Abstract
Nowadays, the presence of acrylamide in lots of fried and baked foods raises concerns due to its potential to cause toxicity and cancer in animals and human. Consequently, a number of papers have focused on evaluation of various chemicals in reduction of acrylamide in various food sources, as well as decreasing its related toxicities. In addition, plants are important sources of diverse metabolites demonstrating either possible effectiveness in acrylamide toxicity or reduction of acrylamide content in food sources. In this paper, we have criticized all relevant studies in terms of acrylamide mitigation from food by phytochemicals and antioxidants, and the influence of herbal medicines and phyto-pharmaceuticals on reduction of acrylamide toxicity in both animals and human.
Keywords: Acrylamide, Antioxidants, Baked foods, Fried foods, Phytoceuticals
Introduction
The chemical formula C3H5NO represents a well-known compound named “acrylamide” (acrylic amide; prop-2-enamide), which is a white odorless water soluble crystal, although it can be solved in other chemical solvents like ethanol, ether, and chloroform. Generally, acidic and basic materials, as well as oxidizing agents together with iron and iron salts, are able to destroy this structure to generate ammonia. However, it can also be decomposed by high temperature to form both carbon monoxide and dioxide, and nitrogen oxides (Sigma-Aldrich 2014).
Today, acrylamide is industrially prepared by the hydrolysis of acrylonitrile using nitrile hydratase. This compound is mainly applied in polyacrylamides production usually employed as a water-soluble thickener. Polyacrylamide is able to produce a water soluble gel, which is very useful in electrophoresis and famous as SDS-PAGE (Sodium dodecyl sulfate polyacrylamide gel electrophoresis). There are some other applications for this polymer such as papermaking, tertiary oil recovery, and the manufacturing of permanent press fabrics. The monomeric acrylamide can be used industrially to provide dyes and other monomers (Office of Pollution Prevention and Toxics. Chemical Summary for Acrylamide. United States Environmental Protection Agency. EPA 749-F-94-005a. Last access: Feb12 (2014).
In 2002, some reports revealed that acrylamide could be found in lots of fried and baked foods. Consequently, recent articles are concerned with whether acrylamide can cause considerable toxicity and cancer in both animals and human or not (Simonne et al. 2014) In this review, we aimed to give an overview on the present background information of acrylamide, its natural contents in food sources, and toxicity in both animals and human, as well as the role of phyto-pharmaceuticalsand antioxidants on mitigation of acrylamide from foods and reduction of its toxicities.
Formation of acrylamide in foods during cooking process
These days, cooking is a respected art form. However, there have been some concerns with the creation of acrylamide during the cooking process. In fact, acrylamide is not found naturally in its sources but can be formed when sugars and amino acids react with each other in carbohydrate (and/or protein)-rich foods during the cooking process due to high temperatures. Actually, this monomeric compound can be found in a number of foods such as potato chips, fries, breads, cereals, even coffee during high temperature cooking including toasting, frying and baking. The darker the color of the fried or baked food (e.g., French fries or toast), the higher the presence of acrylamide (Acrylamide: a natural process in cooking June (2010).
Tareke et al. (2002)) reported the moderate concentration of acrylamide (5–50 ppb) in heated protein-rich foods, while a higher content (150–4000 ppb) in carbohydrate-rich foods (potato, beetroot and selected commercial potato products) for the first time. They also reported different concentrations of acrylamide (1,200, 450 and 410 ppb) in potato chips, biscuits, as well as French fries and crackers, respectively. Moreover, the above mentioned researchers concluded that acrylamide did not exist in the same foods when used as raw materials or boiling water cooked (Tareke et al. 2002). Most references report mean or average acrylamide level in particular periods but some factors like sampling date and distribution of acrylamide content over evaluation period are so effective on quantification process and must be considered carefully (Wenzl and Aklam 2007). Another important problem, dietary intake of acrylamide in different populations, has been evaluated in different previous researches (Claeys et al. 2010; ANSES, 2011; FDA/CFSAN, 2006; OEHHA-CAL/EPA, 2005). Acrylamide level in different food groups on the basis of various reliable references are summarized in Table 1. An appraisal on Table 1 shows that although the measured amounts of acrylamide have been reported in a wide range, French Fries, potato chips, biscuits, roasted coffee and green teas as well as decaffeinated ones, and also different types of breads could be the main sources of oral exposure to this compound.
Table 1.
Acrylamide level in selected food groups based on different reliable references
| Food | Acrylamide concentration (ppb = μg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2, a | 3, b | 4, c | 5, b | 6, b | 7 | 8, d | 9, c | 10, d | |
| Cereals and cereal based products | 343 | 70 (5–1649) | 189 (˂30–1346) | |||||||
| Breakfast cereals | 119.4 | 138 (132–144) | 96 | 86 | 16.2 | ˂10–1649 | 139 (35–325) | 84 (5–545) | ||
| Bread | 30 (25–35) | 446 | 50 (5–1987) | 31 | 34.3 | ˂10–3200 | 16 (3–51) | 30 (12–162) | ||
| Ginger bread | 415 (414–415) | 303 (5–7834) | 501 (5–6141) | |||||||
| Popcorn | 145 (5–3100) | 180 | 328 (205–451) | 416 | ||||||
| Biscuits | 350 | 145 (4–3324) | 37 | 139 | 18–3324 | 380 (27–1573) | 100 (22.5–1035) | |||
| Pizza | 33 | 20 | 41 | |||||||
| Root and tubers | 477 | |||||||||
| Potato chips | 597.5 | 334 | 186 (5–4653) | 466 | 724.1 | 117–4215 | 1115 (˂60–3100) | 268 (5–2310) | ||
| French fries | 338 (336–339) | 413 | 59–5200 | 239 (41–1285) | 320 (˂60–12000) | |||||
| Restaurants | 404.1 | |||||||||
| Oven bake | 697.8 | |||||||||
| Potato crisps | 675 (674–676) | 752 | 528 (5–4215) | 954.5 | 835 (220–2061) | 652 (5–4215) | ||||
| Croquettes | 110 | |||||||||
| Stimulants and analogue | 509 | |||||||||
| Coffee | 255 (200–310) | |||||||||
| Not brewed | 288 | 68 | ||||||||
| Brewed | 7.8 | 13 | 7 | |||||||
| Roasted | 256 (255–257) | 288 | 320 (79–1188) | 45–935 | 212 (172–243) | 310 (101–935) | ||||
| Instant | 1123 | 865 (724–997) | ||||||||
| Decaffeinate | 668 | |||||||||
| Cocoa products | 220 | 80 | ˂2–826 | 442 (176–707) | ||||||
| Green tea (roasted) | 306 | |||||||||
| Vegetables | 17 | |||||||||
| Raw | 4.2 | |||||||||
| Processed | 59 | |||||||||
| Fruits | ||||||||||
| Fresh | ˂1 | 2 | ||||||||
| Processed | 131 | |||||||||
| Canned olive | 242.8 | 414 | 884 | |||||||
| Nuts and oilseeds | 84 | |||||||||
| Almond (roasted) | 320 | |||||||||
| Peanut (roasted) | 27 | |||||||||
| Peanut butter | 88 | |||||||||
| Sunflower | 39.5 | |||||||||
| Condiments and sauces | 71 | |||||||||
| Infant formula | ˂5 | ˂10–130 | ||||||||
| Baby foods | 69 (64–74) | 67 (5–432) | 13 (3–27) | |||||||
| Canned, jarred | 22 | |||||||||
| Dry powder | 16 | |||||||||
| Biscuits | 86 (83–90) | 181 | 79 (5–910) | 106 (5–432) | ||||||
| Cereal based | 51 (45–57) | 65 (3–598) | ||||||||
| Alcoholic beverages | 6.6 | |||||||||
1Food and Drug Administration, Center for Food Safety and Applied Nutrition, FDA/CFSAN, 2006
2European Food Safety Authority, EFSA Journal 2012,10:2938
3Summary and conclusions of the sixty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives, JECFA. Rome, 2005
4European Union database on acrylamide levels in food- update and critical review of data collection, Food Additives and Contaminants, 2007, 24(S1): 5–12
5Office of Environmental Health Hazard Assessment/California Environmental Protection Agency, OEHHA-CAL/EPA., 2005
6French agency for food, environmental and occupational health and safety, ANSES, Second French Total Diet Study (TDS 2), Pesticide residues, additives, acrylamide and polycyclic aromatic hydrocarbons, 2011
7Lineback, et al. 2012
8Food Standard Agency UK, A rolling programme of surveys on process contaminants in UK retail foods, Acrylamide and Furan: Survey 4, 2012
9Joint Institute for Food Safety and Applied Nutrition and National Center for Food Safety and Toxicology, JIFSAN/NCSF, 2002
10Gobel and Kliemant 2007
aValues indicate middle bound and ranges in brackets indicate lower bound and upper bound values in 2010
bMean concentration
cValues indicate median and ranges in brackets indicate minimum and maximum values
dvalues indicate mean and ranges in brackets indicate minimum and maximum values
Role of antioxidants in acrylamide content mitigation in foods
The correlation between antioxidant phytochemicals and acrylamide can be considered from two points of view. The first one describes antioxidants as exogenous additives, while the second one views them as endogenous secondary metabolites.
-
Using additives like organic acids, amino acids and mono- and divalent cations, as a mitigating strategy to control acrylamide content in foods, has been fully discussed in “CIAA toolbox for acrylamide” and different previous review studies (Capuano and Fogliano 2011; CIAA 2011; Friedman and Levin 2008; Capuano and Fogliano 2011; Zhang and Zhang 2007a; Zhang et al. 2009b).
Lack of sufficient studies and discordance in available results, impede logical judgment about the effectiveness of antioxidants. This is especially true regarding phenolic compounds such as flavonoids, phenolic acids, and tannins, as exogenous controllers on food acrylamide level. In some cases, it seems that antioxidant activity is not mainly responsible for changing the acrylamide content. This conflicting situation may be attributed to the chemical diversity of antioxidants. In fact, according to the unique structure of each antioxidant, it can play a specific role in either the promotion or reduction of acrylamide formation, sometimes regardless of its antioxidant potency.
Despite some reports about the beneficial effect of curcumin as a potent antioxidant to reduce acrylamide formation, a recent study showed that it can facilitate acrylamide production. This is due to the carbonyl moieties, which react with aspargine to finally produce acrylamide. On the other hand, naringenin, with weak antioxidant activity, can strongly react with the amide group of intermediates in the Maillard reaction and block acrylamide formation (Jin et al. 2013; Hamzalioglu et al. 2013; Zhang and Zhang 2007a). Moreover, there are some other parameters influencing acrylamide production including reaction condition, solubility, concentration, and preparation method for antioxidants, which can confusingly cause opposite results for the same kind of agent (Jin et al. 2013).
-
A limited studies supposed a negative correlation between acrylamide formation and phenolic (anti-oxidative) composition of its raw natural source (Evers and Deuber 2012). Moreover, Zhu et al. (2010) revealed that hydroxycinnamoylquinic/hydroxycinnamoyl derivatives are the major phenolic compounds in potatoes. They also showed that total phenolic content was reversely correlated with aspargine (−0.590) and total sugar quantity (−0.488) in raw potato and acrylamide content (−0.691) in processed form. Kalita et al. (2013) concluded that acrylamide content in fried potato has a negative relation to total phenolic (−0.367) and chlorogenic acid content (−0.359) of raw material. However, the presence of excess sugar can block this beneficial effect.
A few studies have focused on groups of phytochemicals other than phenolics like sulfur or nitrogen containing natural products. The acrylamide reducing activity of garlic is attributed to sulfur groups of alliin and allicin, or their free radical scavenging activity (Casado et al. 2010; Jin et al. 2013; Yuan et al. 2011). Moreover, a nitrogenous compound, piperin, weakly reduces acrylamide content in the aspargine/glucose model system (Zhu et al. 2009).
Besides pure phytochemicals with antioxidant activity, there are different reports about the effects of some plants and herbal extracts as additives on acrylamide mitigation. Most of these effects are attributed to phytochemicals with antioxidant power, carbonyl trapping activity, amino acid precipitation effect, and other supposed mechanisms of action. For example, there are significant positive correlations between DPPH-scavenging capacity (0.992) and reducing power (0.955) of some spices and their potency to reduce acrylamide formation (Jin et al. 2013; Salazar et al. 2012a; Zhu et al. 2011; Ciesarova et al. 2008; Cheng et al. 2010). Tables 2 and 3 have summarized some studies about natural compounds and medicinal plant effects on acrylamide content mitigation in model systems or foods.
Table 2.
Effect of natural compounds on acrylamide content mitigation
| Phytochemical name | Used system | Acrylamide reduction (%) | Other parameters | References |
|---|---|---|---|---|
| Rutin (1 mmol/ml, 100 μl) | Model (aspargine/glucose) | 49.4 | 180 °C for 15 min | Zhu et al. 2009 |
| P-coumaric acid | 53.4 | |||
| Gallic acid | 47.7 | |||
| Quercetin | 38.4 | |||
| Chlorogenic acid | 25.5 | |||
| (+)-Catechin | 23.5 | |||
| Hesperedin | 16.7 | |||
| Naringenin | 13.9 | |||
| Piperine | 13 | |||
| Ferulic acid | - | |||
| Hesperetin | - | |||
| Caffeic acid | Model (aspargine/glucose) | - | 180–200 °C for 7 min | Bassama et al. 2010 |
| Catechin | - | |||
| Cinnamic acid | - | |||
| Ferulic acid | Slight promotion | |||
| Coumaric acid | - | |||
| Gallic acid | - | |||
| Epicatechin | - | |||
| Tyrosol | Model (aspargine/glucose) | Up to 50 | 180 °C for 20 min | Kotsiou et al. 2010 |
| Oleuropein | - | |||
| Gallic acid | - | |||
| Ferulic acid | Model (aspargine/glucose) | Up to 50 | 105–125 ± 2 °C for 10–60 min | Kotsiou et al. 2011 |
| Caffeic acid | Up to 50 | |||
| Gallic acid | Up to 70 | |||
| Protocatechuic acid | Up to 70 | |||
| Curcumin (0.05–2 % w/w) | Model (starch based) | Up to 47 | 190 °C for 20 min | Zhu et al. 2011 |
| Proanthocyanidins | Up to 62 | |||
| Eugenol (0.25–4 % w/w) | Food (cooky) | Up to 42 | 210 °C for 15 min | |
| Cinnamaldehyde | Up to 34 | |||
| Curcumin | Up to 40 | |||
| Allicin (0.0375 %) | Model (asparagine/sugar) | >50 | 700 Watt for 20 min | Yuan et al. 2011 |
| Rutin (10−2—10–9 mol/L) | Model (asparagine/sugar) | Up to 60 | 180 °C for 5 min | Cheng et al. 2013 |
| Kaempferol-3-O-glucoside | Up to 40 | |||
| Quercetin | Up to 50 | |||
| Quercetin-3-O-glucoside | Up to 60 | |||
| Myricetin | Up to 66 | |||
| Kaempferol | Up to 50 | |||
| Ferulic acid | Food (potato chips) | 76.71 | 190 ± 5 °C for 6 min | Abdel-Monem et al. 2013 |
| Protocatechuic acid | 31.81 | |||
| Caffeic acid | 73.33 | |||
| Catechin | 90.90 | |||
| Gallic acid | 98.03 | |||
| Phytic acid (0.1 M) | Model (β-alanine/glucose) | – | 100–105 °C for 180 or 300 min | Wang et al. 2013 |
| Caffeic acid (0.05 M) | Model (aspargine/fructose) | 30 | Model: 180 °C for 15 min | |
| Food: 185 °C for 15 min | Oral et al. 2014 | |||
| Food (biscuit) | 19 | |||
| Chlorogenic acid | Model (aspargine/fructose) | 45 | ||
| Food (biscuit) | 15 | |||
| Ellagic acid | Model (aspargine/fructose) | 69 | ||
| Food (biscuit) | 19 | |||
| Epicatechin | Model (aspargine/fructose) | 74 | ||
| Food (biscuit) | 17 | |||
| Oleuropein | Model (aspargine/fructose) | - | ||
| Food (biscuit) | 17 | |||
| Punicalagin | Model (aspargine/fructose) | 85 | ||
| Food (biscuit) | 10 | |||
| Tyrosol | Model (aspargine/fructose) | 51 | ||
| Food (biscuit) | 17 | |||
| Chlorogenic acid (Phenol type) (0.5–50 μmol/ml) | Model (aspargine/glucose) | – | 160 °C for 5–20 min | Cai et al. 2014 |
| Chlorogenic acid (Quinone type) | Up to 55 | |||
| Homo orientin (10−6-10 mmol/L) | Model (low moisture aspargine/glucose) | Up to 70 | 180 °C for 15 min | Zhang and Zang, 2008 |
| Epigallocatechin gallate | Up to 70 |
Table 3.
Effect of medicinal plants on acrylamide content mitigation
| Plant name | Used part | Acrylamide reduction (%) | Used system | Other parameters | References |
|---|---|---|---|---|---|
| Capsicum annuum L. var. Aviculare | Oleoresin of fruit (for frying) | – | Model (aspargine/glucose) | 180 °C | Salazar et al. 2012a |
| 26/77 | Food (fried potato-5 min/tortilla chips-2.30 min) | ||||
| Camellia sinensis (L.) Kuntze. | Leaf (3 g/kg) | – | Olive Sterilization in computer controlled retort | 121 °C for 15 min | Casado et al. 2010 |
| Rosmarinus officinalis L. | |||||
| Origanum vulgare L. | |||||
| Allium sativum L. | Blanched bulb (15 g/kg) | 30.62 | |||
| Raw (15 g/kg) | – | ||||
| Mentha canadensis L. | Aerial part | 74.5 | Model (aspargine/glucose) | 180 °C for 15 min,Aqueous extract (0.1 mg /ml, 100 μl) | Zhu et al. 2009
Kotsiou et al. 2010 Arribas-Lorenzo et al. 2009 Arribas-Lorenzo and Morales 2012 Zhu et al. 2011 |
| Cuminum cyminum L. | Seed | 73.1 | |||
| Illicium verum Hook. f. | Fruit | 68.9 | |||
| Coriandrum sativum L. | Seed | 61.5 | |||
| Lycium barbarum L. | Fruit | 60.1 | |||
| Eugenia caryophylata Thunb. | Bud | 58.7 | |||
| Prunella vulgaris L. | Inflorescence | 56.3 | |||
| Lens culinaris L. | Seed | 55.6 | |||
| Capsicum frutescens L. | Fruit | 55.5 | |||
| Vitis vinifera L. | Seed | 54.1 | |||
| Citrus hystrix DC. | Leaf | 50.7 | |||
| Murraya koenigii L. | Leaf | 49.7 | |||
| Salvia officinalis L. | Aerial part | 49.2 | |||
| Rheum officinalis Baill. | Root | 48.7 | |||
| Foeniculum vulgare Mill. | Seed | 48.1 | |||
| Rhus chinensis Mill. | Gall | 47.6 | |||
| Petroselinum crispum L. | Leaf | 47.5 | |||
| Camellia sinensis L. | Leaf | 46.8 | |||
| Origanum vulgare L. | Leaf | 46.1 | |||
| Chrysanthemum morifolium Ramat. | Flower | 43.7 | |||
| Curcuma longa L. | Rhizome | 42.2 | |||
| Fagopyrum esculentum Moench. | Grain | 37.6 | |||
| Glycine max L. | Seed | 36.9 | |||
| Gardenia jasminoides Ellis. | Fruit | 35.8 | |||
| Cymbopogon citrates Stapf. | Stem | 34.8 | |||
| Matteuccia strothiopteris (L.) Todaro. | Rhizome | 32.9 | |||
| Myristica fragrans Houtt. | Fruit | 31.7 | |||
| Punica granatum L. | Peel/Husk | 30.1 | |||
| Rosa chinensis Jacq. | Flower | 28.1 | |||
| Rubus chingii Hu. | Fruit | 24.3 | |||
| Cinnamomum zeylanium N. | Cortex/Bark | 23 | |||
| Acacia catechu (L.f.) Willd | Aerial part | 22.7 | |||
| Morus alba L. | Fruit | 14.5 | |||
| Crataegus pinnatifida Bge. | Fruit | 11 | |||
| Ilex cornuta Lindl. et Paxt. | Leaf | – | |||
| Olea europaea L. | Fruit virgin oil | – | Model (aspargine/glucose) | 180 °C for 20 min, Phenolic extract | |
| Origanum vulgare L. | Leaf | 49 | |||
| Olea europaea L. | Fruit virgin oil | Up to 20 | Food (cooky) | 190 °C for 16 min | |
| Allium cepa L. | Raw bulb | Up to 30 | Food (cooky) | 210 °C for 15 min, 0.25–4 % w/w | |
| Curcuma longa L. | Raw rhizome | Up to 36 | Food (cooky) | 210 °C for 15 min | |
| Aqueous extract | Up to 32 | ||||
| Cuminum cyminum L. | Raw seed | Up to 37 | Food (cooky) | 210 °C for 15 min | |
| Aqueous extract | Up to 46 | ||||
| Coriandrum sativum L. | Raw seed | Up to 31 | Food (cooky) | 210 °C for 15 min | |
| Aqueous extract | Up to 36 | ||||
| Eugenia caryophylata Thunb. | Bud | Up to 56 | Model (starch based) | 190 °C for 20 min, 0.05–2 % w/w | |
| Up to 50 | Food (cooky) | 210 °C for 15 min | |||
| Cinnamomum zeylanium N. | Cortex/Bark | Up to 44 | Model (starch based) | 190 °C for 20 min | |
| Up to 38 | Food (cooky) | 210 °C for 15 min | |||
| Piper nigrum L. | Seed | 50 | Model (starch based) | 180 °C for 20 min, Hydro- alcoholic extract | Ciesarova et al., 2008 |
| Origanum majorana L. | Leaf | 60 | |||
| Origanum vulgare L. | Leaf | 60 | |||
| Pimenta dioica (L.) Merr. | Fruit | 75 | |||
| Coriandrum sativum L. | Seed | – | Food (ginger cake) | 180 °C for 18 min, powder 2% w/w | Markov et al., 2012 |
| Cinnamomum zeylanium N. | Cortex/Bark | – | |||
| Pimenta dioica (L.) Merr. | Fruit | – | |||
| Illicium verum Hook. f. | Fruit | – | |||
| Myristica fragrans Houtt. | Fruit | 23 | |||
| Foeniculum vulgare Mill. | Seed | 21 | |||
| Pimpinella anisum L. | Seed | 17 | |||
| Eugenia caryophylata Thunb. | Bud | 17 | |||
| Vanilla spp. | Seed | 11 | |||
| Elettaria cardamomum (L.) Maton | Seed | 9 | |||
| Piper nigrum L. | Seed | 9 | |||
| Zingiber officinale Roscoe. | Rhizome | 5 | |||
| Camellia sinensis L. | Leaf | 43 | Food (cooky) | 190 °C for 7 min, Polyphenols (0.1 g/kg) | Li et al., 2012 |
| Phyllostachys nigravar. henonis (Mitford) Stapf ex Rendle. | 63.9 | 190 °C for 7 min, Antioxidants (0.2 g/kg) | |||
| Malus domestica Borkh. | Fruit | 35 | Model (aspargine/glucose) | 160 °C for 30 min, Ethanolic extract, 35 mg/ml, 2 ml | Cheng et al., 2010
Abdel-Monem et al., 2013 |
| Garcinia mangostana L. | Fruit | – | |||
| Euphoria longana Lamk. | Fruit | – | |||
| Hylocereus undatus (Haworth) Britton & Rose. | Fruit | – | |||
| Vaccinium corymbosumL. | Fruit | – | |||
| Phyllostachys nigra var. henonis (Mitford) Stapf ex Rendle. | Leaf | 84.6 | |||
| Food (potato chips) | 190 °C for 7 min | ||||
| Psidium guajava L. | 84 | ||||
| Rosmarinus officinalis L. | 85.7 | ||||
| Origanum vulgare L. | 92.8 | ||||
| Olea europaea L. | 75.7 | ||||
| Vaccinium oxycoccos L. | 59.6 | ||||
| Camellia sinensis L. | 56.5 | ||||
| Phyllostachys nigra var. henonis (Mitford) Stapf ex Rendle. | Leaf | 43.4 | |||
| Model (aspargine/glucose) | 180 °C, 10−6 mg/ml reaction solution | Zhang et al., 2008b | |||
| Camellia sinensis L. | 32.3 | ||||
| Olea europaea L. | Fruit virgin oil | Food (fried crisps) | 180 ± 1 °C for 5–15 min | Napolitano et al., 2008 | |
| Phyllostachys nigravar. henonis (Mitford) Stapf ex Rendle. | Leaf | Up to 74 | Food (potato crisps) | 170 ± 1 °C for 4 min, Antioxidants (0.001–1 % w/w) | Zhang et al., 2007a |
| Up to 76 | Food (French fries) | ||||
| Phyllostachys nigravar. henonis (Mitford) Stapf ex Rendle. | Leaf | Up to 59 | Food (fried chicken wing) | 170 ± 1 °C for 4 min, Antioxidants (0.001–1 % w/w) | Zhang et al., 2007b |
| Phyllostachys nigravar. henonis (Mitford) Stapf ex Rendle. | Leaf | Up to 83 | Food (bread) | 180 ± 3 °C (0.002–4.9 g of 30 % Hydro-alcoholic extract/kg flour) | Zhang and Zang, 2007b |
| Camellia sinensis L. | Up to 72.5 | ||||
| Phyllostachys nigra | |||||
| var. henonis (Mitford) Stapf ex Rendle. | Leaf | Up to 74.4 | Model (low moisture aspargine/glucose) | 180 °C for 15 min (10−6–10 mg/ml of antioxidants extract) | Zhang and Zang, 2008 |
| Camellia sinensis L. | Up to 74.3 | ||||
| Amaranthus hypochondriacus L. | Seed flour | – | Model (aspargine/glucose) | 180 °C for 10 min (0–55 mg) | Salazar et al., 2012b |
| Seed protein | Up to 40 | ||||
| Seed flour | – | Food (cooky) | 190 °C for 7–9 min (8 g) | ||
| Seed protein | |||||
| Seed flour | – | Food (tortilla chips) | 190 °C for 30–45 s or 205 °C for 4–5.25 min (5 % w/w) | ||
| Seed protein | – | ||||
| Rosmarinus officinalis L. | Leaf | Up to 38 | Food (deep fried potato) | 180 °C for 5 min (1,000 mg/kg of extract) | Urbancic et al., 2013 |
Toxicity of Acrylamide
As mentioned in previous parts, acrylamide has been used as a chemical intermediate to produce polyacrylamide in different industries since the 1950s. Its toxic effects had been researched since then, but it specially attracted scientists’ attention after 2002. They found that different foods, especially cereal based foods, which cooked at high temperatures, were the main source of human exposure to acrylamide. Different research studies confirmed that acrylamide and its biotransformed metabolite, glycidamide, are hazardous neurotoxins, reproductive toxins, and carcinogens in addition to their danger for other organs like the liver, kidney, intestines and lungs (Capuano and Fogliano 2011; Lopachin 2004).
Neurotoxicity
The proposed mechanisms for acrylamide neurotoxicity are central and peripheral nerve terminal degeneration, harmful effects on the cerebral cortex, thalamus and hippocampus, myodegeneration of muscles, decreasing release of neurotransmitter and interference with kinesin motor protein function and nerve signal transportation (Capuano and Fogliano 2011). Clinical manifestations of this toxicity are peripheral neuropathy, skeletal muscle weakness, numbness of movement organs, and ataxia. It has been typically concerned with occupational exposure, but recent studies have signalized the role of dietary exposure (CIAA 2011; Friedman and Levin 2008).
Reproductive toxicity
In this case, acrylamide can degenerate epithelial cells of seminiferous tubules and harmfully affect the number and shape of sperm, probably because of its interfering effects on kinesin motor protein in the flagella of sperm (Jin et al. 2013; Tyl and Friedman 2003; Tyl et al. 2000; Zhang and Zhang 2007a).
Carcinogenicity
Acrylamide was classified as a probable carcinogen for humans by the “IARC International Agency for Research on Cancer 1994 (Group 2A). National Toxicology and Report on carcinogens (2011) set acrylamide as "reasonably anticipated to be a human carcinogen". These classifications are supported by several studies, which show that acrylamide and glycidamide can form covalent adducts with DNA, hemoglobin, and different functional groups of proteins like SH, inducing gene mutation and chromosomal aberration, increasing benign and malignant neoplasm in rodents (Casado et al. 2010; Kalita et al. 2013; Pelucchi et al. 2011; Salazar et al. 2012a; Zhu et al. 2010).
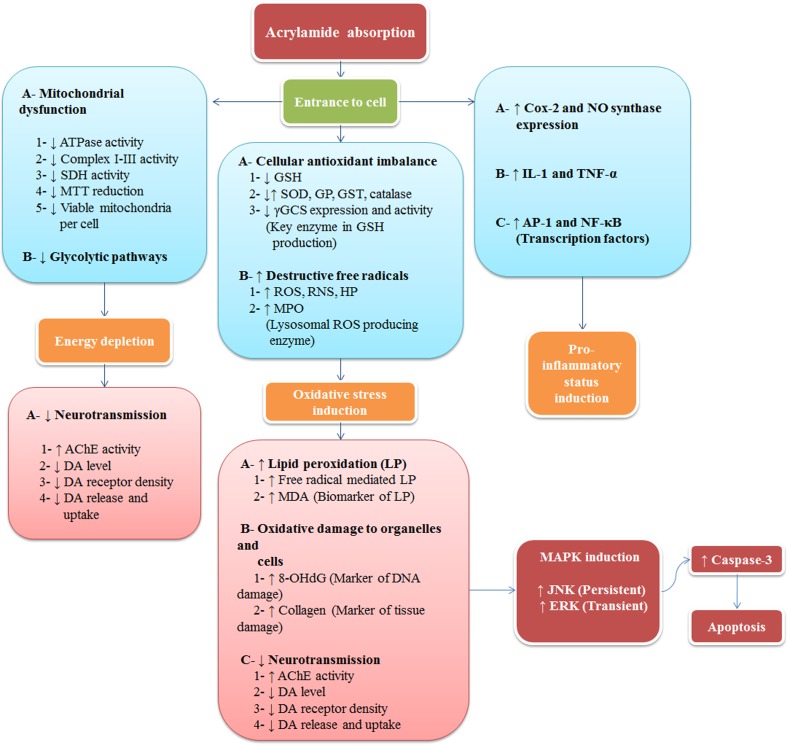
A current meta-analysis of epidemiologic study about acrylamide exposure and human cancers concluded that the risk of cancer does not increase by dietary or occupational exposure to acrylamide except for kidney cancer. This study attributed the risk underestimation to the low sensitivity of epidemiologic studies to detect a small increase in risk, exposure misclassification, and other methodological limitations and biases. It finally justified actions aimed at reducing human exposure to acrylamide and recommended further studies especially on kidney cancer (Yuan et al. 2011; Rice 2005). Figure 1 describes some subsequent intracellular events of acrylamide entrance to cells.
Fig. 1.
Subsequent intracellular events of acrylamide entrance to cell, AChE AcethylCholin Esterase Activity; AP-1 Activator Protein 1; COX-2 Cyclooxygenase 2, DA Dopamine, ERK Extracellular Signal-Regulated Kinase, γ-GCS: γ-Glutamylcysteine Synthetase, GSH Glutathione; IL-1, Interleukin 1, NK c-Jun N-terminal Kinase, MAPK Mitogen-Activated Protein Kinase, MDA Malondialdehyde, MTT, Methylthiazol Tetrazolium Assay, 8-OHdG, 8-Hydroxydeoxyguanosine, NF-κB, Nuclear Factor kappa B, RNS, reactive nitrogen species, ROS, reactive oxygen species, SDH Succinate Dehydrogenase, SOD Superoxide Desmutase, TNF-α: Tumor Necrosis Factor Alpha
Effective natural compounds and phytomedicines against acrylamide toxicity
Regarding the importance of finding an effective way to constrain acrylamide hazards, several studies have been carried out to evaluate the plant extracts and the phytochemical effects on different aspects of acrylamide toxicity. Results of some studies about the beneficial effects of plants and their phytochemicals on acrylamide, showed induced toxicity in different models. These results are summarized in Table 4. According to these data, increasing reduced glutathione, inhibiting reactive oxygen species formation and oxidative stress, and reducing cell apoptosis are general proposed mechanisms for protective activities of phytoconstituents against acrylamide toxicity.
Table 4.
Effective natural compounds and medicinal plants against acrylamide neurotoxicity
| Treatment | Used part | Model | Significant effects of treatment on acrylamide toxicity | Proposed mechanism of action | References |
|---|---|---|---|---|---|
| Acorus calamus L. | Hydroalcoholic extract of rhizome (25 mg/kg) | Rat | Decreasing hind limb paralysis, recovery of motor behavioral parameters, increase GSH content in corpus striatum | Detoxification with GSH | Shukla et al., 2002 |
| Panax ginseng C.A.Mayer | Standardized extract (20 mg/kg) | Rat | Recovery of brain antioxidant biochemical parameters like SOD activity | Inhibiting lipid peroxidation, increase SOD activity and free radical scavenging | Mannaa et al. 2006 |
| Zingiber officinale Roscoe. | Ethanolic extract of rhizome (0.25 mg) | Mice | Improving histological structure of the nervous tissues in the spinal cord and cerebellum, | Decrease of lipid peroxidation, Detoxification with GSH | El-Tantavi, 2007 |
| Glycin max (L.) Merr. | Dark soy sauce (0.5 ml/kg) | Rat | Improving body weight gain, relative brain weight, gait abnormalities, axonal degeneration and brain biochemical parameters like SOD activity | Antioxidant activities | Xichun and Minai, 2009 |
| Genistein | Pure compound (25,50 mg/kg) | Rat | Decreasing oxidative stress and neuron injuries and apoptosis | Antioxidant and anti- apoptotic activities | Cheng-mei et al., 2009 |
| Allium sativum L. | Powder (1.5 % w/w) | Rat | Decreasing total lipids and triglycerides and NO contents and increase phospholipids and GSH contents and SOD, GP, CAT and GST activity, decrease MAO activity and TBARS in brain | Detoxification with GSH, antioxidant activities, increasing neurotransmission due to inhibition of MAO activity | Ghareeba et al., 2010 |
| Swietenia mahagoni L. | Methanolic extract of leaf (100,200 mg/kg) | Rat | Decreasing neuropathy and oxidative stress markers like TBARS, increase GSH, inhibit histopathological changes in sciatic nerve like axonal degeneration and derangement of nerve fibers | Detoxification with GSH, antioxidant activities | Vanitha et al. 2012 |
| Crocus sativus L. | Purified crocin from stigma (10,20,50 μM) | PC12 cell line | Decreasing cytotoxicity, DNA fragmentation, Bax-Bcl-2 ratio, cell apoptosis and intracellular ROS (with 50 μM) | Antioxidant and anti-apoptotic activities | Mehri et al., 2012 |
| Epigallocatechin-3 -gallate | Pure compound (5–100 μmol/L) | CGN cell line | Decreasing cytotoxicity, Bax-Bcl-2 ratio and cell apoptosis, increase SOD activity | Antioxidant and anti- apoptotic activities | Liu et al. 2012 |
| Eugenol | Pure compound (10,25 μM) | Drosophila melanogaster | Decreasing mortality, locomotor dysfunction and oxidative markers like MDA, ROS, PC and HP, increase GSH and dopamine content, modulate AChE activity in head region | Detoxification with GSH, antioxidant activities, increase of neurotransmission and anti-apoptotic activity due to inhibiting AChE | Prasad and Muralidhara, 2012 |
| soeugenol | |||||
| Eugenol | Pure compound (10 mg/kg) | Rat | Decreasing oxidative markers like MDA, ROS and HP, AChE activity and Ca2+ and NO content in brain and sciatic nerve, increase GSH and DA content and ATPase activity in brain and sciatic nerve, increase feed intake, body weight, restore gait abnormalities | Detoxification with GSH, antioxidant and anti-inflammatory activities | Prasad and Muralidhara 2013 |
| Isoeugenol | |||||
| Bacopa monnieri (L.) Pennell. | Ethanolic extract of leaf (2–6 μg/ml) | N27 neuronal cell line | Restoring cell loss and GSH depletion | Detoxification with GSH, antioxidant activities | Shinomol et al., 2013 |
| Ethanolic extract of leaf (0.05 % v/w) | Drosophila melanogaster | Improving locomotor dysfunction and GSH depletion in head | |||
| Ethanolic extract of leaf (2 %) | Mice | Restoring brain oxidative damage and GSH depletion | |||
| Chrysin | Pure compound (0.5–5 μM) | PC12 cell line | Decrease in cytotoxicity | Antioxidant and anti-apoptotic activities | Mehri et al. 2014 |
| Pure compound (12.5,25,50 mg/kg) | Rat | Improving gait abnormalities (with 50 mg/kg) | |||
| Geraniol | Pure compound (5,10 μM) | Drosophila melanogaster | Decrease mortality and locomotor deficits, oxidative markers like MDA, ROS, HP and AChE activity, increase activity of antioxidant enzymes like TRR, GST, SOD and CAT, increase GSH, TSH and dopamine content, modulate HP and mitochondrial dysfunction in head and body regions | Antioxidant and neurogenerative activities, detoxification with GSH, increase neurotransmission and anti-apoptotic activity due to inhibit AChE | Prasad and Muralidhara 2014 |
| Curcumin |
PC Phytochemical, GSH Glutathione, SOD Superoxide dismutase, TBARS Thiobarbituric acid reactive substance, ROS Reactive oxygen species, AChE Acetylcholinesterase, MDA Malondialdehyde, HP Hydroperoxides, MAO Monoamine oxidase, NO Nitric oxide, DA Dopamine, TSH Total thiol, TRR Thioreduxine reductase, PC Protein carbonyls
Tables 5 and 6 exhibit the most reported medicinal and food plants as well as natural compounds against different aspects of acrylamide related toxicity.
Table 5.
Effective natural compounds and medicinal plants against acrylamide-related reproductive toxicity, genotoxicity, and general toxicity
| Treatment | Used part | Model | Significant effects of treatment on acrylamide toxicity | Proposed mechanism of action | References |
|---|---|---|---|---|---|
| Reproductive toxicity | |||||
| Phenylethyl isothiocyanate | Pure compound (0.5 %) | Rat | Decrease exfoliation of immature spermatocytes into the lumen of seminiferous tubules and degeneration of tubules | Inhibit CYP2E1 and acrylamide conversion to glicydamide in testis | Lee et al., 2005 |
| Camellia sinensis (L.) Kuntze. | Water extract of leaf (70 mg/kg) | Rat | Restore serum testosterone level and harmful histopathological changes in testis like tubular endothelium thickening, germ cell degeneration, atrophy of semeniferous tubules and production of multinucleated giant cells | Antioxidant activity in serum, liver and testicular level | Yassa et al., 2013 |
| Genotoxicity | |||||
| Aloysia triphylla (L′Her.) Britton | Water extract of leaf (5 %) | Mice | Decrease genotoxicity, increase total antioxidant capacity in plasma | Antioxidant activity due to polyphenols, decrease acrylamide epoxidation or detoxification of glicylamide | Zamorano-Ponce et al., 2006 |
| General toxicity | |||||
| Azadirachta indica A.Juss | Ethanolic extract of leaf (200 mg/kg) | Rat | Restore GSH, MDA and CAT in liver and modulate histological injuries in liver, kidney, lung, heart, spleen and stomach | Detoxification with GSH, Antioxidant and anti- inflammatory activities | Mogda et al., 2008 |
| Camellia sinensis (L.) Kuntze. | Extracted catechins (1.5 %) | ||||
| Resveratrol | Pure compound (30 mg/kg) | Rat | Decrease ALT, AST, LDH, BUN, creatinine, TNFα, IL-1, IL-6 and indicator of DNA damage, 8-OHdG, in serum, increase GSH and decrease collagen, MDA and MPO in brain, lung, liver, kidney and testis | Inhibit neutrophil infiltration, antioxidant and anti -inflammatory activities, Detoxification with GSH | Alturfan et al., 2012 |
| Carica papaya L. | Water extract of fruit (250 mg/kg) | Rat | Decrease MDA and increase GSH, SOD and CAT in stomach, liver and kidney tissues | Detoxification with GSH, Antioxidant activity due to flavonoids and phenolic acids | Sadek, 2012 |
| Allium sativum L. | 2.5,5 % | Rat | Decrease MDA and increase GSH, SOD and in kidney, spleen, testis and brain, decrease urea, creatinine, LDH and alkaline phosphatase and increase total protein and albumin in serum | Detoxification with GSH, antioxidant activities | El-Halim and Mohamed, 2012 |
| Solanum nigrum L. | Leaf and fruit powder (2 g/kg of diet) | Rat | Decrease mortality, urea, creatinine, LDH, ALT and AST, improve weight gain, total protein, albumin and globulin, IgG, IgM, | Antioxidant activity due to flavonoids and phenolic acids | Soliman, 2013 |
| Cyanidin-3-glucoside | Pure compound (10–100 μM) | MDA-MB-231 cell line | Decrease cytotoxicity, LDH leakage, ROS formation, oxidative stress, GSH depletion and GST and GP activity, increase GPx1 and GSTP1 and decrease CYP2E1expression, increase γGCS expression as a marker of GSH synthesis | Detoxification with GSH, antioxidant activities, Inhibit CYP2E1 and acrylamide conversion to glicydamide | Song et al., 2013 |
| Allium sativum L. | Powder (54 mg/kg) | Rat | Restore serum triglyceride, cholesterol, HDL, SOD, GP and CAT | Antioxidant activity due to allicin, ascorbic acid, flavonoids, anthocyanins and phenolics | El-Shafey et al., 2013 |
| Hibiscus sabdariffa L. | Powder (81 mg/kg) | ||||
| Allicin | Pure compound (5,10,20 mg/kg) | Mice | Decrease TBARS and MPO and increase SOD and GSH in kidney, liver and brain, decrease ALT, AST, LDH, BUN, TNFα, IL-1, IL-6 and ROS and increase IL-10 in serum | Detoxification with GSH, antioxidant activities | Zhang et al., 2013 |
LDH Lactate dehydrogenase, ROS Reactive oxygen species, GSH Glutathione, GST Glutathione-S-Transferase, GP Glutathione peroxidase, ALT Alinine transaminase, AST Aspartate transaminase, MDA Malondialdehyde, OD Superoxide dismutase, CAT Catalase, MPO Myeloperoxidase, BUN Body urea nitrogen, TNF Tumor necrosing factor, IL Interleukin, γGCS Gamma-glutamyl cysteine synthase, 8-OHdG 8-Hydroxydeoxyguanosine
Table 6.
Effective natural compounds and medicinal plants against acrylamide-related intestinal and hepatotoxicity
| Treatment | Used part | Model | Significant effects of treatment on acrylamide toxicity | Proposed mechanism of action | References |
|---|---|---|---|---|---|
| Intestinal toxicity | |||||
| Hydroxytyrosol | Pure compound (5–40 μM) | Caco-2 cell line | Decrease LDH, ROS and apoptosis inducers like JNK and its active form p-JNK contents and caspase-3, GP and GR activity, increase cell viability | Antioxidant and anti-apoptotic activities due to polyphenols | Rodriguez-Ramiro et al., 2011a |
| Theobroma cocoa | Polyphenolic extract (10 μg/ml) | Caco-2 cell line | Decrease caspase-3 activity, cell apoptosis, GSH depletion and ROS formation, increase GST and γGCS activities, increase ERK and its active form p-ERK which favor survival signals and inhibit p-JNK increase (epichatechin is the weakest) | Antioxidant and anti-apoptotic activities due to polyphenols | Rodriguez-Ramiro et al., 2011b |
| Epicatechin | Pure compound (10 μM) | ||||
| Procyanidin B2 | Pure compound (10 μM) | ||||
| Solanum | Raw fiber (2 %) | Mice | Increase mitotic number,improve number of proliferating cells and decrease number of apoptotic cells in intestine epithelium, improve histomorphology parameters of epithelium, submucosa, mucosa and neurofilaments especially intestine absorptive surface (higher activity with heated form) | Reduction of chemical absorption due to binding them and enhance fecal passage | Dobrowolski et al., 2012;Woo et al., 2007 |
| Heated fiber (2 %) | |||||
| Myrcitrin | Pure compound (2.5–10 μg/ml) | Caco-2 cell line | Increase cell viability, attenuate intracellular ROS | Antioxidant activity and decrease oxidative stress | Chen et al., 2013 |
| Hepatotoxicity | |||||
| Hydroxytyrosol | Pure compound (12.5-50 μM) | Hep-G2 cell line | Decrease micronucleus frequency, genotoxicity and ROS formation, prevent GSH depletion, enhance γGCS expression | Detoxification with GSH, antioxidant activities | Zhang et al., 2008a |
| Curcumin | Pure compound (2.5 μg/ml) | Hep-G2 cell line | Decrease cytotoxicity, ROS formation, DNA damage, micronucleus frequency, | Antioxidant activity especially against hydrogen peroxide | Cao et al., 2008 |
| Hydroxytyrosol | Pure compound (12.5–50 μM) | Hep-G2 cell line | Increase cell viability, decrease DNA damage, ROS level, 8-OHdG as a marker of oxidative DNA damage and GSH depletion | Detoxification with GSH, antioxidant activities | Zhang et al., 2009a |
| Allium sativum L. | Powder (1.5 % w/w) | Rat | Decrease total lipids and triglycerides, TBARS and NO level, increase GSH and SOD, GP and GST activity in liver | Detoxification with GSH, antioxidant activities | Ghareeb et al. 2010 |
| Honey | Aqueous solution (0.01–10 mg/ml) | Hep-G2 cell line | Increase cell viability | Antioxidant activity due to polyphenols | Mademtzoglou et al., 2010 |
| Digera muricata | Methanolic extract (100,150,200 mg/kg) | Rat | Restore ALT, AST, ALP, LDH, BUN, cratinine, bilirubin, total protein and albumin, CAT, SOD, GP and GST | Antioxidant activity due to phenolics and flavonoids | Khan et al., 2011 |
| Allicin | Pure compound (3.75,7.5,15 μM) | Mice hepatocyte | Decrease cytotoxiciy, MDA and 8-OHdG, increase GSH content and SOD activity | Detoxification with GSH, antioxidant activities, Inhibit CYP2E1 and acrylamide conversion to glicydamide | Zhang et al., 2012 |
| Pure compound (5,10,20 mg/kg) | Mice | Increase GSH content and SOD activity, decrease MDA and 8-OHdG contents | |||
LDH Lactate dehydrogenase, ROS Reactive oxygen species, GP Glutathione peroxidase, GR Glutathine reductase, JNK c-Jun N-amino terminal kinase, ERK Extracellular regulated kinase, GSH Glutathione, GST Glutathione-S-transferase, γGCS: Gamma-glutamyl cysteine synthase, 8-OHdG: 8-Hydroxydeoxyguanosine, TBARS: Thiobarbituric acid reactive substance, CAT Catalase, BUN Body urea nitrogen, MDA Malondialdehyde
Discussion
Today, there is no doubt that antioxidants and other phytochemicals play considerable roles in either the prevention and cure of several illnesses, or reduction of toxicity induced by chemicals or environmental factors (Kahkeshani et al. 2013; Rahimi et al. 2005; Manayi et al. 2014; Mostafalou and Abdollahi 2013; Saeidnia and Abdollahi 2013a,b; Saeidnia and Abdollahi 2012). In this review, we aimed to focus on two important roles of antioxidants and phytochemicals on the reduction of acrylamide in food resources, and its toxicity in animals and humans as well.
Reduction of acrylamide content in foods
Among the various mechanisms by which chemicals are supposed to mitigate acrylamide from food sources, free radical scavenging activity is outstanding, particularly in the case of using phytoceuticals like polyphenols or plant derived antioxidants (Jin et al. 2013; Kalita et al. 2013; Salazar et al. 2012a,b; Zhu et al. 2010). An appraisal on Table 2, which summarized the findings of several studies on potentially active antioxidant compounds and acrylamide reduction, reveals that phenolic acids like p-coumaric acid, gallic acid, ferulic acid and caffeic acid could reduce the acrylamide content close to half or even lower (Abdel-Monem et al. 2013; Kotsiou et al. 2011; Zhu et al. 2009). The predominant hypothesis is that antioxidant compounds can decrease acrylamide formation but as mentioned in previous parts, there is still a controversy about correlation of acrylamide content and antioxidant activity in some cases. This problem can be explained considering the acrylamide formation reaction, which is partly complex. Acrylamide is produced in a series of multiple steps reactions between asparagine and a carbonyl compound with production of different intermediates. Thus, antioxidant compounds can interfere in various parts of this reaction according to their molecular structure and functional groups, and this interference can increase or decrease acrylamide production regardless of antioxidant activity (Jin et al. 2013).
As mentioned previously, an example is curcumin, which changes acrylamide content contrary to its antioxidant activity because of its specific functional groups (Hamzalioglu et al. 2013). There are other factors rather than antioxidant structure, which can affect acrylamide content. For example, some phenolic acids like chlorogenic acid can convert sucrose into more reactive intermediates like 5-Hydroxymethylfurfural that reacts with aspargine with more tendency, and increases acrylamide production (Kocadagl et al. 2012). On the other hand, some flavonoids like naringenin or epicatechin decrease acrylamide production through reaction with different intermediates of Maillard reaction and trapping them (Cheng et al. 2009; Totlani and Peterson 2005; Totlani and Peterson 2006).
Jin et al. (2013) have comprehensively discussed the relationship between antioxidants and acrylamide formation. They concluded that antioxidants can affect four parts of Maillard reaction: 1- reactive carbonyl pool as substrate, 2- asparagine as substrate, 3- intermediates and 4- acrylamide as product. Antioxidants can decrease or increase reaction between two substrates, and activate or deactivate them. Moreover, they can affect intermediates or final product, and change acrylamide production trend.
Different studies showed that increasing concentration of antioxidant couldn’t mitigate acrylamide formation in all cases and sometimes the higher concentration surprisingly increased acrylamide production (Kotsiou et al. 2010; Yuan et al. 2011; Zhu et al. 2010).
In addition to the role of antioxidants and their structures, other parameters related to reaction condition like temperature and heating time, pH and moisture can dramatically affect acrylamide content. This highlights the importance of usage the standard model systems with defined reaction condition for experiments (Jin et al. 2013).
Among a number of medicinal plants subjected to investigations (in different studies) for reducing the acrylamide content in food, Mentha canadensis, Phyllostachys nigra, Pimenta dioica, Illicium verum, Camellia sinensis, Origanum vulgare and Olea europaea exhibited considerable mitigation activity (Abdel-Monem et al. 2013; Zhang et al. 2007a,b; Zhu et al. 2009). Previous studies showed that in some cases, crude aqueous extracts were able to inhibit acrylamide formation more than their pure constituents especially poly phenols. Therefore, beside poly phenolic compounds, other phytochemicals might play role in mitigation of acrylamide not only by free radical scavenging activity but also by other mechanisms like trapping carbonyl moiety or/and precipitating aspargine and constituents like proteins, peptides, saccharides and monovalent/divalent cations (Oral et al. 2014; Cheng et al. 2010; Zhu et al. 2009; Zhu et al. 2010).
Reduction of acrylamide toxicity
Tables 4 and 6 show that plants with high contents of polyphenols and antioxidant activity like Theobroma cocoa, Solanum nigrum, Panax ginseng, Digera muricata, Crocus sativus, Glycin max, and Zingiber officinalis, as well as antioxidant compounds like myrcitrin, genistein, resveratrol, and curcumin have been reported to be effective in the reduction of acrylamide toxicity in cell lines and/or animal studies. Although the proposed mechanisms of action for each plant extract or phytochemical are illustrated in Tables 4 and 6, it seems that most of them may act via multiple mechanisms such as anti-apoptotic and anti-inflammatory activities (Zhang et al. 2007a a,b).
Conclusion
Acrylamide, which can be formed in fried and baked foods due to high temperatures, is demonstrated to be a source of different toxicities in animals and humans.
There are some chemicals exhibiting mitigation activity to reduce the acrylamide contents in food, of which phytoceuticals and phytomedicines have been studied and proved to possess such activity via different mechanisms, such as free radical scavenging activity.
Beside the positive role of antioxidants in acrylamide reduction in food, the exact role of antioxidants in this regard is still under question due to some controversial reports.
Antioxidative activity is one of the probable mechanisms, by which phenolic-containing herbal medicines are able to reduce the acrylamide toxicity in both in vitro and in vivo studies, although other mechanisms such as anti-apoptotic, anti-inflammatory, and the reduction of chemical absorption due to enhanced fecal passage are suggested.
Acknowledgments
This paper is the outcome of a financially non-supported study. Special thanks to Miss Sherlin Haghnazari for revising the English language of this article.
Conflict of interest statement
Authors have no competing interests.
References
- Abdel-Monem S, Ismial A, Mohammed Ali RF, Askar M, Mahmoud Samy W. Impact of pre-treatments on the acrylamide formation and organoleptic evolution of fried potato chips. Am J Biochem Biotech. 2013;9:90–110. doi: 10.3844/ajbbsp.2013.90.101. [DOI] [Google Scholar]
- Acrylamide: a natural process in cooking (June 2010). Food insights, your nutrition and food safety resource. http://www.foodinsight.org/Newsletter/Detail.aspx?topic=Acrylamide_A_Natural_Process_in_Cook (Last access Feb10, 2014)
- Alturfan AA, Tozan-Beceren A, Sehirli AO, Demiralp E, Sener G, Omurtag GZ. Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol Biol Rep. 2012;39:4589–4596. doi: 10.1007/s11033-011-1249-5. [DOI] [PubMed] [Google Scholar]
- Arribas-Lorenzo G, Fogliano V, Morales FJ. Acrylamide formation in a cookie system as influenced by the oil phenol profile and degree of oxidation. Eur Food Res Technol. 2009;229:63–72. doi: 10.1007/s00217-009-1026-z. [DOI] [Google Scholar]
- Arribas-Lorenzo G, Morales FJ. Recent insights in acrylamide as carcinogen in foodstuffs. In: Fishbein JC, Heilman JM, editors. Advances in molecular toxicology. New York: Elsevier; 2012. pp. 163–184. [Google Scholar]
- Bassama J, Brat P, Bohuon P, Boulanger R, Günata Z. Study of acrylamide mitigation in model system: Effect of pure phenolic compounds. Food Chem. 2010;123:558–562. doi: 10.1016/j.foodchem.2010.04.071. [DOI] [Google Scholar]
- Cai Y, Zhang Z, Jiang S, Yu M, Huang C, Qiu R, Zou Y, Zhang Q, Oua S, Zhoua H, Wang Y, Bai W, Li Y. Chlorogenic acid increased acrylamide formation through promotion of HMF formation and 3-aminopropionamide deamination. J Hazard Mater. 2014;268:1–5. doi: 10.1016/j.jhazmat.2013.12.067. [DOI] [PubMed] [Google Scholar]
- Cao J, Liu Y, Jia L, Jiang L, Geng CY, Yao XF, Kong Y, Jiang BN, Zhong LF. Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J Agric Food Chem. 2008;56:12059–12063. doi: 10.1021/jf8026827. [DOI] [PubMed] [Google Scholar]
- Capuano E, Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): a review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci Technol. 2011;44:793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- Casado FJ, Sanchez AH, Montano A. Reduction of acrylamide content of ripe olives by selected additives. Food Chem. 2010;119:161–166. doi: 10.1016/j.foodchem.2009.06.009. [DOI] [Google Scholar]
- Chen W, Feng L, Shen Y, Su H, Li Y, Zhuang J, Zhang L, Zheng X. Myricitrin inhibits acrylamide-mediated cytotoxicity in human Caco-2 cells by preventing oxidative stress. BioMed Res Int. 2013;2013:724183. doi: 10.1155/2013/724183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ren Y, Zhang Y, Zhang Y. Effect of flavonol on reduction of acrylamide in Maillard reaction and its correlation with antioxidant capacity. J Zhejiang Univ. 2013;39:178–184. doi: 10.1631/jzus.B1200041. [DOI] [Google Scholar]
- Cheng KW, Zeng X, Tang YS, Wu JJ, Liu Z, Sze KH. Inhibitory mechanism of naringenin against carcinogenic acrylamide formation and nonenzymatic browning in Maillard model reactions. Chem Res Toxicol. 2009;22:1483–1489. doi: 10.1021/tx9001644. [DOI] [PubMed] [Google Scholar]
- Cheng KW, Shi JJ, SY OU, Wang M, Jiang Y. Effects of fruit extracts on the formation of acrylamide in model reactions and fried potato crisps. J Agric Food Chem. 2010;58:309–312. doi: 10.1021/jf902529v. [DOI] [PubMed] [Google Scholar]
- Cheng-mei, J., Wen-hong, Z., Hong, Z., Ling, S., Dan-ni, W., 2009. The effect of genistein on cerebellar neuronal apoptosis induced by acrylamide. J. Bengbu Med. College, 01. http://en.cnki.com.cn/Article_en/CJFDTOTAL-BANG200901001.htm
- CIAA (2011). Acrylamide toolbox 2011. http://www.fooddrinkeurope.eu/uploads/ publications_documents/Toolboxfinal260911.pdf. (Last access: Feb 10, 2014)
- Ciesarova Z, Suhaj M, Horvathova J. Correlation between acrylamide contents and antioxidant capacities of spice extracts in a model potato matrix. J Food Nutr Res. 2008;47:1–5. [Google Scholar]
- Claeys W, Baert K, Mestdagh F, Vercammen J, Daenens P, De Meulenaer B, Maghuin-Rogister G, Huyghebaert A. Assessment of the acrylamide intake of the Belgian population and the effect of mitigation strategies. Food Addit Contam. 2010;27(9):1199–1207. doi: 10.1080/19440049.2010.489577. [DOI] [PubMed] [Google Scholar]
- Dobrowolski P, Huet P, Karlsson P, Eriksson S, Tomaszewska E, Gawron A, Pierzynowski SG. Potato fiber protects the small intestinal wall against the toxic influence of acrylamide. Nutrition. 2012;28:428–435. doi: 10.1016/j.nut.2011.10.002. [DOI] [PubMed] [Google Scholar]
- El-Halim SS, Mohamed MM. Garlic powder attenuates acrylamide-induced oxidative damage in multiple organs in rat. J Appl Sci Res. 2012;8:168–173. [Google Scholar]
- El-Shafey AAM, Seddek MN, El-Ezaby MM, Seliem MME, Abd El-Maksoud MAE. Protctive effects of garlic “Allium sativum” and karkada “Hibiscus sabdarrifa” on acrylamide treated male albino rats. Egypt J Exp Biol. 2013;9:101–107. [Google Scholar]
- El-Tantavi HGM. The protective role of ginger (Zingiber officinale) against acrylamide induced neurotoxicity in mice. Egypt J Histol. 2007;30:325–336. [Google Scholar]
- Evers, D., Deuber, H., 2012. Potato antioxidant compounds: Impact of cultivation methods and relevance for diet and health, nutrition, well-being and health, in: Bouayed, J. (Ed), Nutrition, InTech, doi:10.5772/31077
- Friedman M, Levin CE. Review of methods for the reduction of dietary content and toxicity of acrylamide. J Agr Food Chem. 2008;56:6113–6140. doi: 10.1021/jf0730486. [DOI] [PubMed] [Google Scholar]
- Ghareeb DA, Khalil AA, Elbassoumy AM, Hussien HM, Abo-Sraia MM. Ameliorated effects of garlic (Allium sativum) on biomarkers of subchronic acrylamide hepatotoxicity and brain toxicity in rats. Tox Environ Chem. 2010;92:1357–1372. doi: 10.1080/02772240903348187. [DOI] [Google Scholar]
- Gobel A, Kliemant A. The German minimization concept for acrylamide. Food Addit Contamin. 2007;24(S1):82–90. doi: 10.1080/02652030701452116. [DOI] [PubMed] [Google Scholar]
- Hamzalioglu A, Mogol BA, Lumaga RB, Fogliano V, Gokmen V. Role of curcumin in the conversion of asparagine into acrylamide during heating. Amino Acids. 2013;44:1419–1426. doi: 10.1007/s00726-011-1179-5. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) Some industrial chemicals. IARC Monogr Eval Carcinog Risk Chem Hum. 1994;60:389–433. [Google Scholar]
- Jin C, Wu X, Zhang Y. Relationship between antioxidants and acrylamide formation: a review. Food Res Int. 2013;51:611–620. doi: 10.1016/j.foodres.2012.12.047. [DOI] [Google Scholar]
- Kahkeshani N, Farahanikia B, Mahdaviani P, Abdolghaffari A, Hassanzadeh G, Abdollahi M, Khanavi M. Antioxidant and burn healing potential of Galium odoratum extracts. Res Pharm Sci. 2013;8:197–203. [PMC free article] [PubMed] [Google Scholar]
- Kalita D, Holm DG, Jayanty SS. Role of polyphenols in acrylamide formation in the fried products of potato tubers with colored flesh. Food Res Int. 2013;54:753–759. doi: 10.1016/j.foodres.2013.08.023. [DOI] [Google Scholar]
- Khan MR, Afzaal M, Saeed N, Shabbir M. Protective potential of methanol extract of Digera muricata on acrylamide induced hepatotoxicity in rats. Afr J Biotech. 2011;10:8456–8464. doi: 10.5897/AJB10.2194. [DOI] [Google Scholar]
- Kocadagl T, Goncuoglu N, Hamzalioglu A, Gökmen V. In depth study of acrylamide formation in coffee during roasting: role of sucrose decomposition and lipid oxidation. Food Function. 2012;3:970–975. doi: 10.1039/c2fo30038a. [DOI] [PubMed] [Google Scholar]
- Kotsiou K, Tasioula-Margari M, Capuano E, Fogliano V. Effect of standard phenolic compounds and olive oil phenolic extracts on acrylamide formation in an emulsion system. Food Chem. 2011;124:242–247. doi: 10.1016/j.foodchem.2010.06.025. [DOI] [Google Scholar]
- Kotsiou K, Tasioula-Margari M, Kukurova K, Ciesarova Z. Impact of oregano and virgin olive oil phenolic compounds on acrylamide content in a model system and fresh potatoes. Food Chem. 2010;123:1149–1155. doi: 10.1016/j.foodchem.2010.05.078. [DOI] [Google Scholar]
- Lee KY, Shibutani M, Kuroiwa K, Takagi H, Inoue K, Nishikawa H, Miki T, Hirose M. Chemoprevention of acrylamide toxicity by antioxidative agents in rats-effective suppression of testicular toxicity by phenylethyl isothiocyanate. Arch Toxicol. 2005;79:531–541. doi: 10.1007/s00204-005-0656-6. [DOI] [PubMed] [Google Scholar]
- Li D, Chen Y, Zhang Y, Lu B, Jin C, Wu X, Zhang Y. Study on mitigation of acrylamide formation in cookies by 5 antioxidants. J Food Sci. 2012;77:1144–1149. doi: 10.1111/j.1750-3841.2012.02949.x. [DOI] [PubMed] [Google Scholar]
- Lineback DR, Coughlin JR, Stadler RH. Acrylamide in foods: a review of the science and future considerations. Annu Rev Food Sci Technol. 2012;3:15–35. doi: 10.1146/annurev-food-022811-101114. [DOI] [PubMed] [Google Scholar]
- Liu, C., Jiang C., Zhou, L., 2012. Protective effect of epigallocatechin-3-gallate on apoptosis of rat cerebellar granule neurons induced by acrylamide. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 37, 944–950 [DOI] [PubMed]
- Lopachin RM. The changing view of acrylamide neurotoxicity. Neurotoxicology. 2004;25:617–630. doi: 10.1016/j.neuro.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Mademtzoglou D, Haza AI, Coto AL, Morales P. Rosemary, heather and heterofloral honeys protect towards cytotoxicity of acrylamide in human hepatoma cells. Revista Complutense de Ciencias Veterinarias. 2010;4:12–32. [Google Scholar]
- Manayi A, Saeidnia S, Gohari AR, Abdollahi M (2014) Methods for the discovery of new anti-aging products - targeted approaches. Expert Opin. Drug Discov. [Epub ahead of print] [DOI] [PubMed]
- Mannaa F, Abdel-Wahhab AM, Ahmed HH, Park MH (2006) Protective role of Panax ginseng extract standardized with ginsenoside Rg3 against acrylamide-induced neurotoxicity in rats. J. Appl. Toxicol. 26, 198–206 [DOI] [PubMed]
- Markov L, Ciesarov Z, Kukurov K, Zielinski H, Przygodzka M, Bednrikov A, Simko P. Influence of various spices on acrylamide content in buckwheat ginger cakes. Chem Papers. 2012;66:949–954. [Google Scholar]
- Mehri S, Abnous K, Mousavi SH, Motamed Shariaty V, Hosseinzadeh H. Neuroprotective Effect of Crocin on Acrylamide-induced Cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–235. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehri, S., Karami, H.V., Vahdati Hassani, F., Hosseinzadeh, H., 2014. Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo Assessments. Iran. Biomed. J. 18, doi:10.6091/ibj.1291.2013 [DOI] [PMC free article] [PubMed]
- Mogda KM, Ibrahim EM, El-Kholy MM, El-Madawy SA. Antioxidant and histopathological effect of catechin and neem leaves extract in acrylamide toxicity of rats. Egypt J Comp Path Clinic Path. 2008;21:290–313. [Google Scholar]
- Mostafalou S, Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Morales F, Sacchi R, Fogliano V. Relationship between virgin olive oil phenolic compounds and acrylamide formation in fried crisps. J Agric Food Chem. 2008;56:2034–2040. doi: 10.1021/jf0730082. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program, Report on carcinogens. (2011). Twelfth Edition, pp. 25–28
- Office of Pollution Prevention and Toxics. Chemical Summary for Acrylamide. United States Environmental Protection Agency. EPA 749-F-94-005a. (Last access: Feb12, 2014)
- Oral AR, Dogan M, Sarioglu K. Effects of certain polyphenols and extracts on furans and acrylamide formation in model system, and total furans during storage. Food Chem. 2014;142:423–429. doi: 10.1016/j.foodchem.2013.07.077. [DOI] [PubMed] [Google Scholar]
- Pelucchi C, Vecchia CL, Bosetti C, Boyle P, Boffetta P. Exposure to acrylamide and human cancer-a review and meta-analysis of epidemiologic studies. Ann Oncol. 2011;22:1487–1499. doi: 10.1093/annonc/mdq610. [DOI] [PubMed] [Google Scholar]
- Prasad SN, Muralidhara M. Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster – Its amelioration with spice active enrichment: Relevance to neuropathy. NeuroToxicology. 2012;33:1254–1264. doi: 10.1016/j.neuro.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Prasad SN, Muralidhara M. Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochem Res. 2013;38:330–345. doi: 10.1007/s11064-012-0924-9. [DOI] [PubMed] [Google Scholar]
- Prasad SN, Muralidhara M. Neuroprotective effect of geraniol and curcumin in an acrylamide model of neurotoxicity in Drosophila melanogaster: Relevance to neuropathy. J Insect Physiol. 2014;60:7–16. doi: 10.1016/j.jinsphys.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rice JM. The carcinogenicity of acrylamide. Mutat Res. 2005;580:3–20. doi: 10.1016/j.mrgentox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ramiro I, Angeles Martin M, Ramos S, Bravo L, Goya L. Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress. Toxicology. 2011;288:43–48. doi: 10.1016/j.tox.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ramiro I, Ramos S, Bravo L, Goya L, Angeles Martin M. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J Nutr Biochem. 2011;22:1186–1194. doi: 10.1016/j.jnutbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Saeidnia S, Abdollahi M. Antioxidants: friends or foe in prevention or treatment of cancer: the debate of the century. Toxicol Appl Pharmacol. 2013;271:49–63. doi: 10.1016/j.taap.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Saeidnia S, Abdollahi M. Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicol Appl Pharmacol. 2013;273:442–455. doi: 10.1016/j.taap.2013.09.031. [DOI] [PubMed] [Google Scholar]
- Saeidnia S, Abdollahi M. Who plays dual role in cancerous and normal cells? Natural antioxidants or free radicals or the cell environment. Int J Pharmacol. 2012;8:711–712. doi: 10.3923/ijp.2012.711.712. [DOI] [Google Scholar]
- Sadek KM. Antioxidant and immunostimulant effect of Carica papaya L. aqueous extract in acrylamide intoxicated rat. Acta Inform Med. 2012;20:180–185. doi: 10.5455/aim.2012.20.180-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R, Arambula-Villa G, Hidalgo FJ, Zamora R. Mitigating effect of piquin pepper (Capsicum annuum L. var. Aviculare) oleoresin on acrylamide formation in potato and tortilla chips. LWT Food Sci. Technol. 2012;48:261–267. [Google Scholar]
- Salazar R, Arambula-Villa G, Vazquez-Landaverde P, Hidalgo FJ, Zamora R. Mitigating effect of amaranth (Amarantus hypochondriacus) protein on acrylamide formation in foods. Food Chem. 2012;135:2293–2298. doi: 10.1016/j.foodchem.2012.06.089. [DOI] [PubMed] [Google Scholar]
- Shinomol GK, Raghunath N, Bharath MMS, Muralidhara M. Prophylaxis with Bacopa monnieri attenuates acrylamide induced neurotoxicity and oxidative damage via elevated antioxidant function. Cent Nerv Syst Agents Med Chem. 2013;13:3–12. doi: 10.2174/1871524911313010003. [DOI] [PubMed] [Google Scholar]
- Shukla PK, Khanna VK, Ali MM, Maurya RR, Handa SS, Srimal RC. Protective effect of Acorus calamus against acrylamide induced neurotoxicity. Phytother Res. 2002;16:256–260. doi: 10.1002/ptr.854. [DOI] [PubMed] [Google Scholar]
- Sigma-Aldrich Co. (2014) Acrylamide. http://www.sigmaaldrich.com/catalog/product/fluka/01700?lang=en®ion=CA (Last access: Feb 6, 2014)
- Simonne, A.H, Archer, D.L. Acrylamide in foods: a review and update. University of Florida. IFAS Extention. FCS8759. http://edis.ifas.ufl.edu/fy578. (Last access: Feb1, 2014)
- Soliman GZA. Protective effect of Solanum nigrum, vitamin C or melatonin on the toxic effect of acrylamide on rats. J Pharm Biol Sci. 2013;5:47–54. [Google Scholar]
- Song J, Zhao M, Liu X, Zhu Y, Hu X, Chen F. Protection of cyanidin-3-glucoside against oxidative stress induced by acrylamide in human MDA-MB-231 cells. Food Chem Toxicol. 2013;58:306–310. doi: 10.1016/j.fct.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Tareke E, Rydberg P, Karlsson P, Ericksson S, Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50:4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- Totlani VM, Peterson DG. Reactivity of epicatechin in aqueous glucoseglycine Maillard model system: Quenching of C2, C3 and C4 sugar fragments. J Agric Food Chem. 2005;53:4130–4135. doi: 10.1021/jf050044x. [DOI] [PubMed] [Google Scholar]
- Totlani VM, Peterson DG. Epicatechin carbonyl-trapping reactions in aqueous Maillard systems: Identification and structural elucidation. J Agric Food Chem. 2006;54:7311–7318. doi: 10.1021/jf061244r. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Friedman MA. Effects of acrylamide on rodent reproductive performance. Reprod Toxicol. 2003;17:1–13. doi: 10.1016/S0890-6238(02)00078-3. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Marr MC, Myers CB, Ross WP, Friedman MA. Relationship between acrylamide reproductive and neurotoxicity in male rats. Reprod Toxicol. 2000;14:147–157. doi: 10.1016/S0890-6238(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Urbancic, S., Kolar, M.H., Dimitrijevic, D., Vidrih, R., 2013. Stabilisation of sunflower oil and reduction of acrylamide formation of potato rosemary extract during deep-fat frying, LWT Food Sci. Technol. http://dx.doi.org/10.1016/j.lwt.2013.11.002
- Vanitha, S., Thiagarajan, V.R.K., Muthuraman, A., Krishnan, S., Aruna, A., Tharabai, R., 2012. Pharmacological evaluation of methanolic leaf extract of Swietenia mahagoni on acrylamide-induced neuropathic pain in rats. Toxicol. Ind. Health. 1–10, doi: 10.1177/0748233713491808 [DOI] [PubMed]
- Wang H, Zhou Y, Ma J, Zhou Y, Jian H. The effects of phytic acid on the Maillard reaction and the formation of acrylamide. Food Chem. 2013;141:18–22. doi: 10.1016/j.foodchem.2013.02.107. [DOI] [PubMed] [Google Scholar]
- Wenzl T, Aklam E. EU database of acrylamide levels in food- update and critical review of data collection. Food Addit Contam. 2007;24(S1):5–12. doi: 10.1080/02652030701216479. [DOI] [PubMed] [Google Scholar]
- Woo GH, Shibutani M, Kuroiwa K, Lee KY, Takahashi M, Inoue K, Fujimoto H, Hirose M. Lack of preventive effects of dietary fibers or chlorophyllin against acrylamide toxicity in rats. Food Chem Toxicol. 2007;45:1507–1515. doi: 10.1016/j.fct.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Xichun Z, Minai Z. Protective role of dark soy sauce against acrylamide-induced neurotoxicity in rats by antioxidative activity. Toxicol Mech Methods. 2009;19:369–374. doi: 10.1080/15376510902806167. [DOI] [PubMed] [Google Scholar]
- Yassa HA, George SM, Refaiy AEM, Abdel Moneim EM. Camellia Sinensis (Green Tea) extract attenuate acrylamide induced testicular damage in Albino rats. Environ Toxicol. 2013 doi: 10.1002/tox.21846. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shu C, Zhou B, Qi X, Xiang J. Impact of selected additives on acrylamide formation in asparagine/sugar Maillard model systems. Food Res Int. 2011;44:449–455. doi: 10.1016/j.foodres.2010.09.025. [DOI] [Google Scholar]
- Zamorano-Ponce E, Morales C, Ramos D, Sepulveda C, Cares S, Rivera P, Fernandez J, Carballo MA. Anti-genotoxic effect of Aloysia triphylla infusion against acrylamide-induced DNA damage as shown by the comet assay technique. Mut Res. 2006;603:145–150. doi: 10.1016/j.mrgentox.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cao J, Jiang L, Geng C, Zhong L. Protective effect of hydroxytyrosol against acrylamide-induced cytotoxicity and DNA damage in HepG2 cells. Mut Res. 2009;664:64–68. doi: 10.1016/j.mrfmmm.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen J, Zhang X, Wu X, Zhang Y. Addition of Antioxidant of Bamboo Leaves (AOB) Effectively reduces acrylamide formation in potato crisps and french fries. J Agric Food Chem. 2007;55:523–528. doi: 10.1021/jf062568i. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang L, Geng C, Yoshimura H, Zhong L. Inhibition of acrylamide genotoxicity in human liver-derived HepG2 cells by the antioxidant hydroxytyrosol. Chem Biol Interact. 2008;176:173–178. doi: 10.1016/j.cbi.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ren YP, Zhang Y. New research developments on acrylamide: analytical chemistry, Formation mechanism, and mitigation recipes. Chem Rev. 2009;109:4375–4397. doi: 10.1021/cr800318s. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang E, Chen F, Yan H, Yuan Y. Potential protective effects of oral administration of allicin on acrylamide induced toxicity in male mice. Food Funct. 2013;4:1229–1236. doi: 10.1039/c3fo60057b. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu W, Wu X, Zhang X, Zhang Y. Addition of antioxidant from bamboo leaves as an effective way to reduce the formation of acrylamide in fried chicken wings. Food Addit Contam. 2007;24:242–251. doi: 10.1080/02652030601064839. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ying T, Zhang Y. Reduction of acrylamide and its kinetics by addition of antioxidant of bamboo leaves (AOB) and extract of green tea (EGT) in asparagine–glucose microwave heating system. J Food Sci. 2008;73:60–66. doi: 10.1111/j.1750-3841.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y. Effect of natural antioxidants on kinetic behavior of acrylamide formation and elimination in low-moisture asparagine–glucose model system. J Food Eng. 2008;85:105–115. doi: 10.1016/j.jfoodeng.2007.07.013. [DOI] [Google Scholar]
- Zhang Y, Zhang Y. Formation and reduction of acrylamide in Maillard reaction: A review based on the current state of knowledge. Crit Rev Food Sci Nutr. 2007;47:521–542. doi: 10.1080/10408390600920070. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y. Study on reduction of acrylamide in fried bread sticks by addition of antioxidant of bamboo leaves and extract of green tea. Asia Pac J Clin Nutr. 2007;16:131–136. [PubMed] [Google Scholar]
- Zhang L, Zhang H, Miao Y, Wu S, Ye H, Yuan Y. Protective effect of allicin against acrylamide-induced hepatocyte damage in vitro and in vivo. Food Chem Toxicol. 2012;50:3306–3312. doi: 10.1016/j.fct.2012.05.060. [DOI] [PubMed] [Google Scholar]
- Zhu F, Cai YZ, Keb J, Corkea H. Compositions of phenolic compounds, amino acids and reducing sugars in commercial potato varieties and their effects on acrylamide formation. J Sci Food Agric. 2010;90:2254–2262. doi: 10.1002/jsfa.4079. [DOI] [PubMed] [Google Scholar]
- Zhu F, Cai YZ, Keb J, Corke H. Dietary plant materials reduce acrylamide formation in cookie and starch-based model systems. J Sci Food Agric. 2011;9:2477–2483. doi: 10.1002/jsfa.4491. [DOI] [PubMed] [Google Scholar]
- Zhu F, Cai YZ, Keb J, Corkea H. Evaluation of the effect of plant extracts and phenolic compounds on reduction of acrylamide in an asparagine/glucose model system by RP-HPLC-DAD. J Sci Food Agric. 2009;89:1674–1681. doi: 10.1002/jsfa.3640. [DOI] [Google Scholar]