions were substrate concentration of 8.07 %, incubation time of 65.82 h and temperature of incubation of 32.37 °C and the polygalacturonase activity achieved was 6.6 U/ml. The high polygalacturonase activity achieved by using banana peel as substrate might be attributed to the presence of higher level of pectin i.e. 13.05 % in the dried banana peel, which might have assisted in the production of pectic enzymes.
Table 3.
Solutions for Optimization of processing parameters for obtaining highest polygalacturonase activity by Desirability function
| Sl. No. | SC, % | T, h | T, °C | Polygalacturonase activity | Desirability |
|---|---|---|---|---|---|
| 1 | 8.07 | 65.82 | 32.37 | 6.6 | 0.88 |
Polygalacturonase (PG) activity and protein content
Pectinase was produced at optimum condition in sufficient amount to carry out further experiments. Some part of the pectinase thus produced was partially purified using ethanol. The polygalacturonase activity and protein content of the partially purified pectinase are presented in Table 4. The protein content of the partially purified pectinase was found to be more than two times that of crude pectinase, which might be due to precipitation of the enzyme proteins particularly pectinase by ethanol as ethanol changes the solubility of protein in water and the proteins precipitates (Hewedi et al. 1985). It was further observed that although the protein content in partially purified pectinase doubled, the polygalacturonase activity increased by more than three times of that of crude pectinase, indicating that the protein molecules separated by ethanol were mostly of the enzyme pectinase and the proportion of protein other than pectinase was higher in crude enzyme. This might be attributed to the higher molecular weight and lower solubility of polygalacturonase in ethanol compared to other proteins present in crude enzyme which facilitated the separation of polygalcturonase. Therefore, it could be assumed that ethanol can be effectively used to partially separate pectinase molecules from crude enzyme preparations.
Table 4.
polygalacturonase activity and protein content of crude and partially purified pectinase
| Type of enzyme | Polygalacuronase activity, U/ml | Protein content, μg/ml |
|---|---|---|
| Crude enzyme | 6.7 | 6.02 |
| Partially purified enzyme | 20.6 | 12.12 |
SDS-PAGE profile of pectinase
SDS-PAGE profiling of crude and partially purified pectinase were carried out and compared with pure pectinase (Himedia, India) and ammonium sulfate (60 %) fractionated pectinase (Fig. 2). Protein bands for partially purified pectinase (L4) and pure pectinase were found to be almost similar in pattern. Proteins bands of crude pectinase (L2) were less intensely stained than pure and partially purified pectinases, while, ammonium sulfate fractionated pectinase was least stained, as also observed by Díaz et al. (2011) indicating that partial purification with ethanol is an effective method for separation of enzyme protein. The SDS-PAGE profile showed that the molecular weight of proteins present in the different pectinases varied from 34 to 42 kDa, which might be due to presence of other proteins in the crude extract and partially purified enzyme. Similar values were also reported by Kumar et al. (2012) for pectinase from Aspergillus foetidus.
Fig. 2.
SDS-PAGE Analysis of different enzyme extracts obtained with different methods of purification. L1- Molecular weight markers as specified in the left of the figure; L2- Crude enzyme; L3- Ammonium sulphate precipitated enzyme; L4- Ethanol precipitated enzyme; L5- Pure pectinase (Himedia, India)
Effect of pectinase treatment on clarification of banana juice
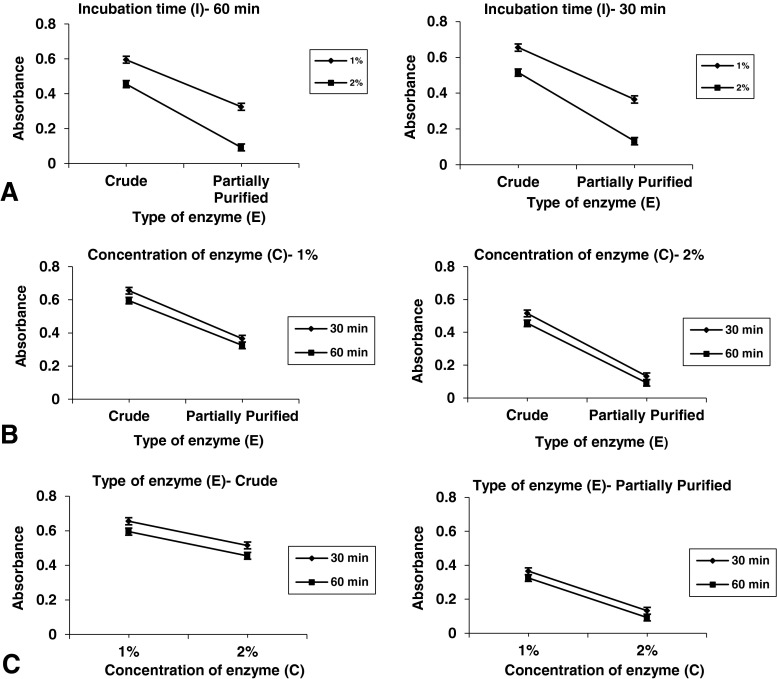
Table 5 shows the effect of different treatment combinations using crude and partially purified enzyme on absorbance, titrtable acidity and reducing sugar content of clarified banana juice. It has been observed that highest clarification i.e. lowest absorbance (0.10) was obtained when partially purified enzyme was used at 2 % concentration and incubation time was 60 min which was not significantly different from that when the time of incubation was 30 min signifying that increasing time of incubation has no significant effect on clarification process for partially purified enzyme. Similarly highest reducing sugar content (13.5 %) in the clarified juice was obtained when partially purified enzyme was used at 2 % concentration and incubation time was 60 min. The reduction in absorbance and increase in reducing sugar content with time and concentration of pectinase might be explained by the fact that the rate of degradation of cell wall pectin to polygalacturonic acid was higher when the concentration was more and with time higher amount of pectin was degraded (Dorreich 1993; Kyamuhangire et al. 2002) There was no significant difference in titratable acidity between the treatment combinations, which showed that titratable acidity is independent of enzyme type (E), time of incubation (I) and concentration of enzyme (C).
Table 5.
Combinations of treatment conditions and their responses
| Type of enzyme (E) | Concentration of enzyme (C), % | Time of incubation (I), min | Absorbance | Acidity, % | Reducing sugar content, % |
|---|---|---|---|---|---|
| Crude enzyme | 1 | 30 | 0.65 ± 0.03a * # | 0.54 ± 0.04a | 8.63 ± 0.25e |
| Partially purified enzyme | 1 | 30 | 0.37 ± 0.02e | 0.55 ± 0.04a | 9.20 ± 0.30d |
| Crude enzyme | 2 | 30 | 0.52 ± 0.03c | 0.53 ± 0.03a | 8.93 ± 0.32de |
| Partially purified enzyme | 2 | 30 | 0.12 ± 0.02f | 0.58 ± 0.02a | 12.37 ± 0.25b |
| Crude enzyme | 1 | 60 | 0.60 ± 0.04b | 0.55 ± 0.02a | 10.17 ± 0.15c |
| Partially purified enzyme | 1 | 60 | 0.33 ± 0.03e | 0.54 ± 0.02a | 10.47 ± 0.31c |
| Crude enzyme | 2 | 60 | 0.45 ± 0.03d | 0.53 ± 0.03a | 10.47 ± 0.31c |
| Partially purified enzyme | 2 | 60 | 0.10 ± 0.02f | 0.57 ± 0.05a | 13.50 ± 0.26a |
*Values of responses as Mean ± S.D. of three replications
#Responses followed by same superscript small letters within a column are not significantly different (p < 0.05)
Effect two factor interaction on clarity (Absorbance) of banana juice
Clarity of banana juice was significantly affected by enzyme type (E), time of incubation (I) and concentration of enzyme (C), while the interaction of only enzyme type and concentration of enzyme was significant (Table 6). It has been observed from the ANOVA that two factor interaction model was significant and had a non-significant lack of fit.
Table 6.
Analysis of variance (ANOVA) for two-factor interaction models of various responses
| Absorbance | Acidity, % | Reducing sugar content, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SS | F-value | p-value | SS | F-value | p-value | SS | F-value | p-value | |
| Model | 0.88 | 227.53 | < 0.0001* | 7.92 × 10−3 | 1.51 | 0.2340 | 60.65 | 142.40 | < 0.0001* |
| E | 0.64 | 995.54 | < 0.0001* | 3.50 × 10−3 | 4.01 | 0.0616 | 20.17 | 284.12 | < 0.0001* |
| C | 0.21 | 325.07 | < 0.0001* | 5.04 × 10−4 | 0.58 | 0.4581 | 17.34 | 244.29 | < 0.0001* |
| I | 0.02 | 23.32 | 0.0002* | 1.04 × 10−4 | 0.12 | 0.7343 | 11.21 | 157.88 | < 0.0001* |
| E × C | 0.01 | 20.32 | 0.0003* | 3.50 × 10−4 | 4.01 | 0.0616 | 11.76 | 165.68 | < 0.0001* |
| E × I | 6.00 × 10−4 | 0.93 | 0.3476 | 2.04 × 10−4 | 0.23 | 0.6352 | 0.17 | 2.35 | 0.1438 |
| C × I | 0.00 | 0.00 | 1.0000 | 1.04 × 10−4 | 0.12 | 0.7343 | 6.67 × 10−3 | 0.094 | 0.7630 |
| Residual | 0.011 | 0.02 | 1.21 | ||||||
| Lack of Fit | 1.67 × 10−3 | 2.88 | 0.1092 | 4.17 × 10−6 | 4.48 × 10−3 | 0.9474 | 6.67 × 10−3 | 0.089 | 0.7694 |
*Significant terms (p < 0.05)
E enzyme type; I time of incubation; C concentration of enzyme; SS Sum of squares
The effect of two factor interaction on absorbance of clarified banana juice are shown in Fig. 2. It could be seen that for both 30 and 60 min of incubation (Fig. 3a) significant differences were present in the absorbance of the clarified juices for the two enzymes with partially purified enzyme being more effective in reducing the absorbance of the raw juice for both the levels of concentration of enzyme. Partially purified enzyme evinced no significant differences when time was increased from 30 to 60 min (Fig. 3b) for both 1 and 2 % concentration of enzyme implying that increasing the incubation time will not affect the clarification process. But, for crude enzyme significant differences were observed when time was increased from 30 to 60 min. The effect of both time and concentration of enzyme were significant for crude enzyme on the clarification process (Fig. 3c). As the time of incubation and concentration of the enzyme were increased, reduction in the absorbance of banana juice was achieved, which might be explained by higher rate of degradation of pectin with higher concentration of enzyme (Dorreich 1993). On the other hand, for partially purified enzyme the effect of concentration was significant i.e. with increase in concentration better clarification was achieved. No significant differences were observed with time for a particular concentration of enzyme, which shows that 30 min is sufficient for partially purified enzyme for hydrolyzing the pectin present in banana juice.
Fig. 3.
Effect of two-factor interaction on clarity (absorbance) of banana juice (a) Effect of interaction of E and C at different levels of I; (b) Effect of interaction of E and I at different levels of C; and (c) Effect of interaction of C and I at different levels of E. E enzyme type; I time of incubation; C concentration of enzyme Error Bars represent LSD at p < 0.05
Effect of two factor interaction on titratable acidity of clarified juice
Pectinase treatment did not affect the acidity of the clarified juices. It was observed that effect of enzyme type, time of incubation and concentration of enzyme or their interactions were not significant (Table 6). Effect of two factor interaction on acidity of clarified juice showed no significant differences between treatment results with change in enzyme type, or time of incubation, or concentration of enzyme (Fig. 4). Similar results were also observed by Vaidya et al. (2009) for kiwifruit juice treated with different enzymes.
Fig. 4.

Effect of two-factor interaction on acidity of clarified banana juice (a) Effect of interaction of E and C at different levels of I; (b) Effect of interaction of E and I at different levels of C; and (c) Effect of interaction of C and I at different levels of E. E enzyme type; I time of incubation; C concentration of enzyme Error Bars represent LSD at p < 0.05
Effect of two factor interaction on reducing sugar content of clarified juice
Analysis of variance (ANOVA) for two factor interaction model for reducing sugar content of clarified juice shows that the model was significant with a non-significant lack of fit (Table 6). It was also observed that the effects of enzyme type, time of incubation, and concentration of enzyme and the interaction of enzyme type and time of incubation was significant on reducing sugar content.
The effect of two factor interaction of enzyme type, time of incubation and concentration of enzyme on reducing sugar content of clarified banana juice are shown in Fig. 4. It was observed that for both the levels of time of incubation no significant differences between the two levels of concentration of enzyme were present in the reducing content of juice when crude enzyme was used (Fig. 5a). Although reducing sugar content in the clarified juice increased significantly for partially purified enzyme when concentration of enzyme was increased from 1 to 2 % for each level of time of incubation. Significant differences were also not observed between crude and partially purified enzyme when concentration of enzyme was 1 %. The reason for such behavior might be due to the amount of enzyme present in crude enzyme was already less, and increasing the concentration from 1 to 2 % which was almost similar with the amount of enzyme present in 1 % of partially purified enzyme, that did not affect the hydrolysis process much. The effect of interaction of enzyme type and time of incubation on reducing sugar content showed significant differences in reducing sugar content with time of incubation for each level of enzyme type when concentration of enzyme was 1 %, but reducing sugar content was not affected by enzyme type for particular level of time of incubation (Fig. 5b). When the concentration of enzyme was 2 % reducing sugar content significantly increased with increase in time of incubation as well as change in enzyme type. It was observed that for crude enzyme the reducing sugar content increased significantly with increase time of incubation and remained unaffected by change in concentration of enzyme (Fig. 5c). In partially purified enzyme the effect of both time of incubation and concentration of enzyme were significant. Reducing sugar content increased significantly with increase in time of incubation as well as concentration of enzyme.
Fig. 5.
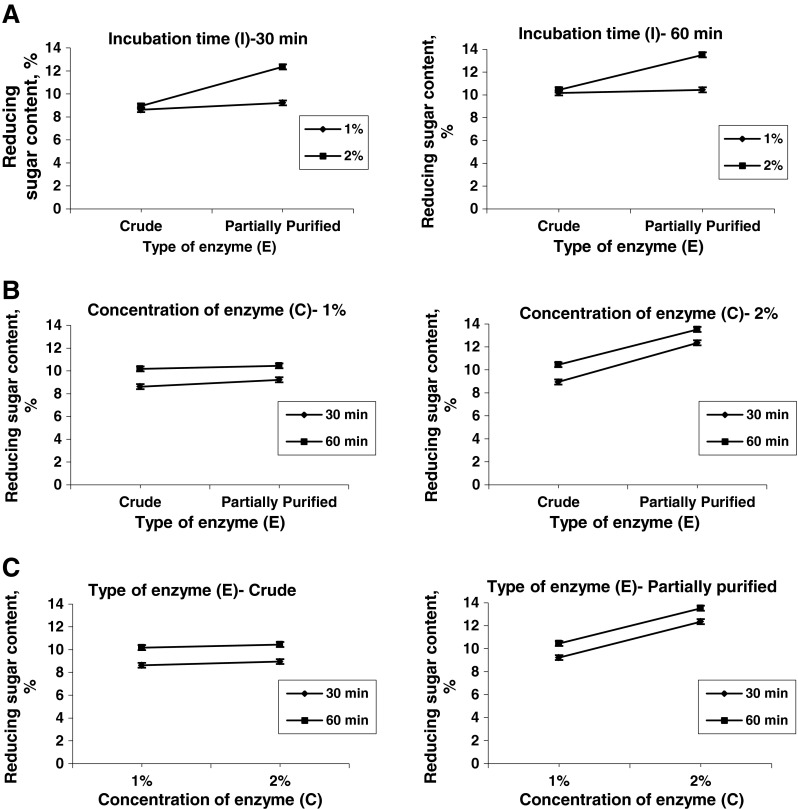
Effect of two-factor interaction on reducing sugar content of clarified banana juice (a) Effect of interaction of E and C at different levels of I; (b) Effect of interaction of E and I at different levels of C; and (c) Effect of interaction of C and I at different levels of E. E enzyme type; I time of incubation; C concentration of enzyme Error Bars represent LSD at p < 0.05
Conclusion
Banana peels, which is a waste product and was used as substrate for production of crude pectinase from A. niger in the present study, may be an efficient carbon source and has good potential as a substrate for pectinase production as they are cost effective, renewable and available in large quantities. The optimum values of concentration of substrate, incubation time and temperature of incubation obtained were 8.07 %, 65.82 h and 32.37 °C respectively, and the maximum polygalacturonase activity achieved was 6.6 U/ml of crude enzyme. Partial purification with ethanol, which is a simple process for separation of protein, further increases the concentration of enzyme thereby, reducing the volume of crude enzyme and making it more effective for food application, particularly for clarification of juices. Partial purification also makes the enzyme convenient to store.
References
- AOAC . Official methods of analysis. 15. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Bailley JE, Ollis DF. The kinetics of enzyme-catalyzed reactions. In: Biochemical Engineering Fundamentals. New York: McGraw Hill; 1986. pp. 86–156. [Google Scholar]
- Bari MR, Alizadeh M, Farbeh F. Optimizing endopectinase production from date pomace by Aspergillus niger PC5 using response surface methodology. Food Bioproducts Process. 2010;8:67–72. doi: 10.1016/j.fbp.2009.03.004. [DOI] [Google Scholar]
- Dhabekar A, Chandak A. Utilization of banana peels and beet waste for alcohol production asiatic journal of biotechnology resources. Asiatic J Biotechnol Res. 2010;1:8–13. [Google Scholar]
- Díaz AB, Bolívar J, de Ory I, Caro I, Blandino A. Applicability of enzymatic extracts obtained by solid state fermentation on grape pomace and orange peels mixtures in must clarification. LWT - Food Sci Technol. 2011;44:840–846. doi: 10.1016/j.lwt.2010.12.006. [DOI] [Google Scholar]
- Dorreich K (1993) New fruit juice technologies with enzymes. Proceedings of the 23rd Symposium of International Federation of Fruit Juice Producers. Budapest. Pp 51–62
- Hewedi MM, Mulvihill DM, Fox PF. Recovery of milk protein by ethanol precipitation. Ire J Food Sci Technol. 1985;9:11–23. [Google Scholar]
- Kasozi G, Kasisira LL (2005) Design and performance of a banana juice extractor. African Crop Science Conference Proceedings 7:1381–1384
- Khairnar Y, Krishna VK, Boraste A, Gupta N, Trivedi S, Patil P, Gupta G, Gupta M, Jhadav A, Mujapara A, Joshi B, Mishra D. Study of pectinase production in submerged fermentation using different strains of Aspergillus niger. Int J Microbiol Res. 2009;1:13–17. doi: 10.9735/0975-5276.1.2.13-17. [DOI] [Google Scholar]
- Kumar YS, Kumar PV, Reddy OVS. Pectinase production from mango peel using Aspergillus foetidus and its application in processing of mango juice. Food Biotechnol. 2012;26:107–123. doi: 10.1080/08905436.2012.670830. [DOI] [Google Scholar]
- Kyamuhangire W, Myhre H, Sørensen HT, Pehrson R. Yield, characteristics and composition of banana juice extracted by the enzymatic and mechanical methods. J Sci Food Agric. 2002;82:478–482. doi: 10.1002/jsfa.1052. [DOI] [Google Scholar]
- Lee WC, Yusof S, Hamid NSA, Baharin BS. Optimizing conditions for hot water extraction of banana juice using response surface methodology (RSM) J Food Eng. 2006;75:473–479. doi: 10.1016/j.jfoodeng.2005.04.062. [DOI] [Google Scholar]
- Liew Abdullah AG, Sulaiman NM, Aroua MK, Mohd Noor MJ. Response surface optimization of conditions for clarification of carambola fruit juice using a commercial enzyme. J Food Eng. 2006;81:65–71. doi: 10.1016/j.jfoodeng.2006.10.013. [DOI] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall J. Protein measurement with the Folin 16 phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mankarios AT, Friend J. Polysaccharide degrading enzymes of Botrytis allii and Sclerotium cepivorum: enzyme production in culture and the effect of the enzymes on isolated onion cell walls. Physiol Plant Pathol. 1980;17:93–104. doi: 10.1016/0048-4059(80)90010-7. [DOI] [Google Scholar]
- Mantovani CF, Geimba MP, Brandelli A. Enzymatic clarification of fruit juices by fungal pectin lyase. Food Biotechnol. 2005;19:173–181. doi: 10.1080/08905430500316284. [DOI] [Google Scholar]
- Martin N, Souza SR, Silva R, Gomes E. Pectinase production by fungal strains in solid state fermentation using agro-industrial by product. Braz Arch Biol Technol. 2004;47:813–819. doi: 10.1590/S1516-89132004000500018. [DOI] [Google Scholar]
- Mieszczakowska-Frąc M, Markowski J, Zbrzeźniak M, Pìocharski W. Impact of enzyme on quality of blackcurrant and plum juices. LWT - Food Sci Technol. 2012;49:251–256. doi: 10.1016/j.lwt.2011.12.034. [DOI] [Google Scholar]
- Mohapatra D, Mishra S, Sutar N. Banana and its by-product utilization: an overview. J Sci Ind Res. 2010;69:323–329. [Google Scholar]
- Mrudula S, Anitharaj R. Pectinase production in solid-state fermentation by aspergillus niger using orange peel as substrate. Glob J Biotechnol Biochem. 2011;6:64–71. [Google Scholar]
- Mukesh Kumar DJ, Saranya GM, Suresh K, Priyadharshini DA, Rajakumar R, Kalaichelvan PT. Production and optimization of Pectinase from Bacillus sp. MFW7 using cassava waste. Asian J Plant Sci Res. 2012;2:369–375. [Google Scholar]
- Panis B, Thinh NT (2001) Cryopreservation of Musa Germplasm. INIBAP Technical Guidelines 5. INIBAP, Montpellier
- Patil SR, Dayanand A. Production of pectinase from deseeded sunflower head by Aspergillus niger in submerged and solid-state conditions. Bioresour Technol. 2006;97:2054–2058. doi: 10.1016/j.biortech.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Pilnik W, Voragen AGJ. Pectic enzymes in fruit and vegetable juice manufacture. In: Nagodawithama T, Reed G, editors. Enzymes in food processing. New York: Academic; 1993. pp. 363–399. [Google Scholar]
- Pinelo M, Zeuner B, Meyer AS. Juice clarification by protease and pectinase treatments indicates new roles of pectin and protein in cherry juice turbidity. Food Bioproducts Process. 2010;88:259–265. doi: 10.1016/j.fbp.2009.03.005. [DOI] [Google Scholar]
- Ramadan MF, Moersel JT. Impact of enzymatic treatment on chemical composition, physicochemical properties and radical scavenging activity of goldenberry (Physalis peruviana L.) juice. J Sci Food Agric. 2007;87:452–460. doi: 10.1002/jsfa.2728. [DOI] [Google Scholar]
- Ramli S, Alkarkhi AFM, Yong YS, Easa AM. Utilization of banana peel as a functional ingredient in yellow noodle. Asian J Food Agro-Industry. 2009;2:321–329. [Google Scholar]
- Ranganna S. Manual of analysis of fruit and vegetable products. New Delhi: Tata McGraw-Hill Publ. Co. Ltd.; 1977. p. 634. [Google Scholar]
- Rombouts FM, Pilnik W. Pectic enzymes. In: Rose AH, editor. Economic microbiology. Microbial enzymes and bioconversions. London: Academic; 1980. pp. 227–282. [Google Scholar]
- Sandri IG, Fontana RC, Barfknecht DM, da Silveira MM. Clarification of fruit juices by fungal pectinases. LWT - Food Sci Technol. 2011;44:2217–2222. doi: 10.1016/j.lwt.2011.02.008. [DOI] [Google Scholar]
- Sin HN, Yusof S, Hamid NSA, Rahman RA. Optimization of enzymatic clarification of sapodilla juice using response surface methodology. J Food Eng. 2006;73:313–319. doi: 10.1016/j.jfoodeng.2005.01.031. [DOI] [Google Scholar]
- Somogyi M. Determination of reducing sugars by Nelson-Somogyi method. J Biol Chem. 1952;200:245. [Google Scholar]
- Sun Y, Wang Z, Wu J, Chen F, Liao X, Hu X. Optimising enzymatic maceration in pretreatment of carrot juice concentrate by response surface methodology. Int J Food Sci Technol. 2006;41:1082–1089. doi: 10.1111/j.1365-2621.2006.01182.x. [DOI] [Google Scholar]
- Taskin E, Eltem R. The Enhancement of polygalacturonase and polymethylgalacturonase production on solid-state conditions by Aspergillus foetidus. Food Biotechnol. 2008;22:203–217. doi: 10.1080/08905430802262533. [DOI] [Google Scholar]
- Vaidya D, Vaidya M, Sharma S, Ghanashyam Enzymatic treatment for juice extraction and preparation and preliminary evaluation of Kiwifruits wine. Nat Prod Rad. 2009;8:380–385. [Google Scholar]
- Wang W-D, Xu S-Y, Jin M-K. Effects of different maceration enzymes on yield, clarity and anthocyanin and other polyphenol contents in blackberry juice. Int J Food Sci Technol. 2009;44:2342–2349. doi: 10.1111/j.1365-2621.2007.01637.x. [DOI] [Google Scholar]