Abstract
The pleothera of micro organisms obtained from contaminated food cultured in a starch broth was effectively tested against antibacterial agents, i.e. nisin, lysozyme and chelating agent EDTA. A variety of combination treatments of these antimicrobial agents and their incorporation in Starch based active packaging film according to their permissibility standards was done. 4 variables of Nisin concentration (ranging from 0 to 750 IU/ml), 3 variables of lysozyme concentration (ranging from 0 to 500 IU/ml) and 3 variables of EDTA concentration from (0 to 20 μM) were chosen. Bacterial inhibition by combination of different levels of different factors without antimicrobial films was evaluated using a liquid incubation method. The samples were assayed for turbidity at interval of 2, 4 and 24 h to check effectiveness of combined effects of antimicrobial agents which proved a transitory bactericidal effect for short incubation times. Zone of Inhibition was observed in the antimicrobial films prepared by agar diffusion method. Statistical analysis of experimental data for their antimicrobial spectrum was carried out by multi regression analysis and ANOVA using Design-Expert software to plot the final equation in terms of coded factors as antimicrobial agents. The experimental data indicated that the model was highly significant. Results were also evaluated graphically using response surface showing interactions between two factors, keeping other factor fixed at values at the center of domain. Synergy was also determined among antibacterial agents using the fractional inhibitory concentration (FIC) index which was observed to be 0.56 supporting the hypothesis that nisin and EDTA function as partial synergistically. The presented work aimed to screen in quick fashion the combinatorial effect of three antimicrobial agents and evaluating their efficacy in anti microbial film development.
Keywords: Active packaging, Antimicrobial activity, Ethylenediaminetetraacetate, Food preservation, Lysozyme, Nisin, Response Surface Methodology
Introduction
Active packaging is one of the innovative food packaging concepts that have been introduced as a response to the changes in current consumer demands and market trends. Packaging is termed as “active” when it performs some desired role other than to provide an inert barrier to the external environment (Hotchkiss 1997). The extra functions include oxygen scavenging, antimicrobial (AM) activity, moisture scavenging, ethylene scavenging, ethanol emitting and so on which extend shelf-life or improve safety or sensory properties while maintaining the quality of the food. Recent food-borne microbial outbreaks are driving a search for innovative ways to inhibit microbial growth in the food while maintaining quality, freshness and safety. AM food packaging is one of the special applications of active food packaging that controls inside food and atmospheric conditions actively and responsively (Muhammad et al. 2005).
Nisin, a naturally occurring antimicrobial peptide was discovered in 1928 (Hurst 1967; Monteville and Chen 1998). Nisin is produced by certain strains of Lactococcus lactis and is the only lanthionine bacteriocin with Food and Drug Administration approved GRAS (Generally Regarded As Safe; US21CFR170.30-Food Additives) status (FDA 1988) and now used as a biopreservative in 57 countries around the world. Nisin’s antimicrobial mechanism has been extensively studied and well documented (Winkowski et al. 1996). Nisin inhibits spore germination and growth of gram positive bacteria (Vessoni Penna and Moraes 2002; Thomas et al. 2002). Nisin first binds to the cell membrane through ionic interactions of the C-terminus and then forms pores in the membrane by the penetration of the hydrophobic N-terminus. This results in disruption of the proton motive force and leakage of cellular materials (Bruno et al. 1992; Okereke and Montville 1992; Breukink et al. 1998). The effectiveness of nisin against gram-negative cells is generally low owing to the inability of nisin to cross lipo-polysaccharide (LPS) outer membrane. However, when used in combination with chelators such as ethylenediaminetetraacetic acid (EDTA), nisin is also effective against gram-negative bacteria (Stevens et al. 1991; Branen and Davidson 2004). Nisin solubility and stability increases substantially with increasing acidity. Nisin is stable at pH 2 and can be autoclaved at 121 °C. Under alkaline pH there is an increasing loss of activity, with complete inactivation after 30 min at 63 °C at pH 11 (Hansen et al. 1991). Because it is non-toxic, heat stable and does not contribute to off-flavours, nisin is commercially used in a variety of foods including dairy, eggs, vegetables, meat, fish, beverages and cereal-based products to inhibit growths of foodborne pathogens including Listeria monocytogenes (Schillinger et al. 1996).
Lysozyme, has also gained considerable interest for use in food preservative and packaging systems, because it is a natural enzyme produced by animals and it activity targets a specific cellular structure of microorganisms. It is a single peptide protein with a molecular weight of 14.6 kDa, which catalyzes the hydrolysis of 1,4-glycosidic linkages between N-acetylmuramic acid and N-acetylglucosamine of cell wall peptidoglycan (Proctor and Cunningham 1988). However, the application of lysozyme in foods has been limited, because gram-negative bacteria are protected by an outer membrane, which is relatively resistant to antimicrobial activities of lysozyme (Holley and Gill 2003).
Ethylenediaminetetraacetate has been used in cell lysis protocol and several food products as a chelating agent to prevent oxidation (Hansen et al. 2001). Although the mechanism of antibacterial effectiveness by EDTA is not fully understood, its chelating property to divalent cations like Ca2+ or Mg2+ should be involved (Shelef and Seiter 1993). EDTA facilitate the detachment of cells from biofilm and enhance the killing of biofilm-producing microorganisms by depriving Mg2+ associated with lipopolysaccharides and depriving Ca2+, and Fe2+, which are essential factors for microbial growth (Banin et al. 2006).
Direct addition of nisin into foods results in an immediate reduction of bacterial populations but may not prevent the recovery of injured cells or the growth of cells that were not destroyed by direct addition if residues of the antimicrobial are rapidly depleted (Zhang et al. 2004). Nisin, at various concentrations alone and with other antimicrobial agents incorporated into polyethylene or other edible polymer films, was effective against various microorganisms, including L. plantarum, L. monocytogenes, E. coli and Salmonella spp. (Padgett et al. 1998; Cutter et al. 2001; Eswaranandam et al. 2004). Combining the bacteriocin directly into a polymer material may provide several advantages as a bacteriocin delivery mechanism. First, only the necessary amount of bacteriocin, chelating agent would be used. Secondly, these agents would not be a direct additive to the food product, but may be classified as an indirect food additive. Thirdly, as the plasticizer material containing the nisin, lysozyme and EDTA was made from an edible and biodegradable starch polymer, environmental advantages associated with disposal and breakdown of plastic would be circumvented (Siragusa et al. 1999).
Food preservation demands combinations of mild treatments and their evaluations. The effectiveness of hurdles in combination with the different microorganisms is tested usually in multifactorial challenge tests. Even with the use of multifactorial test design till now only limited number of test microbes and combined preservation measures were tested. One of the causes is well known: the cell count enumeration based investigations are extremely time consuming and laborious (Kiss et al. 2007).
Statistical methods provide an alternative methodology to optimize a particular process by considering the mutual interactions among the variables and give an estimate of combined effect of these variables on final results. Response surface methodology (RSM) is one such technique based on the fundamental principles of statistics, such as randomization, replication and duplication, which simplifies the optimization by studying the mutual interactions among the variables over a range of values in a statistically valid manner. This is generally known as full factorial design. Application of factorial designs and RSM is a common practice in biotechnology for the optimization of media components and culture conditions. These optimization methods involve four major steps; performing statistically designed experiments, estimating coefficients in a mathematical model, predicting the response, and checking the adequacy of the model. Availability of user-friendly software packages has made this technique increasingly popular for optimization studies (Chauhan et al. 2006). The objective of the present study is to screen in quick fashion the combinatorial effect of these three antimicrobial agents and also evaluating their efficacy in anti microbial film development.
Materials and methods
Microbial source
The food sample mixture containing cooked rice with pulses with approximate composition of 60–70 % carbohydrate, 10–20 % protein, 10 % moisture and 2 % fat content were allowed to get contaminated from the open environmental sources for 2 days. After serial dilution, spread plate technique was employed and confluent layer of cells were grown on petri plates containing Starch media and and on screening by gram staining (Gram 1884), majority of the consortia was observed to be gram negative cocci. Culture was maintained in Starch broth (0.5 % peptone, 0.3 % beef extract, and 0.2 % starch) at 4 °C.
Antimicrobials agents
Nisin obtained from (Himedia, Mumbai, India) was reported to be 2.5 % pure with the remaining components listed being sodium chloride and denatured milk solids. Lysozyme was obtained from Medox, India and disodium EDTA was obtained from Remeck, India. Standard stock solution of nisin containing 9*104 IU/ml, lysozyme containing 2* 104 U/ml and 100 mM EDTA was prepared separately in 1 X phosphate buffer saline.
Starch/antimicrobial agents film preparation
For antimicrobial film preparation, 10.4 % Starch (Sisco Research Lab, India) was dissolved in 20 % ethanol using magnetic stirrer and 4 % glycerine (CDH) was dispersed into it. This mixture was stirred using magnetic beads and after that, mild heating at 40 ºC was done in water bath to dissolve the starch polymer totally and the film solution was divided into equal aliqouts. Different combinations of antimicrobial agents as listed in Table 1 were added to it to set the desired concentration range of chosen three anti microbial agents and 250 μl of the film solution prepared from each of the twelve solution sets was casted onto a thin layer plate overlaid with a parafilm® M and allowed to air dry overnight at room temperature. The films were peeled off and placed on agar plates having culture density set to 106 CFU/ml.
Table 1.
Level wise distribution of retained factors for the determination of the inhibitory activities directly as well as incorporated in starch based antimicrobial film on CFU/ml in Starch Broth media
| Factors | Levels | Experimental values |
|---|---|---|
| Nisin | 4 | 0,2,500,5,000,7,500 IU/ml |
| Lysozyme | 3 | 0, 1,000, 5,000 U/ml |
| EDTA | 3 | 0, 10, 20 mM |
Bacterial inhibition testing
Bacterial inhibition by combination of different levels of different factors without antimicrobial films was evaluated using a liquid incubation method as described by Appendini and Hotchkiss (2002) and with antimicrobial films was done by agar diffusion method (Chung et al. 2003). For the liquid incubation test, working cultures were prepared by keeping the initial cell concentration at 106 CFU/ml and incubating with different combinations of antibacterial agents at 37 °C, 180 rpm and turbidity was evaluated after 2, 4, 24 and 48 h. One milliliter of the inoculated medium was sampled at each sampling time. Inoculated medium without any antimicrobial agent served as a positive control. In the agar diffusion test, each film sample was placed on the surface of a starch agar plate overlaid with the 1.5 % w/v of agar. The seed density of overlay was approximately 106 CFU/mL. The agar plates were incubated at 37 °C for 24 h. Diameters of inhibition zone around film specimen determine antimicrobial activity and diffusivity of agents in each film sample.
Determining fractional inhibitory concentration index
Synergy was determined using the fractional inhibitory concentration (FIC) index. This index is calculated by utilizing the minimun inhibitory concentrations (MIC) of the antimicrobial compounds alone and the respective MIC when the compounds are combined. The formula is ([A]/MICA) + ([B]/MICB), where MICA and MICB are the MIC of the compounds alone and [A] and [B] are the MIC of the compounds when used together. This calculation is interpreted as synergy (<0.5), partial synergy (>0.5 but <0.75), additive effect (>0.75 but <1.0), indifference (>1.0 but <4.0), and antagonism (>4.0) (Berenbaum 1981). FIC index was determined between Nisin and EDTA as antimicrobial agents against food spoilage micro organism.
Microtitre plate assay
A Biorad iMark™ 96 well microplate absorbance reader was used to monitor optical density of culture at 600 nm to determine MIC and FIC index. Three sets of 1:1 serial dilution was prepared from stock of 7,500 IU Nisin, 100 mM EDTA and wells used to evaluate the effects of the two antimicrobials combined contained a 50:50 mixture of each of the two prepared stock solutions in starch broth and culture was added to the wells to achieve a final concentration of 2.5 *105 CFU/mL with a positive control in which no antimicrobial agent was added and keeping starch broth as blank. The microtitre plate was incubated at 37 °C for 2 h and OD at 600 nm was read following a 10-s shake cycle. The sampling was done in evaluation mode where four plate readings per one of measurement are performed and the average data of their plate readings is generated as a plate data.
Statistical analysis
Antimicrobial experiments on spoilage bacteria with different combinations of three factors using liquid incubation method were conducted in triplicate on different days, each observations were noted with and without film treatment (n = 6). Data points were expressed as the mean ± SD. Statistical analysis of experimental data was carried out by multiregression analysis and ANOVA using the Design-Expert® 8.0.7.1 (Stat-Ease Inc., Minneapolis, U.S.A.) Software. After incubation at 37 °C, samples were assayed for turbidity measurement after 2 h, 4 h, 24 h and 48 h. Statistical significance of the polynomial model equation generated was determined by Fisher’s test value, and the proportion of variance explained by the model was given by the multiple coefficient of determination, R squared (R2) i.e. Regression value. The data was also represented graphically by surface responses showing interactions between two factors, while the other factors were fixed at values at the centre of the domain. Values at a significance level of 0.05 was used for experimental design, regression and graphical analysis of the data obtained.
Results and discussion
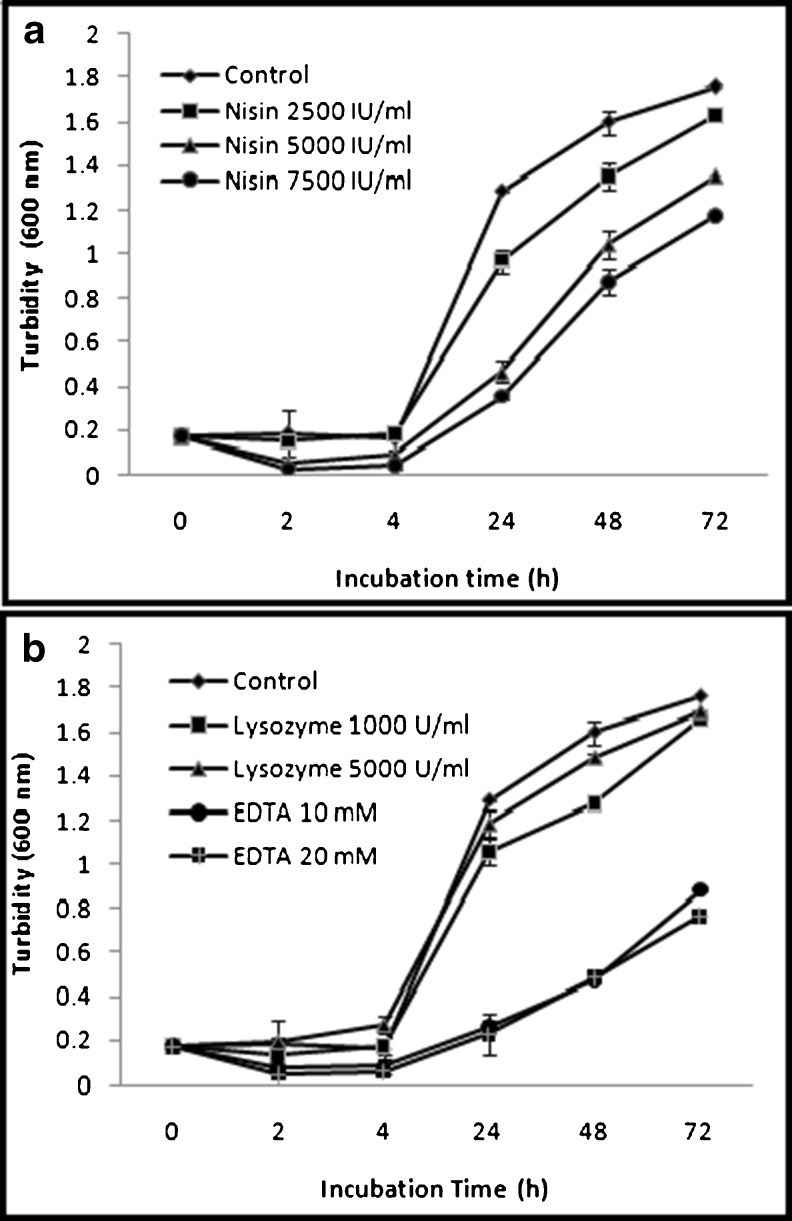
Growth curves of spoilage micro organism in Starch Broth media, with and without addition of antibacterial agents were determined. Figures 1 and 2 shows the inhibitory activity of nisin, lysozyme and EDTA added alone against the growth of micro organism. The maximum reduction was observed up to 4 h. The population of cells treated by agents remained significantly lower than the control through 72 h, in the liquid incubation method. These antimicrobial agents had a transitory bactericidal effect for short incubation times. However, inhibition of growth was not observed during longer incubation times. Similar results had also been observed which had indicated an immediate bactericidal effect, with reduction of a population by 1–3 log cycles on addition of nisin, and with little or no affect upon further incubation (Boussouel et al. 1999; Jin and Zhang 2008; Harris et al. 1991).
Fig. 1.
a. The effect of direct addition of nisin at different concentrations on the growth of spoilage micro organism in comparison to control in starch broth at 37 °C. b. The effect of direct addition of lysozyme and EDTA at different concentration on the growth of spoilage micro organism in starch broth at 37 °C. Error bars represent the SD of the mean from three separate tests
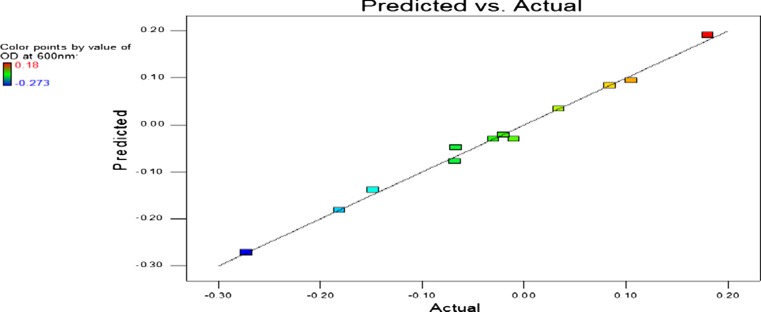
Fig. 2.
Parity plot of predicted versus actual experimental values of the response represented by 3 blocks for determining turbidity of cell growth at 600 nm
Statistical analysis of combined effects of nisin, lysozyme and EDTA on spoilage micro organism in starch media
The results obtained were evaluated using Design Expert 8.0.7.1. Every response of combinatorial antimicrobial agents shown as 12 sets in Table 2 and conducted in triplicates at 2, 4 and 24 h of incubation was represented by a full quadratic model with 7 coefficients for three variables (Table 3) describing interaction terms and relationships between responses and experimental factors are represented with the polynomial quadratic equation:
Table 2.
Fully factorial design consisting of 12 sets for the study of 3 experimental factors in coded units was evaluated using Design Expert 8 software
| Set | Nisin(IU/ml) | Lysozyme(U/ml) | EDTA(mM) |
|---|---|---|---|
| 1 | 7,500 | 5,000 | 20 |
| 2 | 7,500 | 5,000 | 10 |
| 3 | 7,500 | 1,000 | 20 |
| 4 | 7,500 | 1,000 | 10 |
| 5 | 5,000 | 5,000 | 20 |
| 6 | 5,000 | 5,000 | 10 |
| 7 | 5,000 | 1,000 | 20 |
| 8 | 5,000 | 1,000 | 10 |
| 9 | 2,500 | 5,000 | 20 |
| 10 | 2,500 | 5,000 | 10 |
| 11 | 2,500 | 1,000 | 20 |
| 12 | 2,500 | 1,000 | 10 |
Table 3.
Coefficients of the quadratic model for three variables: nisin (from 2,500 to 7,500 IU/ml), lysozyme (1,000 and 5,000 U/ml) and EDTA concentrations (10 and 20 mM) as determined by inhibition assays after incubation at 37 °C for 2, 4 and 24 h
| Factors | 2 h | 4 h | 24 h |
|---|---|---|---|
| Intercept | −0.018 | 0.007 | −0.066 |
| A-Nisin | −0.118 | −0.116 | −0.124 |
| B-Lysozyme | 0.004 | 0.009 | 0.018 |
| C-EDTA | −0.025 | −0.016 | −0.038 |
| AB | −0.035 | −0.042 | 0.000 |
| AC | 0.011 | −0.007 | −0.018 |
| BC | −0.012 | −0.020 | −0.045 |
| A2 | −0.003 | −0.021 | 0.024 |
| R2 | 0.97 | 0.94 | 0.95 |
| R2A | 0.91 | 0.83 | 0.86 |
Where ƞj is the dependent variable; βj0 is the constant coefficient; Xi are the coded independent variables; βji are the linear coefficients, βjij are the second-order interaction coefficients and βjii are the quadratic coefficients.
Coefficients preceded by a negative sign (as indicated in Table 3) signified that the increase in the designed factor decreased the response (antimicrobial activity). As per the parameters in this experiment conduct, the lysozyme with the value of 0.004, showed that it was insufficient to inhibit cell growth when not used in combination. The nisin with negative coefficient value (−0·118) had a significant negative effect (P ≤ 0·001) on the result of the response evaluated and that is anti-microbial effect. EDTA (−0.025) had also significant inhibitory effects (P ≤ 0·06) on cell growth which decreased as nisin impact values (−0.124) and EDTA (−0.038) increased during the first 24 h i.e. the agents had capability to attain their anti microbial effect upto 24 h. For a given concentration of food grade chelator i.e. EDTA, the effect of nisin increased with increasing concentration. EDTA is usually used in combination with other preservatives and promotes the suppression of L. monocytogenes in meat (Monk et al. 1996; Parente et al. 1998), and other pathogenic organisms but sometimes EDTA seems to modulate the antibacterial activity of antimicrobials such as nisin (Zhang and Mustapha 1999). The obtained results from (Corbo et al. 2010) proved antioxidant activity by the combined lysozyme, nisin and EDTA agents as cause for prevention of growth of food spoilage micro organisms.
The parity plot showed a satisfactory correlation between the experimental values and predictive values as shown in Figure 2 wherein, the points cluster around the diagonal line which indicates the good fit of the model, since the deviation between the experimental and predictive values was very less.
The regression equation indicated that coefficient of determination (R- square) was 0.98 (a value of R2 > 0.75 indicates the aptness of the model). The “Predicted R-Squared” of 0.79 is in reasonable agreement with the “Adjusted R-Squared” of 0.94. The experimental data was statistically analyzed using Fischer’s statistical test for ANOVA and the results indicated that the model was highly significant. The Model F-value of 21.86 implies the model is significant (Table 4). There is only a 4.44 % chance that a “Model F-Value” this large could occur due to noise. Values of “Prob > F” less than 0.05 indicate model terms are significant. In this case A: nisin, C: EDTA are significant model terms suggesting this model can be used to navigate the design space. Final Equation designed in Terms of Coded Factors:
Table 4.
Results of ANOVA for response surface quadratic model
| Source | Sum of squares | df | Mean square | F Value | p-value (Prob > F) |
|---|---|---|---|---|---|
| Block | 0.086 | 2 | 0.043 | ||
| Model | 0.091 | 7 | 0.013 | 21.86 | 0.0444 |
| A:Nisin | 0.051 | 1 | 0.051 | 85.84 | 0.0114 |
| B:Lysozyme | 0.000 | 1 | 0.000 | 0.06 | 0.8347 |
| C-EDTA | 0.012 | 1 | 0.012 | 20.07 | 0.0464 |
| AB | 0.011 | 1 | 0.011 | 18.48 | 0.0501 |
| AC | 0.000 | 1 | 0.000 | 0.20 | 0.6963 |
| BC | 0.001 | 1 | 0.001 | 0.99 | 0.4243 |
| A2 | 0.001 | 1 | 0.001 | 1.74 | 0.3177 |
| B2 | 0.000 | 0 | |||
| C2 | 0.000 | 0 | |||
| Residual | 0.001 | 2 | 0.001 | ||
| Cor Total | 0.178 | 11 |
Turbidity value at 600 nm = −0.019−0.011*A + 0.002*B - 0.032*C - 0.04*AB + 0.005*AC - 0.007*BC- 0.021*A2.
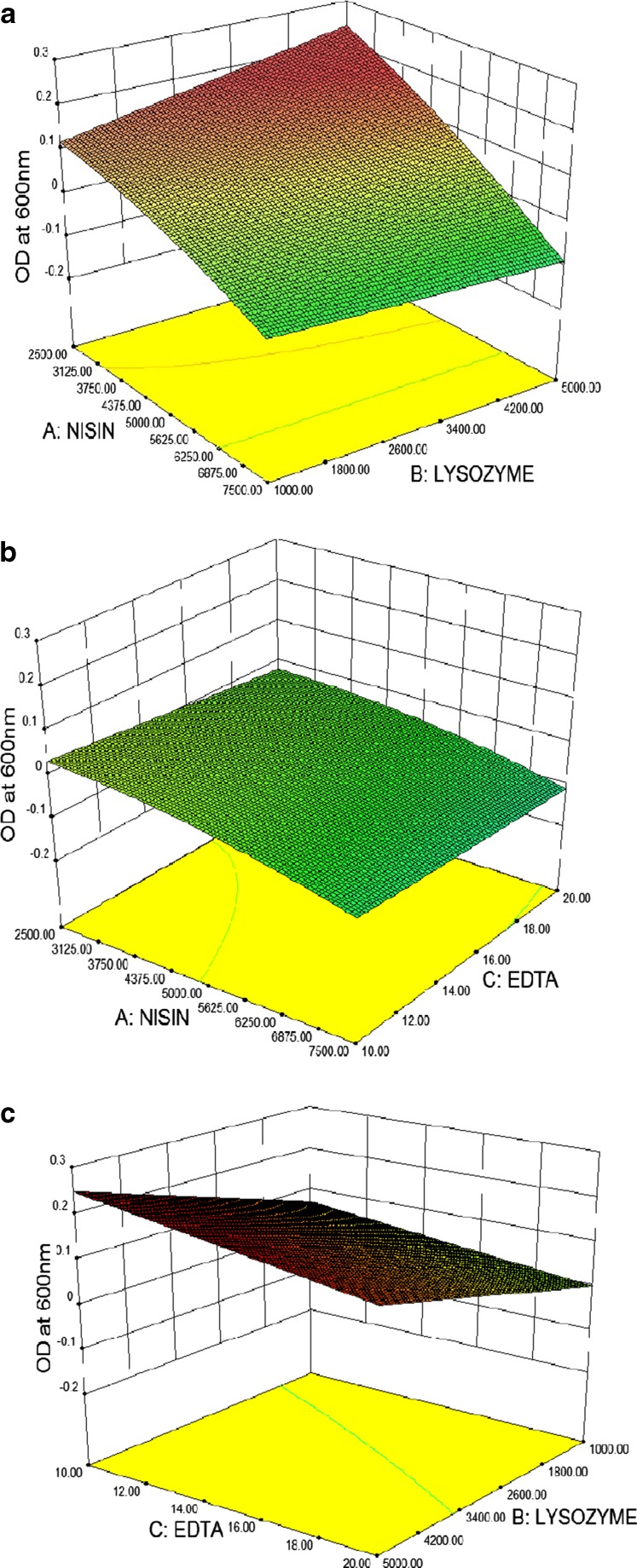
The three dimensional response surfaces were plotted to study the interaction among the three antimicrobial factors selected and to determine the optimum concentration of each for attaining maximum antimicrobial activity. The plots were generated by plotting the response using z-axis against two independent variables while keeping the other independent variables at their 0-level. The coordinates of the central point within the highest contour levels in each of the figures 3 (a, b, c) correspond to the optimum concentrations of the respective components.
Fig. 3.
a. Response surface plot showing the effect of nisin and lysozyme on growth of microbes when EDTA held at zero level. b. Response surface plot showing the effect of nisin and EDTA on growth of microbes when lysozyme held at zero level. C. Response surface plot showing the effect of EDTA and lysozyme on growth of microbes when nisin held at zero level
FIC Index
The results of this study indicate that nisin used in combination with EDTA can be an effective approach to control the growth of food contaminating micro organisms. In the present study, spoilage causing micro organism was inhibited by approximately 1,000 IU/ml of nisin and 100 mM of EDTA in 1 X PBS and starch broth. Ultimately, the permeabilization of the gram-negative cell wall by EDTA would increase the possibility for nisin to be exposed to the inner membrane and cause cell damage or death as referred for other antibacterial peptides (Murdock et al. 2007; Branen and Davidson 2004).
In these studies as reflected in Tables 4 and 5, synergistic inhibition was observed when 468.75 IU/m1 of nisin was used in combination with 6.25 mM of EDTA and 937.5 IU/m1 of nisin and 100 mM EDTA when used alone inhibited or quench the growth. The FIC was observed to be 0.56. These data support the hypothesis that nisin and EDTA function as partial synergistically to inhibit the growth of pathogenic bacteria. This data also suggest that the synergistic effect demonstrated is the result of chelation of ions by EDTA due to which nisin acts well whereas the role of lysozyme was not an impact factor in inhibiting the growth of micro organisms which is also consistent with the work reported by (Holley and Gill 2003) on nutrient media and in contrast to the reported observation of increased antimicrobial activity by combination of EDTA and lysozyme (Shively and Hartsell 1964a, b; Hughey and Johnson 1987; Ellison and Giehl 1991; Payne et al. 1994) in which micro organisms were suspended in buffer, rather than any growth media that corresponds to cells suspended under the conditions of starvation.
Table 5.
Minimum inhibitory concentration (MIC) and fractional inhibitory concentration (FIC) calculations for nisin and EDTA
| For Spoilage micro organism | |
|---|---|
| MIC Alone | |
| Nisin (IU/ml) | 937.5 |
| EDTA (mM) | 100 |
| MIC Combined | |
| Nisin (IU/ml) | 468.75 |
| EDTA (mM) | 6.25 |
| FIC index | 0.56 |
Bacterial inhibition by starch based active packaging film containing nisin, lysozyme and EDTA
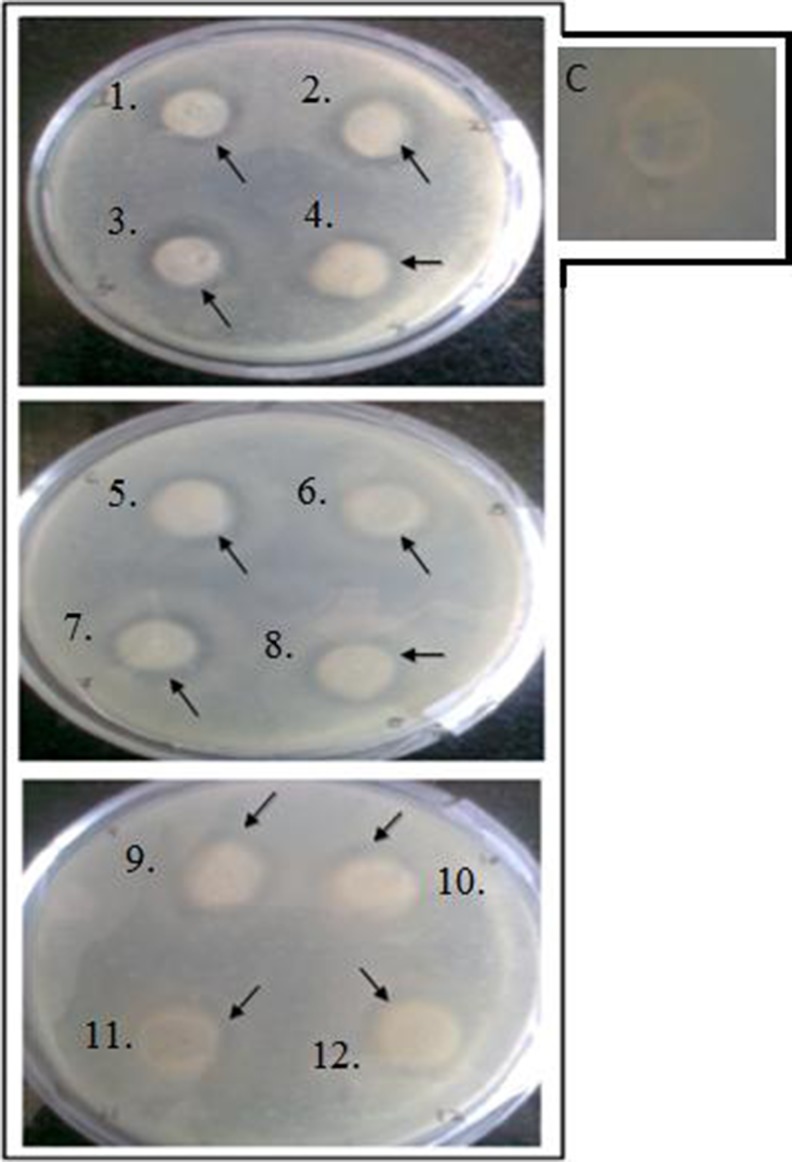
The agar diffusion test was designed to test an antimicrobial film for solid foods packaging. Antimicrobial activity of the film was expressed in terms of inhibition zone. Out of the polymer films tested in this study, the control polymer films, containing starch and glycerol as plasticizer did not exhibit any antimicrobial activity (shown as inset in Figs. 3 and 4); however, 12 different combinations of Starch + nisin (2,500, 5,000, 7,500 IU/ml) + lysozyme (1,000, 5,000 U/ml) + EDTA (10, 20 mM) as per sets mentioned in Table 2 did exhibit activity as indicated by presence of zones of inhibition around film in this plate overlay assays (Fig. 4) which indicates that antimicrobial agents were released and diffused from the starch polymer film surface into the solid phase. The least antimicrobial activity was detected in the 12th set containing less concentration of each agent. The ratio of the diameter of inhibition zone to the diameter of the film specimen (12 mm) was 1.63 for the starch film coated with combination of antibacterial agents. Paper coated nisin with binder had showed tremendous potential for preserving the microbial and chemical quality of perishable foods, and thus, extending their shelf life (Lee et al. 2004). (Hettiarachchy et al. 2008) had also demonstrated that use of an edible film coating containing nisin, organic acids, and natural extracts is a promising means of controlling the growth and recontamination of L. monocytogenes, S. typhimurium, and E. coli O157:H7 type pathogenic micro organisms upto 28 days. The (Ko et al. 2001) results had also showed that nisin-incorporated edible films have more potency at lower pH conditions.
Fig. 4.
Results of bacterial growth inhibition by agar diffusion method with 12 mm diameter pieces of film reflecting 12 different combinations of three agents (as shown as 12 sets in Table 2) incubated at 37 °C, 48 h. Arrows indicate areas of detectable antimicrobial activity with C as control shown in inset around which no inhibition zone was observed as it is starch-glycerol film without incorporation of any antimicrobial agents
Conclusion
Although active biofilms provide a method for increasing the shelf life of food products and prevent food spoilage but there is a need for further optimization to be procured and in this an improvement need to be made in terms of the combinations of different antimicrobial agents which can be used in the biofilms for packaging. The equation obtained by statistical analysis of combined effects of nisin, lysozyme and EDTA on spoilage microorganism in starch media can further be used to calculate the maximum effective combination of the three ingredients used for making biofilm and still further work is being done in the same direction to practically check the predicted results of the equation and also then to check the effect of other antimicrobials in combination with these three.
References
- Appendini P, Hotchkiss JH. Review of antimicrobial packaging food packaging. Innov Food Sci Emerg Technol. 2002;3:113–126. doi: 10.1016/S1466-8564(02)00012-7. [DOI] [Google Scholar]
- Banin E, Brady KM, Greenberg EP. Chelator induced dispersal and killing Pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol. 2006;72:2064–2069. doi: 10.1128/AEM.72.3.2064-2069.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- Boussouel N, Mathieu F, Benoit V, Linder M, Revol-Junelles AM, Milliere JB. Response surface methodology an approach to predict the effects of a lactoperoxidase system, Nisin, alone or in combination, on Listeria monocytogenes in skim milk. J Appl Microbiol. 1999;86:642–652. doi: 10.1046/j.1365-2672.1999.00707.x. [DOI] [PubMed] [Google Scholar]
- Branen JK, Davidson PM. Enhancement of nisin, lysozyme and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int J Food Microbiol. 2004;90:63–74. doi: 10.1016/S0168-1605(03)00172-7. [DOI] [PubMed] [Google Scholar]
- Breukink E, van Kraaij C, van Dalen A, Demel RA, Siezen RJ, de Kruijff B, Kuipers OP. The orientation of nisin in membranes. Biochemistry. 1998;37:8153–8162. doi: 10.1021/bi972797l. [DOI] [PubMed] [Google Scholar]
- Bruno ME, Kaiser A, Montville TJ. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992;58:2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan K, Trivedi U, Patel KC. Application of response surface methodology for optimization of lactic acid production using date juice. J Microbiol Biotechnol. 2006;16(9):1410–1415. [Google Scholar]
- Chung D, Papadakis SE, Yam KL. Evaluation of a polymer coating containing triclosan as the antimicrobial layer for packaging materials. Int J Food Sci Technol. 2003;38:165–169. doi: 10.1046/j.1365-2621.2003.00657.x. [DOI] [Google Scholar]
- Corbo MR, Mastromatteo M, Lucera A, Sinigaglia M. Use of Lysozyme, Nisin, and EDTA combined treatments for maintaining quality of packed ostrich patties. J Food Sci. 2010;75:178–186. doi: 10.1111/j.1750-3841.2010.01556.x. [DOI] [PubMed] [Google Scholar]
- Cutter CN, Willett JL, Siragusa GR. Improved antimicrobial activity of nisin incorporated polymer film by formulation change and addition of food grade chelator. Lett Appl Microbiol. 2001;33:325–328. doi: 10.1046/j.1472-765X.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Ellison RT, Giehl JJ. Killing of gram negative bacteria by lactoferrin and lysozyme. J Clin Investig. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaranandam S, Hettiarachchy NS, Johnson MG. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy, protein film against Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella gaminara. J Food Sci. 2004;69:78–84. [Google Scholar]
- Food and Drug Administration (FDA) Nisin preparation: affirmation of GTAS status as a direct human food ingredient. Fed Regist. 1988;53:11247–11251. [Google Scholar]
- Gram HC. Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten. Fortschr Med. 1884;2:185–189. [Google Scholar]
- Hansen JN, Chung Y, Liu W (1991) Biosynthesis and mechanism of the action of nisin and subtilin. ESCOM Science Publishers In: Nisin and novel lantibiotics, Leiden., pp. 287–302
- Hansen LT, Austin JW, Gill TA. Antibacterial effect of protamine in combination with EDTA and refrigeration. Int J Food Microbiol. 2001;66:149–161. doi: 10.1016/S0168-1605(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Fleming HP, Klaenhammer TR. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A, and UAL 500 to nisin. J Food Prot. 1991;54:836–840. doi: 10.4315/0362-028X-54.11.836. [DOI] [PubMed] [Google Scholar]
- Hettiarachchy NS, Gadang VP, Johnson MG, Owens C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J Food Sci. 2008;73:389–394. doi: 10.1111/j.1750-3841.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- Holley RA, Gill AO. Interactive inhibition of meat spoilage and pathogenic bacteria by lyozyme, nisin and EDTA in the presence of nitrite and sodium chloride at 24 °C. Int J Food Microbiol. 2003;80:251–259. doi: 10.1016/S0168-1605(02)00171-X. [DOI] [PubMed] [Google Scholar]
- Hotchkiss JH. Food-packaging interactions influencing quality and safety. Food Addit Contam. 1997;14:601–607. doi: 10.1080/02652039709374572. [DOI] [PubMed] [Google Scholar]
- Hughey VL, Johnson EA. Antimicrobial activity of lysozyme against bacteria involved in food spoilage and food-borne disease. Appl Environ Microbiol. 1987;53:2165–2170. doi: 10.1128/aem.53.9.2165-2170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Functions of nisin and nisin like basic proteins in the growth cycle of Streptococcus lactis. Nature. 1967;214:1232–1234. doi: 10.1038/2141232a0. [DOI] [PubMed] [Google Scholar]
- Jin T, Zhang H. Biodegradable polylactic acid polymer with nisin for use in antimicrobial food packaging. J Food Sci. 2008;73:127–134. doi: 10.1111/j.1750-3841.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Kiss IF, Márialigeti K, Zukál E, Makk J (2007) Method for measurement of antibacterial activity of Nisin. Bulletin USAMV-CN. 63 - 64/2007
- Ko S, Janes ME, Hettiarachchy NS, Johnson MG. Physical and chemical properties of edible films containing nisin and their action against Listeria monocytogenes. J Food Sci. 2001;66:1006–1011. doi: 10.1111/j.1365-2621.2001.tb08226.x. [DOI] [Google Scholar]
- Lee DS, Lee CH, An DS, Lee SC, Park HJ. A coating for use as an antimicrobial and antioxidative packaging material incorporating nisin and alpha-tocopherol. J Food Eng. 2004;62:323–329. doi: 10.1016/S0260-8774(03)00246-2. [DOI] [Google Scholar]
- Monk JD, Beuchat LR, Hathcox AK. Inhibitory effects of sucrose monolaurate, alone and in combination with organic acids, on Listeria monocytogenes and Staphylococcus aureus. J Appl Bacteriol. 1996;81:7–18. doi: 10.1111/j.1365-2672.1996.tb03276.x. [DOI] [PubMed] [Google Scholar]
- Monteville TJ, Chen Y (1998) Mechanistic action of pediocin and nisin: recent progress and unresolved questions. Appl Microbiol Biotechnol 511–519 [DOI] [PubMed]
- Muhammad II, Razali F, Liza MS (2005) Study of an active antimicrobial system using a bio-switch concept. Project Report. Universiti Teknologi Malaysia (Unpublished)
- Murdock CA, Cleveland J, Matthews KR, Chikindas ML. The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett Appl Microbiol. 2007;44:255–261. doi: 10.1111/j.1472-765X.2006.02076.x. [DOI] [PubMed] [Google Scholar]
- Okereke A, Montville TJ. Nisin dissipates the proton motive force of the obligate anaerobe Clostridium sporogenes PA 3679. Appl Environ Microbiol. 1992;58:2463–2467. doi: 10.1128/aem.58.8.2463-2467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett T, Han IY, Dawson PL. Incorporation of food antimicrobial compounds into biodegradable packaging films. J Food Prot. 1998;61:1330–1335. doi: 10.4315/0362-028x-61.10.1330. [DOI] [PubMed] [Google Scholar]
- Parente E, Giglio MA, Ricciardi A, Clementi F. The combined effect of nisin, leucocin F10, pH, NaCl and EDTA on the survival of Listeria monocytogenes in broth. Int J Food Microbiol. 1998;40:65–75. doi: 10.1016/S0168-1605(98)00021-X. [DOI] [PubMed] [Google Scholar]
- Payne KD, Oliver SP, Davidson PM. Comparison of EDTA and apo-lactoferrin with lysozyme on the growth of foodborne pathogenic and spoilage bacteria. J Food Prot. 1994;57:62–65. doi: 10.4315/0362-028X-57.1.62. [DOI] [PubMed] [Google Scholar]
- Proctor VA, Cunningham RE. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit Rev Food Sci Nutr. 1988;26:359–395. doi: 10.1080/10408398809527473. [DOI] [PubMed] [Google Scholar]
- Schillinger U, Geisen R, Holzapfel WH. Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci Technol. 1996;7:158–164. doi: 10.1016/0924-2244(96)81256-8. [DOI] [Google Scholar]
- Shelef LA, Seiter J. Enhancement of the activity of novobiocin against Escherichia coli by lactoferrin. J Dairy Sci. 1993;82:492–499. doi: 10.3168/jds.S0022-0302(99)75259-8. [DOI] [PubMed] [Google Scholar]
- Shively JM, Hartsell SE. Bacteriolysis of the Pseudomonads:I. Agents potentiating lysis. Can J Microbiol. 1964;10:905–909. doi: 10.1139/m64-117. [DOI] [PubMed] [Google Scholar]
- Shively JM, Hartsell SE. Bacteriolysis of the Pseudomonads: II. Chemical treatments affecting the lytic response. Can J Microbiol. 1964;10:911–915. doi: 10.1139/m64-118. [DOI] [PubMed] [Google Scholar]
- Siragusa GR, Cutter CN, Willett JL. Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol. 1999;16:229–235. doi: 10.1006/fmic.1998.0239. [DOI] [Google Scholar]
- Stevens KA, Sheldon BW, Klapes NA, Klaenhamme TR. Nisin treatment for inactivation of Salmonella species and other gram- negative bacteria. Appl Environ Microbiol. 1991;57:3613–3615. doi: 10.1128/aem.57.12.3613-3615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LV, Ingram RE, Bevis HE, Davies A, Milne CF, Delves-Broughton J. Effective use of nisin to control Bacillus and Clostridium spoilage of a pasteurized mashed potato product. J Food Prot. 2002;65:1580–1585. doi: 10.4315/0362-028x-65.10.1580. [DOI] [PubMed] [Google Scholar]
- Vessoni Penna TC, Moraes DA. The effect of nisin on growth kinetics from activated Bacillus cereus spores in cooked rice and milk. J Food Prot. 2002;65:419–422. [PubMed] [Google Scholar]
- Winkowski K, Ludescher RT, Montville TJ. Physiochemical characterization of the nisin-membrane interaction with liposomes derived from Listeria monocytogenes. Appl Environ Microbiol. 1996;62:323–327. doi: 10.1128/aem.62.2.323-327.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Mustapha A. Reduction of Listeria monocytogenes and Escherichia coli O157:H7 numbers on vacuum packaged fresh beef treated with nisin or nisin combined with EDTA. J Food Prot. 1999;62:1123–1127. doi: 10.4315/0362-028x-62.10.1123. [DOI] [PubMed] [Google Scholar]
- Zhang YC, Yam KL, Chikindas ML. Effective control of Listeria monocytogenes by combination of nisin formulated and slowly released into a broth system. Int J Food Microbiol. 2004;90:15–22. doi: 10.1016/S0168-1605(03)00168-5. [DOI] [PubMed] [Google Scholar]