Abstract
Chitin is one of the most abundant bioactive biopolymer on earth. It is commercially extracted from seafood processing crustacean shell byproducts by harsh thermochemical treatments. The extraction conditions, the source and pretreatment of raw material significantly affect its quality and bioactivity. In this investigation response surface methodology (RSM) has been applied to optimize and evaluate the interaction of variables for extraction of high quality chitin from shrimp processing raw byproducts. Variables such as, concentration of HCl (%, v/v) 4.5 (for wet) and 4.9 (for dry), reaction time 3 h, solid liquid ratio of HCl (w/v) 1:5.5 (for wet) and 1:7.9 (for dry) with two treatments achieved >98 % demineralization of shrimp byproduct. Variables such as, concentration of NaOH 3.6 % (w/v), reaction time 2.5 h, temperature 69.0 ± 1 °C, solid liquid ratio of NaOH 7.4 (w/v) and two treatments accomplished >98 % deproteinization of demineralized byproducts. Significant (p ≤ 0.05–0.001) interactive effects were observed between different variables. Chitin obtained in these conditions had residual content (%, w/w) of ash <0.4 and protein <0.8 and the degree of N-acetylation was >93 % with purity of >98 %. In conclusion, the optimized conditions by RSM can be applied for large scale preparation of high quality chitin from raw shrimp byproduct.
Keywords: Chitin, Chitin extraction, Shrimp byproduct, Response surface methodology
Introduction
Chitin, poly [β-(1 → 4)-N-acetyl-D-glucosamine], is one of the most abundant biopolymer on earth, next to cellulose (Duarte et al. 2002; Synowiecki and Al-Khateeb 2003). Although chitin is widely distributed in nature, till date, the major source of commercial chitin comes from marine crustacean because a large amount of raw materials are available as byproducts of seafood processing (Aranaz et al. 2009; Kurita 2006; Synowiecki and Al-Khateeb 2003; Nidheeshet al. 2013) and extraction of chitin and its derivatives from crustacean byproducts should minimize the seafood byproducts disposal problems (Synowiecki and Al-Khateeb 2003; Nidheesh et al. 2014)
Shrimp is one of the most important internationally traded fishery commodities in terms of value. In many tropical developing countries, it is the most valuable fishery export; the employment aspect is also significant. The recent world shrimp catch is about 3.4 million tonnes per year, with Asia as the most noteworthy area for shrimp fishing. World production of shrimp, both captured and farmed, is about six million tonnes, of which about 60 % enters the world market (Gillett 2008). In addition to edible parts, wastes amount to as high as 60–80 % of the whole shrimp and crabs. Except some are processed to make cheap feeds of shrimp and crab shells powder, most wastes are discarded at will (Wang et al. 2010; Wang et al. 2011). These not recovered and unused wastes could become potential precious bioresources, if they are processed/converted by modern biotech to make highly value-added products (Jayakumar et al. 2010; Wang et al. 2010).
Chitin and its principal derivative chitosan have numerous applications, due to their versatile biological and functional properties (Jayakumar et al. 2007; Jayakumar et al. 2010; Kurita 2006; Synowiecki and Al-Khateeb 2003; Nidheesh and Suresh 2014). Some of the applications require specific architectures, and the effectiveness was shown to depend on purity and consistent quality of chitin (Percot et al. 2003; Synowiecki and Al-Khateeb 2003). Chitin in crustacean shell is strongly associated with various organic and inorganic materials, and they all have to be quantitatively removed to attain the highly purified chitin necessary for biological applications (Percot et al. 2003). Usually, preparation of chitin from various crustacean byproducts mainly involves removal of minerals and proteins with 2–5 % acids or bases at higher temperatures (Kurita 2006; Percot et al. 2003; Synowiecki and Al-Khateeb 2003). Although the production of chitin was commercialized for decades, little information has been reported in the literature about the optimization of isolation process, and there is no uniform method for the isolation of chitin from crustacean byproducts (Chang and Tsai 1997; Percot et al. 2003; Shimahara and Takiguchi 1988). About a decade before, Percot et al. (2003) has reported that a cost effective, fast and easily controlled industrial process is required for extraction of chitins with high purity and consistent qualities for specific applications particularly in food and biomedicine. In spite of that, not much work has been published over the last decade on the effective process for the extraction of high quality chitin from seafood processing byproducts. Response surface methodology (RSM) is a powerful and useful statistical technique to identify/quantify interactions between the variables and for optimization of complex chemical, biochemical, and other multifactorial processes (Chang and Tsai 1997; Suresh et al. 2011a; Suresh 2012). Chang and Tsai (1997) has applied RSM for the extraction of chitin from pretreated very fine shell powder (0.177–0.250 mm size) of pink shrimp. However, there has been no report available in published literatures on the optimization process for the extraction of chitin from crustacean crude and raw byproducts using RSM. The main objective of this investigation was to optimize the demineralization and deproteinization process for the extraction of chitin with high quality/purity from the shrimp processing raw byproducts (SPB) using RSM employing central composite design (CCD).
Materials and methods
Raw material and preparation
Shrimp (Penaeus sp.) processing raw byproducts (SPB) composed of cephalothoraxes and carapaces were collected from local seafood market. The SPB was ground in a wet mill (Robot-coupe, 94,305 Vincennes Cedex, France) to a particle size of 1–2 cm. The minced SPB were washed with tap water (1:5, w/v) two times (unless otherwise mentioned) at room temperature (28 ± 2 °C) in order to remove the protein, other organic matters and dirt loosely associated with shrimp shell. A portion of the washed SPB was dried in a forced air drying oven (Kilburn, Mumbai, India) at 55 ± 2 °C for 12 h. The SPB (wet and dried, 1–2 cm size) thus prepared were used as starting material for chitin extraction without any further treatment.
Demineralization of shrimp byproducts
HCl solution was used to remove minerals from SPB. For all design experiments, reactions were carried out in Erlenmeyer flask (500 ml capacity); each having 10 g dry or 25 g wet materials. Four variables [concentration of HCl (X1, %, v/v), reaction time (X2, h), solid liquid ratio of HCl solution (X3, w/v) and number of treatments (X4)], which were expected to have an effect on demineralization of SPB on chitin extraction, screened and identified the most significant variables using a 2-level 2**(4–1) fractional factorial design (FFD). The FFD matrix consisting 20 runs with 4 center points were used. All the independent variables were investigated at a high (+1) and a low (−1) level. All variables taken as central coded value considered as zero. The minimum and maximum ranges of variables were investigated, and the full experimental plans with respect to their values in coded and actual form are presented in Table 1. After treatment, the demineralized SPB were collected by filtration, washed to neutrality with potable tap water, rinsed with deionised water, and dried at 55 °C for 12 h. The rate of demineralization was evaluated by determining ash content in the dried materials and recorded as the response (dependent) variable. The independent variables having major effects on dependent variable were identified on the basis of confidence levels 95 % and above.
Table 1.
Fractional factorial design matrix with independent variables in actual and coded values and observed values of response variable (% demineralization) in the demineralization of wet and dry shrimp byproducts material. X1, concentration of HCl solution (%, v/v); X2, reaction time (h); X3, solid liquid ratio of HCl solution (w/v) and X4, number of treatments
| Run | Independent variables | Response variable, Demineralization (%, w/w) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | For wet material | For dry material | |
| 1 | 0.1 (−1) | 0.5 (−1) | 1 (−1) | 1 (−1) | 9.29 | 12.3 |
| 2 | 4.9 (+1) | 0.5 (−1) | 1 (−1) | 1 (−1) | 23.93 | 19.9 |
| 3 | 0.1 (−1) | 5.5 (+1) | 1 (−1) | 1 (−1) | 11.46 | 11.1 |
| 4 | 4.9 (+1) | 5.5 (+1) | 1 (−1) | 1 (−1) | 7.17 | 19.2 |
| 5 | 0.1 (−1) | 0.5 (−1) | 9 (+1) | 1 (−1) | 8.25 | 10.7 |
| 6 | 4.9 (+1) | 0.5 (−1) | 9 (+1) | 1 (−1) | 83.53 | 87.2 |
| 7 | 0.1 (−1) | 5.5 (+1) | 9 (+1) | 1 (−1) | 17.70 | 15.5 |
| 8 | 4.9 (+1) | 5.5 (+1) | 9 (+1) | 1 (−1) | 90.86 | 89.3 |
| 9 | 0.1 (−1) | 0.5 (−1) | 1 (−1) | 3 (+1) | 24.33 | 20.9 |
| 10 | 4.9 (+1) | 0.5 (−1) | 1 (−1) | 3 (+1) | 82.43 | 23.7 |
| 11 | 0.1 (−1) | 5.5 (+1) | 1 (−1) | 3 (+1) | 24.60 | 18.9 |
| 12 | 4.9 (+1) | 5.5 (+1) | 1 (−1) | 3 (+1) | 57.25 | 17.0 |
| 13 | 0.1 (−1) | 0.5 (−1) | 9 (+1) | 3 (+1) | 16.85 | 26.5 |
| 14 | 4.9 (+1) | 0.5 (−1) | 9 (+1) | 3 (+1) | 75.37 | 99.3 |
| 15 | 0.1 (−1) | 5.5 (+1) | 9 (+1) | 3 (+1) | 37.26 | 29.3 |
| 16 | 4.9 (+1) | 5.5 (+1) | 9 (+1) | 3 (+1) | 92.97 | 100.0 |
| 17 | 2.5 (0) | 3.0 (0) | 5 (0) | 2 (0) | 42.60 | 42.8 |
| 18 | 2.5 (0) | 3.0 (0) | 5 (0) | 2 (0) | 38.72 | 34.2 |
| 19 | 2.5 (0) | 3.0 (0) | 5 (0) | 2 (0) | 41.78 | 42.8 |
| 20 | 2.5 (0) | 3.0 (0) | 5 (0) | 2 (0) | 39.44 | 27.6 |
Based on the identification of variables by the FFD, a CCD of RSM was developed for two variables, viz. X1 and X3 to determine the mutual interaction among the variables and their corresponding optimum levels. The CCD matrix was formulated with six central points and six axial points (with one variable set at extreme ±1.41 level and the other variables at the central point level) and with a total number of 12 runs. The details of experimental design with coded and actual levels of both variables are summarized in Table 3. In the study all other non-significant variables (X2 and X4), were maintained at a constant central point (‘0’coded level) of the levels used in the FFD of screening experiments.
Table 3.
Central composite design matrixes with independent variables in coded and actual values, and observed and predicted values of response variable (% demineralization) in the demineralization of wet and dry shrimp byproducts materials. X1, concentration of HCl solution (%, w/v) and X3, solid liquid ratio of HCl solution (w/v)
| Run | Independent variables | Response variable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 (%, v/v) | X3 (w/v) | Demineralization (%, w/w) | ||||||||
| Wet | Dry | |||||||||
| Ca | Ab | Ac | Ca | Ab | Ac | Observed | Predicted | Observed | Predicted | |
| 1 | −1 | 1 | 1 | −1 | 2 | 2 | 7.9 | −0.8 | 3.5 | 7.9 |
| 2 | −1 | 1 | 1 | +1 | 6 | 7 | 45.9 | 37.6 | 1.3 | −0.5 |
| 3 | +1 | 4 | 5 | −1 | 2 | 2 | 75.3 | 67.1 | 12.9 | 12.9 |
| 4 | +1 | 4 | 5 | +1 | 6 | 7 | 98.4 | 90.5 | 98.5 | 90.1 |
| 5 | −1.41 | 0.38 | 0.17 | 0 | 4 | 5 | 12.8 | 21.3 | 0.0 | −2.5 |
| 6 | +1.41 | 4.62 | 5.82 | 0 | 4 | 5 | 98.8 | 106.7 | 71.8 | 77.1 |
| 7 | 0 | 2.5 | 3 | −1.41 | 1.17 | 1.46 | 2.8 | 11.4 | 3.6 | −1.2 |
| 8 | 0 | 2.5 | 3 | +1.41 | 6.83 | 8.53 | 47.1 | 55.1 | 46.5 | 51.3 |
| 9 | 0 | 2.5 | 3 | 0 | 4 | 5 | 32.1 | 22.4 | 17.0 | 18.4 |
| 10 | 0 | 2.5 | 3 | 0 | 4 | 5 | 16.0 | 22.4 | 15.7 | 18.4 |
| 11 | 0 | 2.5 | 3 | 0 | 4 | 5 | 22.5 | 22.4 | 18.7 | 18.4 |
| 12 | 0 | 2.5 | 3 | 0 | 4 | 5 | 18.9 | 22.4 | 19.4 | 18.4 |
a = coded; b = actual for dry shrimp shell material, c = actual for wet shrimp shell material
Deproteinization of demineralized shrimp byproducts
NaOH solution was used to remove protein from demineralized SPB. For all design experiments, reactions were carried out in Erlenmeyer flask (250 ml capacity), each having 10 g of wet demineralized SPB. Five variables [concentration of NaOH solution (X1, %, w/v), reaction time (X2, h), treatment temperature (X3, °C), solid liquid ratio of NaOH solution (X4, w/v) and number of treatments (×5)], which were expected to have an effect on deproteinization of demineralized SPB screened and identified the most significant variables using a 2-level 2**(5–1) FFD. The FFD matrix consisting 20 runs with 4 center points were used. All the independent variables were investigated at a high (+1) and a low (−1) level. All the variables taken as a central coded value considered as zero. The minimum and maximum ranges of variables investigated and the full experimental plan with respect to their values in coded and actual form are presented in Table 2. After treatment, deproteinized SPB were collected by filtration, washed to neutrality with potable tap water, rinsed with deionised water, and then dried at 55 °C for 12 h. In order to evaluate the extent of deproteinization (%, w/w), the protein content of the dried residues was determined (AOAC 2000) and recorded as response (dependent) variable. The independent variables having major effects on dependent variables were identified on the basis of confidence levels ≥ 95 %.
Table 2.
Fractional factorial design matrix with independent variables in actual and coded values, and observed values of response variable (% deproteinization) in the deproteinization of demineralized shrimp byproducts. X1, Concentration of NaOH solution (%, w/v); X2, reaction time (h); X3, reaction temperature (°C); X4, solid liquid ratio of NaOH solution (w/v); X5, number of treatments
| Run | Independent variables | Response variable | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | Deproteinization (%, w/w) | |
| 1 | 0.10 (−1) | 0.5 (−1) | 30 (−1) | 1 (−1) | 3 (+1) | 6.86 |
| 2 | 4.90 (+1) | 0.5 (−1) | 30 (−1) | 1 (−1) | 1 (−1) | 55.52 |
| 3 | 0.10 (−1) | 4.5 (+1) | 30 (−1) | 1 (−1) | 1 (−1) | 10.45 |
| 4 | 4.90 (+1) | 4.5 (+1) | 30 (−1) | 1 (−1) | 3 (+1) | 33.54 |
| 5 | 0.10 (−1) | 0.5 (−1) | 100 (+1) | 1 (−1) | 1 (−1) | 23.37 |
| 6 | 4.90 (+1) | 0.5 (−1) | 100 (+1) | 1 (−1) | 3 (+1) | 91.00 |
| 7 | 0.10 (−1) | 4.5 (+1) | 100 (+1) | 1 (−1) | 3 (+1) | 17.47 |
| 8 | 4.90 (+1) | 4.5 (+1) | 100 (+1) | 1 (−1) | 1 (−1) | 93.00 |
| 9 | 0.10 (−1) | 0.5 (−1) | 30 (−1) | 9 (+1) | 1 (−1) | 33.14 |
| 10 | 4.90 (+1) | 0.5 (−1) | 30 (−1) | 9 (+1) | 3 (+1) | 50.92 |
| 11 | 0.10 (−1) | 4.5 (+1) | 30 (−1) | 9 (+1) | 3 (+1) | 33.53 |
| 12 | 4.90 (+1) | 4.5 (+1) | 30 (−1) | 9 (+1) | 1 (−1) | 59.98 |
| 13 | 0.10 (−1) | 0.5 (−1) | 100 (+1) | 9 (+1) | 3 (+1) | 57.23 |
| 14 | 4.90 (+1) | 0.5 (−1) | 100 (+1) | 9 (+1) | 1 (−1) | 89.58 |
| 15 | 0.10 (−1) | 4.5 (+1) | 100 (+1) | 9 (+1) | 1 (−1) | 73.57 |
| 16 | 4.90 (+1) | 4.5 (+1) | 100 (+1) | 9 (+1) | 3 (+1) | 100.00 |
| 17 | 2.5 (0) | 2.5 (0) | 65 (0) | 5 (0) | 2 (0) | 71.05 |
| 18 | 2.5 (0) | 2.5 (0) | 65 (0) | 5 (0) | 2 (0) | 73.14 |
| 19 | 2.5 (0) | 2.5 (0) | 65 (0) | 5 (0) | 2 (0) | 74.02 |
| 20 | 2.5 (0) | 2.5 (0) | 65 (0) | 5 (0) | 2 (0) | 63.47 |
Based on the identification of variables by the FFD, a CCD was developed for three variables, viz. X1, X3 and X4 to determine the mutual interactions among the identified variables and their corresponding optimum levels. The CCD matrix was formulated with eight central points and ten axial points (with one variable set at extreme ±1.68 level and the other variables at central point level) leading to 18 experimental run and performed in triplicate. The details of experimental design with coded and actual levels of each variable are summarized in Table 4. In the study all other non-significant variables were maintained at a constant central point (‘0’coded level) of the levels used in the FFD of screening experiments.
Table 4.
Central composite design matrixes with independent variables in coded and actual values, and observed and predicted values of response variable (% deproteinization) in the deproteinization of demineralized shrimp byproducts. X1, concentration of NaOH solution (%, w/v); X3, reaction temperature (°C) and X4, solid liquid ratio of NaOH solution (w/v)
| Run | Independent variables | Response variable | ||||||
|---|---|---|---|---|---|---|---|---|
| X1, | X3 | X4 | Deproteinization (%, w/w) | |||||
| Coded | Actual | Coded | Actual | Coded | Actual | Observed | Predicted | |
| 1 | −1 | 1.1 | −1 | 42 | −1 | 3 | 29.1 | 26.3 |
| 2 | −1 | 1.1 | −1 | 42 | 1 | 7 | 48.9 | 44.9 |
| 3 | −1 | 1.1 | 1 | 84 | −1 | 3 | 61.1 | 61.3 |
| 4 | −1 | 1.1 | 1 | 84 | 1 | 7 | 67.4 | 72.9 |
| 5 | 1 | 3.9 | −1 | 42 | −1 | 3 | 74.3 | 69.1 |
| 6 | 1 | 3.9 | −1 | 42 | 1 | 7 | 79.0 | 79.2 |
| 7 | 1 | 3.9 | 1 | 84 | −1 | 3 | 83.2 | 87.5 |
| 8 | 1 | 3.9 | 1 | 84 | 1 | 7 | 87.6 | 90.7 |
| 9 | −1.68 | 0.1 | 0 | 63 | 0 | 5 | 33.9 | 34.7 |
| 10 | 1.68 | 4.85 | 0 | 63 | 0 | 5 | 87.0 | 85.7 |
| 11 | 0 | 2.50 | −1.68 | 27.68 | 0 | 5 | 36.8 | 44.0 |
| 12 | 0 | 2.5 | 1.68 | 98.32 | 0 | 5 | 90.8 | 83.1 |
| 13 | 0 | 2.5 | 0 | 63 | −1.68 | 1.64 | 66.7 | 68.9 |
| 14 | 0 | 2.5 | 0 | 63 | 1.68 | 8.36 | 89.9 | 87.2 |
| 15C | 0 | 2.5 | 0 | 63 | 0 | 5 | 80.0 | 80.2 |
| 16C | 0 | 2.5 | 0 | 63 | 0 | 5 | 80.8 | 80.2 |
| 17C | 0 | 2.5 | 0 | 63 | 0 | 5 | 80.0 | 80.2 |
| 18C | 0 | 2.5 | 0 | 63 | 0 | 5 | 80.0 | 80.2 |
Statistical analysis of RSM design
All analysis was conducted in triplicate and the results of RSM experiments were analyzed using the statistical software package (Statsoft 1997). The data obtained was subjected to the Analysis of Variance (ANOVA). The responses obtained in CCD were subjected to multiple non-linear regression analysis for obtaining empirical models that relate the response to the independent factors. The results of CCD were used to derive second order polynomial model equation (Eq. 1) .
| 1 |
where Y is the predicted response (% demineralization or % deproteinization); βo is the intercept (regression coefficient); βi is the linear coefficient; βii is the quadratic coefficient; βij is the interaction coefficient. Xi and Xj are the independent variables.
Response surface and contour graphs of RSM design
Three dimensional response surface and contour graphs were drawn to illustrate the main and interactive effects of independent variables. The optimum values of each independent variable for maximum response variable were determined using response surface curves and desirability profile.
Chemical and microbiological analysis
Moisture, fat, protein (Kjeldahl), ash (AOAC 2000) and chitin (Suresh 2012) contents of SPB and chitin materials were determined. The percentage of soluble protein contents in the water extract of SPB was determined by Lowry’s method. The microbiological examination, and sodium and the heavy metal lead content by dry ash method using Atomic Absorption Spectrophotometer (AAS) (Shimadzu AA6701F, Japan) were analyzed (AOAC 2000).
Determination of degree of N-Acetylation of chitin
The degree of N-acetylation of chitin was assessed by the method using Fourier-transform infrared (FTIR) spectroscopy. Chitin-KBr disc was prepared using very well dried mixtures of about 1 mg of the sample and 100 mg of KBr. The FTIR spectra analysis was carried using a NICOLET 5700 FTIR spectrometer (Thermo Electron Cooperation, Madison, WI 53711) with a resolution of 4 cm−1 and 32 accumulations in the frequency range from 4000 to 400 cm−1. The degree of N-acetylation of the samples was calculated using the following equation (Eq. 2) (Baxter et al. 1992).
| 2 |
Decolouration and measurement of colour of chitin
The chitin extracted from SPB at the optimized conditions were treated with different solvents (acetone, absolute ethanol and 1 % (v/v) H2O2 solution) at a solid solvent ratio of 1:10 (w/v) for 10 min, in order to remove the residual fat and pigments. It was filtered through cheese cloth and dried for 12 h at 55 ± 2 °C. The chitin treated with H2O2 was thoroughly washed with deionised water before drying. The colour of chitins was determined instrumentally using Hunter Color Measuring System (Labscan XE, Hunter Associates Laboratory Inc., Reston, VA).
Results and Discussion
Proximate composition of the raw materials
The SPB (original) used in the study contained (%, w/w, dry weight basis, dwb) 39.5 ± 0.4 protein, 23.4 ± 0.7 chitin, 1.2 ± 0.04 fat and 30.4 ± 1.4 ash which was found almost similar to earlier reports (Suresh et al. 2011a, b). However, the differences in chemical/proximate compositions could be related to variations in species, age, harvest location, method of processing and other biological or environmental factors.
Significantly (p ≤ 0.05) high percentage of protein content was observed in the water extract of SPB employing a solid-to-water ratio of 1:5 (w/v) with two washes as compared with other ratios (1:1 and 1:2.5, w/v) (data not shown). Hence, throughout the investigation we followed this method. The washed SPB contained (%, w/w, dwb) 36.8 ± 1.4 protein, 25.4 ± 1.1 chitin, 1.2 ± 0.03 fat and 29.9 ± 0.8 ash. The proteins collected by water washing of SPB can be used for food and feed applications. Distilled water (Percot et al. 2003), alkali soaking followed by water (Chang and Tsai 1997), and potable tap water (Suresh et al. 2011a) has been used by various researchers for washing and also to collect soluble protein from crustaceans’ byproducts.
Extraction of chitin from shrimp processing raw byproducts
Chitin was extracted from SPB initially by demineralization followed by the second step of deproteinization and later in reverse order using the procedure reported earlier (Suresh et al. 2011). It was followed in all further experiments because there was no significant (p ≤ 0.05) difference in the purity of chitin as well as the percentage yield was observed with the changes in the order of demineralization and deproteinization steps (data not shown).
Optimizing demineralization of shrimp byproducts by RSM
The response obtained in terms of % demineralization during screening experiments using FFD is presented in Table 1. Among the variables, the concentration of HCl solution and solid liquid ratio of HCl solution has showed significant (p ≤ 0.05) effect in the demineralization. The tested range of reaction time and number of washing has no significant effect in the demineralization. Similar results were reported by Chang and Tsai (1997). The mean responses obtained along with the predicted responses generated in CCD, is presented in Table 3. Second order polynomial equations derived from the experimental data by multiple regression analysis for demineralization of wet (Y1) and dry (Y2) materials are presented in Eq. 3 and Eq. 4, respectively. The models generated were quadratic type, which accounts for the natural logarithm of response, as a function of two independent variables and their linear, quadratic and interactive functions.
| 3 |
| 4 |
where X1, X3 represent the concentration of HCl solution and solid liquid ratio of HCl solution, respectively.
ANOVA for two variables indicated that the quadratic model derived from CCD could adequately be used to describe the factors for demineralization of SPB under a broad range of operating conditions (Fig. 1, Table 5). Of the two independent variables, concentration of HCl solution has a significant linear (p ≤ 0.001) and quadratic (p ≤ 0.004) effect while the solid liquid ratio of HCl solution have a significant (p ≤ 0.008) linear effect on demineralization of wet SPB (Fig. 1a, Table 5a). No significant interactive effect between concentration of HCl solution and solid liquid of HCl solution was observed in the demineralization of wet SPB (Fig. 1a, Table 5a). From Fig. 2a, it was inferred that the demineralization of wet materials increased along with an increase in concentration of HCl solution and an increase in the solid liquid of HCl solution. At the same time, both independent variables found to have significant (p ≤ 0.00003–0.014) linear and quadratic effects on demineralization of dry shrimp byproducts (Fig. 1b). Significantly (p ≤ 0.0001) high interactive effect was also found between the two independent variables (X1*X3) on the demineralization of dry SPB (Fig. 1b, Table 5b). The coefficient of determination (R2) of the regression equations derived from ANOVA is 0.94551 for the demineralization of wet SPB and 0.98241 for the demineralization of dry SPB, which means that the model can explain 94.55 % and 98.24 % variation, respectively in the response and also indicates the accuracy of the model (a value of R2 > 0.75 indicates the accuracy of the model). The R2 is a proportion of variability in response values explained or accounted by the model (Mantgomery 1984). Though, the lack of fit was significant for the demineralization of dry SPB, the R2 (0.98241) closer to one denotes better correlation between the observed and predicted values.
Fig. 1.
Pareto chart of the central composite design models used for the demineralization of wet shrimp byproducts (a); dry shrimp byproducts (b) and deproteinization of demineralized shrimp byproducts (c). L= linear, Q = Quadratic
Table 5.
ANOVA of central composite design model for chitin isolation
| Sum of square | Degrees of freedom | Mean square | F value | |
|---|---|---|---|---|
| (a) ANOVA for demineralized of wet shrimp byproduct | ||||
| X1 (Linier, L) | 7293.14 | 1 | 7293.140 | 147.4282a |
| X1 (Quadratic, Q) | 2775.86 | 1 | 2775.855 | 56.1129a |
| X3 (L) | 1912.78 | 1 | 1912.779 | 38.6661a |
| X3 (Q) | 188.37 | 1 | 188.369 | 3.8078 |
| X1 × X3 | 55.66 | 1 | 55.662 | 1.1252 |
| Lack of Fit | 545.91 | 3 | 181.970 | 3.6785 |
| Pure error | 148.41 | 3 | 49.469 | |
| Total SS | 12742.34 | 11 | ||
| (b) ANOVA for demineralized of dry shrimp byproduct | ||||
| X1 (L) | 4471.10 | 1 | 4471.098 | 1577.367a |
| X1 (Q) | 573.07 | 1 | 573.066 | 202.173a |
| X3 (L) | 2322.55 | 1 | 2322.546 | 819.376a |
| X3 (Q) | 74.53 | 1 | 74.533 | 26.295a |
| X1 × X3 | 1868.77 | 1 | 1868.773 | 659.288a |
| Lack of Fit | 176.60 | 3 | 58.868 | 20.768a |
| Pure error | 8.50 | 3 | 2.835 | |
| Total sum of square | 10522.66 | 1 | ||
| (c) ANOVA for deproteinization of demineralized shrimp byproduct | ||||
| X1 (L) | 3131.451 | 1 | 3131.451 | 17872.24a |
| X1 (Q) | 634.061 | 1 | 634.061 | 3618.80 a |
| X3 (L) | 1844.774 | 1 | 1844.774 | 10528.74a |
| X3 (Q) | 436.890 | 1 | 436.890 | 2493.48a |
| X4 (L) | 404.373 | 1 | 404.373 | 2307.89a |
| X4 (Q) | 7.538 | 1 | 7.538 | 43.02a |
| X1 × X3 | 137.432 | 1 | 137.432 | 784.37a |
| X1 × X4 | 36.121 | 1 | 36.121 | 206.16a |
| X3 × X4 | 24.146 | 1 | 24.146 | 137.81a |
| Lack of Fit | 236.924 | 5 | 47.385 | 270.44a |
| Pure Error | 0.526 | 3 | 0.175 | |
| Total SS | 6734.800 | 17 | ||
a = significant
a and b: X1, concentration of HCl solution (%, v/v); X2, reaction time (h); X3, solid liquid ratio of HCl solution (w/v) and X4, number of treatments. c: X1, Concentration of NaOH solution (%, w/v); X2, reaction time (h); X3, reaction temperature (°C); X4, solid liquid ratio of NaOH solution (w/v); X5, number of treatments
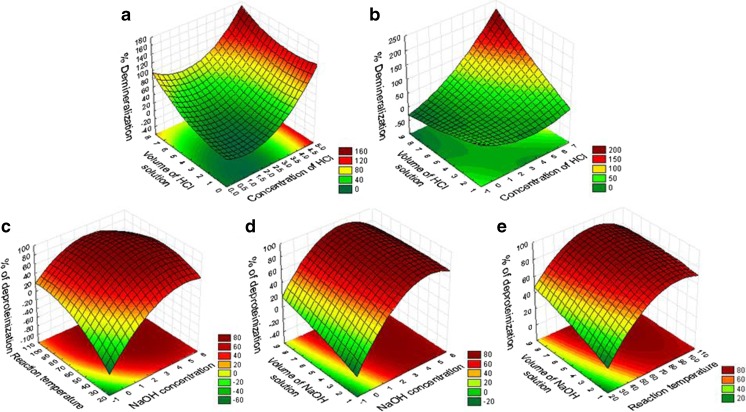
Fig. 2.
Response surface and contour plots of demineralization of wet (a) and dry (b) shrimp byproducts and deproteinization of demineralized of shrimp byproducts (c-e)
The optimum levels of independent variables for maximum demineralization (%, w/w) of SPB were determined using the response surface curves (Fig. 2) and the desirability graph (Fig. 3). From Fig. 2a and Fig. 3a, it can be concluded that the predicted optimal demineralization condition of wet SPB are 4.5 and 1:5.5, respectively the concentration of HCl solution (%, v/v) and solid liquid ratio of HCl solution (w/v). Similarly, from Fig. 2b and Fig. 3b, it can be determined that the predicted optimum for maximum demineralization of dry SPB are concentration of HCl solution 4.9 (%, v/v) and solid liquid ratio of HCl solution 1:7.9 (w/v). It was clearly observed that the optimum levels of two independent variables varied to some extent with respect to the %demineralization of wet and dry SPB.
Fig. 3.
Characteristic of the models used in central composite design for demineralization of wet (a) and dry (b) shrimp raw byproducts and deproteinization of demineralized of shrimp byproducts (c)
Optimization of deproteinization of demineralized SPB by RSM
Table 2 gives the response obtained in terms of deproteinization (%, w/w) during screening experiments using 2-level FFD. Among the variables, the concentration of NaOH solution, reaction temperature and the solid liquid ratio of NaOH solution were identified as significant (p ≤ 0.05) variables affecting deproteinization of demineralized SPB. The tested range of reaction time and number of washing did not result any significant effect on deproteinization. Chang and Tsai (1997) reported that the effect of solid liquid ratio of alkali solution in the deproteinization of SPB was insignificant. Temperature is a critical parameter for the deproteinization with respect to the purity of the obtained chitin (Percot et al. 2003).
The mean response obtained along with the predicted response generated in CCD, is presented in Table 4. The second order polynomial equation (Eq. 5) was derived by multiple regression analysis of the experimental data. The model generated was quadratic type, which accounts for the natural logarithm of response, as a function of three independent variables and their linear, quadratic and interactive functions.
| 5 |
where X1, X3 and X4 represent concentration NaOH solution, reaction temperature and solid liquid ratio of NaOH solution, respectively
ANOVA for the three variables showed that the quadratic model derived from CCD could effectively be used to define the factors for deproteinization of demineralized SPB under a wide range of operating conditions. As shown in Fig. 1c and Table 5c), all the three independent variables such as concentration of NaOH solution (X1), reaction temperature (X3) and solid liquid ratio of NaOH solution (X4), have significant linear and quadratic effect on deproteinization of demineralized materials. Similarly, significant (p ≤ 0.0001) interactive effect was observed between all the three independent variables (X1*X3, X1*X4 and X3*X4) on deproteinization. The coefficient of determination (R2) of the regression equations derived from ANOVA is 0.96474 for deproteinization of demineralized SPB, which means that the model can elucidate 96.47 % variation in the response and also specifies the precision of the model. Though, the lack of fit was significant for deproteinization of demineralized SPB the R2 (0.96471) nearer to one indicates very good relationship between values of observed and predicted response variable.
From Fig. 2c-e, it was inferred that the deproteinization of demineralized SPB increased with an increase in concentration of NaOH solution and reaction temperature for certain point after which it declined. In case of the solid liquid ratio of NaOH solution no such decline was observed. From Fig. 2c-e and Fig. 3c, it can be concluded that the predicted optimum levels of three independent variables (X1, X3 and X4) for maximum deproteinization (%, w/w) of demineralized SPB; are 3.6 % (w/v) concentration of NaOH, 69.0 ± 1 °C reaction temperature and 1:7.4 (w/v) solid liquid ratio of NaOHsolution. In RSM study of deproteinization of shrimp shell powder Chang and Tsai (1997) reported the optimal deproteinization condition occurs at 2.5 N NaOH and 75 °C with minimal solution-to-solid ratio of 5 ml/g.
Optimum conditions predicted by RSM model
The predicted values of the responses viz % demineralization (Y1 and Y2) and % deproteinization (Y3) were calculated using Eq. 1 based on the respective coefficients provided in Eqs. 3–5. The optimum conditions such as, concentration of HCl solution 4.5 % (v/v), reaction time 3 h, solid liquid ratio of HCl solution 1:5.5 (w/v) and two treatments for wet materials, and concentration of HCl solution 4.9 % (v/v), reaction time 3 h, solid liquid ratio of HCl solution 1: 7.9 (w/v) and two treatments, for dry materials fulfill l the conditions to obtain >98 % demineralization of raw SPB. Similarly, the optimum processing conditions, such as, concentration of NaOH solution 3.6 % (w/v), reaction time 2.5 h, treatment temperature 69.0 ± 1 °C, solid liquid ratio of NaOH solution 1:7.4 (w/v) and two treatments accomplish the conditions to attain >99 % deproteinization of demineralized of SPB. In RSM approach, the isolation of chitin from pink shrimp shell powder (0.177–0.250 mm size), Chang and Tsai (1997) have reported that the optimal deproteinization condition occurs at 75 °C, 2.5 N NaOH with a minimal solution to solid ratio of 5 ml/g and the optimal demineralization condition around 1.7 N HCl, with an acid solution to solid ratio of 9 ml/g at ambient temperature. It is very hard to compare, the outcomes of isolation of chitin from shrimp processing raw byproducts obtained by employing RSM, due to considerable differences in proximate composition apparently exist for crustacean byproducts as well as difference in the pretreatment of raw materials. Further, the differences in crustacean species and the method of pretreatment of the raw materials may cause variations in the results of chitin isolation.
Verification of results
Isolation of chitin from SPB at the level of 10, 25, 50, 100, 500, 2500 and 10000 g were carried out at the predicted optimum conditions to deduce the experimental values (Table 6). The resultant chitins were analyzed for residual mineral and protein content (AOAC 2000) and their purity. The purity of the chitin was determined by re-extraction and the percentage recovery of chitin. As shown in Table 6 the experimental values were found to be in agreement with the predicted ones (p ≤ 0.05).
Table 6.
Validation experiment
| Shrimp byproducts (g) | Purity of chitin (%) | Residual mineral (%, w/w) | Residual protein (%, w/w) |
|---|---|---|---|
| 10 | 98.0 ± 0.6 | 0.36 ± 0.06 | 0.52 ± 0.03 |
| 25 | 97.6 ± 0.1 | 0.38 ± 0.04 | 0.56 ± 0.06 |
| 50 | 97.6 ± 0.1 | 0.46 ± 0.05 | 0.68 ± 0.07 |
| 100 | 97.4 ± 0.8 | 0.56 ± 0.05 | 0.81 ± 0.10 |
| 500 | 97.1 ± 0.6 | 0.75 ± 0.08 | 0.86 ± 0.07 |
| 2500 | 97.2 ± 0.4 | 0.37 ± 0.03 | 0.72 ± 0.05 |
| 10000 | 96.7 ± 0.7 | 0.59 ± 0.06 | 0.80 ± 0.04 |
| Chitin obtained by RSM optimization | 97.0 ± 0.7 | 0.4 ± 0.08 | 0.8 ± 0.05 |
Characteristics of chitin extracted
RSM mediated chitin extraction from shrimp processing raw byproducts resulted in 97 ± 0.7 % (w/w) purity with an off white amorphous flakes appearance as wells as odorless and tasteless. In these conditions, the residual content (%, w/w, dwb) of ash (mineral) is 0.4 ± 0.08 and protein is 0.8 ± 0.2 with a moisture content of 2.1 ± 0.2 in the chitin. The FTIR spectrum (Fig. 4) was found to be similar to that of the commercial α-chitin. The percentage of degree of N-acetylation, calculated from the FTIR spectrum, is >93 %. Visual observation and Hunter color analysis of the chitin samples indicated an improved appearance after treatment with solvents as compared to the original product. For Hunter color L values (measures relative lightness), a definite trend was noticed in chitin with the following order of solvent treatment, original (untreated) (69.6) < absolute ethanol (72.3) < H2O2 (73.3). From the AAS results, it was clear that the prepared chitin did not contain sodium and heavy metal lead and microbial count (colony formic unit (CFU)/g) was found to be <10 for total aerobic bacteria, <5 yeast and mold count and 0 for coliform. This result is in accord with specified qualities for food grade chitin (Venugopal 2011).
Fig. 4.
FTIR spectrum of chitin isolated from shrimp processing raw byproducts by response surface methodology approach (a) and commercially available chitin (b)
Conclusion
Chitin is commercially extracted from the abundant seafood processing crustacean byproducts by the conventional acid demineralization and alkali deproteinization treatments. The harsh thermochemical extraction conditions as well as the source and pretreatment of raw material significantly affect its quality and bioactivity. Chitin and its principal derivatives chitosan are used in many fields, and their commercial applications are expanding every year. Even though, there are many reports on chitin extraction from crustacean byproducts, there has been no uniform method for the preparation of chitin with high quality due to the chemical/proximate complexity of the raw materials. The performance of the preparation of chitin from shrimp processing raw byproducts was investigated with a statistical procedure based on RSM in order to elucidate and optimize the factors which may maximize the yield and quality of chitin. The optimum conditions, viz. concentration of HCl solution 4.5 % (v/v), reaction time 3 h, solid liquid ratio of HCl solution 1:5.5 (w/v) and two treatments for wet materials, and concentration of HCl solution 4.9 % (v/v), reaction time 3 h, solid liquid ratio of HCl solution 1: 7.9 (w/v) and two treatments, for dry materials achieve the conditions to obtain >98 % demineralization of raw SPB. Similarly, the optimum processing conditions, viz. concentration of NaOH solution 3.6 % (w/v), reaction time 2.5 h, treatment temperature 69.0 ± 1 °C, solid liquid ratio of NaOH solution 1:7.4 (w/v) and two treatments accomplish the conditions to attain >99 % deproteinization of demineralized of SPB. The optimal conditions attained by RSM would be useful to isolate chitin with high quality (>98 % purity) from shrimp processing raw byproducts.
Acknowledgment
SPV express his gratitude to Council of Scientific and Industrial Research (CSIR), New Delhi, India for the partial financial support (EMPOWER Project) and NT thanks University Grant Commission (UGC), New Delhi, India for award of Research Fellowship.
Contributor Information
T Nidheesh, Email: nidheesht.11@gmail.com.
P V Suresh, Phone: +91-821-2514840, Email: drsureshpv@cftri.res.in, Email: drsureshpv@hotmail.com.
References
- AOAC . Official methods of analysis of AOAC International. 17. Gaithersburg, MD: AOAC International; 2000. [Google Scholar]
- Aranaz I, Mengíbar M, Harris R, Paños I, Miralles B, Acosta N, Heras Á. Functional characterization of chitin and chitosan. Curr Chem Biol. 2009;3(2):203–230. [Google Scholar]
- Baxter A, Dillon M, Anthony Taylor KD, Roberts GA. Improved method for ir determination of the degree of N-acetylation of chitosan. Int J Biol Macromol. 1992;14(3):166–169. doi: 10.1016/S0141-8130(05)80007-8. [DOI] [PubMed] [Google Scholar]
- Chang KLB, Tsai G. Response surface optimization and kinetics of isolating chitin from pink shrimp (Solenocera melantho) shell waste. J Agric Food Chem. 1997;45(5):1900–1904. doi: 10.1021/jf9606870. [DOI] [Google Scholar]
- Duarte ML, Ferreira MC, Marvao MR, Rocha J. An optimized method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int J Biol Macromol. 2002;31(1):1–8. doi: 10.1016/S0141-8130(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Gillett R (2008) Globel study of shrimp fisheries. Food and Agriculture organization (FAO), United Nations, Rome
- Jayakumar R, Nwe N, Tokura S, Tamura H. Sulfated chitin and chitosan as novel biomaterials. Int J Biol Macromol. 2007;40(3):175–181. doi: 10.1016/j.ijbiomac.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Jayakumar R, Prabaharan M, Nair SV, Tamura H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv. 2010;28(1):142–150. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Kurita K. Chitin and chitosan: functional biopolymers from marine crustaceans. Mar Biotechnol. 2006;8(3):203–226. doi: 10.1007/s10126-005-0097-5. [DOI] [PubMed] [Google Scholar]
- Mantgomery DC. Design and analysis of experiments. Singapore: Wiley; 1984. [Google Scholar]
- Nidheesh T, Suresh PV. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014;150:392–399. doi: 10.1016/j.foodchem.2013.10.083. [DOI] [PubMed] [Google Scholar]
- Percot A, Viton C, Domard A. Optimization of chitin extraction from shrimp shells. Biomacromolecules. 2003;4(1):12–18. doi: 10.1021/bm025602k. [DOI] [PubMed] [Google Scholar]
- Shimahara K, Takiguchi Y. Preparation of crustacean chitin. Methods Enzymol. 1988;161:417–423. [Google Scholar]
- Statsoft . Statistica for windows. Tulsa: Statsoft Inc; 1997. [Google Scholar]
- Suresh PV. Biodegradation of shrimp processing bio-waste and concomitant production of chitinase enzyme and N-acetyl-D-glucosamine by marine bacteria: production and process optimization. World J Microbiol Biotechnol. 2012;28(10):2945–2962. doi: 10.1007/s11274-012-1106-2. [DOI] [PubMed] [Google Scholar]
- Suresh PV, Kumar PA, Sachindra NM. Thermoactive β-N-acetylhexosaminidase production by a soil isolate of Penicillium monoverticillium CFR 2 under solid state fermentation: parameter optimization and application for N-acetyl chitooligosaccharides preparation from chitin. World J Microbiol Biotechnol. 2011;27(6):1435–1447. doi: 10.1007/s11274-010-0596-z. [DOI] [PubMed] [Google Scholar]
- Suresh PV, Sachindra NM, Bhaskar N. Solid state fermentation production of chitin deacetylase by Colletotrichum lindemuthianum ATCC 56676 using different substrates. J Food Sci Technol. 2011;48(3):349–356. doi: 10.1007/s13197-011-0252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synowiecki J, Al-Khateeb NA. Production, properties, and some new applications of chitin and its derivatives. Crit Rev Food Sci Nutr. 2003;43:45–171. doi: 10.1080/10408690390826473. [DOI] [PubMed] [Google Scholar]
- Nidheesh T, Kuttappan AKP, Vallabaipatel E, Kandasamy M, Suresh PV (2014) Statistical optimization of solid state fermentation conditions for the enhanced production of thermoactivechitinases by mesophilic soil fungi using response surface methodology and their application in the reclamation of shrimp processing by-products. Ann Microbiol 64(2):617–681
- Venugopal V. Marine Polysaccharides: Food Applications. New York: CRC Press, Taylor and Francis; 2011. [Google Scholar]
- Wang S-L, Hsu W-H, Liang T-W. Conversion of squid pen by Pseudomonas aeruginosa K187 fermentation for the production of N-acetyl chitooligosaccharides and biofertilizers. Carbohydr Res. 2010;345(7):880–885. doi: 10.1016/j.carres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Wang S-L, Tseng W-N, Liang T-W. Biodegradation of shellfish wastes and production of chitosanases by a squid pen-assimilating bacterium, Acinetobacter calcoaceticus TKU024. Biodegradation. 2011;22(5):939–948. doi: 10.1007/s10532-011-9453-5. [DOI] [PubMed] [Google Scholar]