Abstract
The ability of button mushroom (Agaricus bisporus) in changing physical, chemical, microbial and sensory properties of fish patties prepared from sutchi catfish (Pangasius hypophthalmus) was investigated. Two batches of fresh patties were prepared, one batch was treated with 15 % button mushroom (TP) and other batch was a control (CP) without mushroom. The patties were packed in polythene bags and stored under refrigerated condition (6 ± 2 ºC) without adding any preservatives for the estimation of storage stability. The analyses of patties were conducted at regular intervals of 3 days. The results showed that, Peroxide value, Thiobarbituric acid value, Free fatty acids increased significantly in CP at the end of 12 days whereas the TP was within the acceptable limit up to 16 days. Total volatile base nitrogen and Trimethylamine nitrogen also showed a similar trend. The Total plate count and Aerobic spore formers showed an increasing trend in CP when compared to TP. The sensory scores showed that the overall acceptability of CP were lower than TP, which was acceptable even after 16 days of storage. The present study showed that, the quality and storage stability of TP were observed to be in good condition up to 16 days and started deteriorating 20th day onwards, whereas the CP were acceptable only up to 12 days. Therefore it can be recommended that, addition of 15 % of button mushroom to sutchi catfish patty not only increases the nutritional quality but also increases the shelf life of patties under refrigerated storage.
Keywords: Sutchi catfish, Patty, Mushroom, Quality, Shelf life
Introduction
The consumption rate of fish and fishery products has increased around the world due to recognition of their nutritional value (Wang et al. 2010). There is great demand for fish based products specially value added products, ready-to-eat ‘convenience’ form and the prominent among them is battered and breaded products. Lee (1997) suggested that innovative products based on minced meat from seafood can maximize the value of the resources that are available to seafood industry. The current situation in fish processing industry demands a need to introduce new products based on fish mince which are stable, acceptable and nutritious. Addition of fish in the diet not only improves the nutritional quality but also results in increased consumption of meal. Therefore, fish products as a condiment in ‘ready-to-cook’ or ‘ready-to-eat’ form appear to have a good potential (Reddy et al. 2012). Fish and fishery products contain high quality protein and other necessary nutrients; they are low in saturated fatty acids and high in unsaturated fatty acids content (Rathod et al. 2012). Fish patties are popular products in India. However, the other sources than beef and chicken have been suggested for production of patties, since red meat contains high levels of cholesterol (Vicente and Torres 2007) fish muscle can be an alternative due to its high levels of ω-3 fatty acid content and low levels of cholesterol. Therefore, knowledge of suitability of farmed Sutchi catfish mince for production of fish patties is relevant to fish processing industry.
Apart from the dominance of carp culture towards aquaculture production various other fish resources have come into picture and Pangasius is one of the most important species. There has been tremendous growth in production of Sutchi catfish and has reached 1.2 mmt (FAO 2010). Owing to its remarkable growth rate, the production of this catfish in India is estimated to about 200,000 ton per annum. Processing of Pangasius into frozen fillets has been widely practiced in India with great demand in international market. Delicate flavor and firm texture when cooked, absence of fishy odour, small bones and skin makes Pangasius fillet suitable for production of surimi and various mince based products (Orban et al. 2008).
Production of Pangasius has increased in India but fish farmers practicing aquaculture have expressed that the cost of feed, seed, fertilizers and lease rent have gone up considerably. Proportionately the farm gate price of fish has not increased leading to reduced margins. In fact, the fish farmers who are cultivating Pangasius in India are just about making it breakeven (Rathod et al. 2012). During recent year’s value addition have received wider attention because of increased urbanization there is a growing demand for value added products due to social and cultural changes (Pagarkar et al. 2011). Therefore, to increase profitability, development of value added product from Pangasius (P. hypophthalmus) is urgent need of utilization for human consumption.
Extensive studies have been done on the use of various types of fat replacer and plant dietary fibre in processed meat and poultry products in attempts at increasing dietary fibre and lowering fat content. Various types of dietary fibres from cereal and legumes have been utilized in an attempt to improving nutritional quality and at the same time reducing production cost of meat based patties (Wan Rosli et al. 2011). Recently mushrooms (Pleurotus ostreatus) were used as a substitute for pork meat in the development of the Thai glutinous fermented sausage (Chockchaisawasdee et al. 2010). Button mushroom has recently gained attention as a “functional food” because of its unique nutrient profile and potential to confer functional properties. Mushrooms have been used for traditional foods and medicines in Asia. Generally, mushrooms are rich in dietary fiber, minerals, vitamins, and low in fat. Moreover, mushrooms contain various polyphenolic compounds recognized as an excellent as antioxidant such β-glucan, tocopherol, and Vitamin C etc. Niki et al. (1994) have reported that the antioxidant activity of A. bisporus due to the presence of ascorbic acid and phenolic compounds.
The dietary fibres present in the mushroom are associated with the speeding up of the transit time of bowel contents, increases bulk, frequency and ease of faecal voiding. They are also said to protect the body from irritable bowel syndrome and colon cancer. Carbohydrates and fibre from plant based materials have been used in enhancing texture while reducing formulation cost (Pinero et al. 2008). Dietary fibre can also provide several functional properties to food. They improve oil holding capacity, water holding capacity, emulsification and gel formation. Another functional property of dietary fibres is to modify textural properties, stabilize high fat food, improve shelf-life and avoid syneresis (Elleuch et al. 2011).
Although data on patties prepared from marine fishes like Alaskan Pollock (Theragra chalcogramma) and Hoki (Macruronus novaezelandiae) are available (Anon 2010) but those on fresh water fish are meagre. However, Sehgal et al. (2010) have reported recently that, the preparation of patties from Labeo rohita, but the patty prepared using this species has low consumer preference due to the presence of intramuscular spines. Whereas sutchi catfish has favorable attributes like firm texture after cooking, delicate flavor, absence of fishy odour and small bones which, makes Pangasius suitable for production of various minced based products. Therefore the present work is focused on “Influence of button mushroom (Agaricus bisporus)” on quality and refrigerated storage stability of patties prepared from Sutchi catfish (Pangasius hypophthalmus)
Materials and methods
The freshwater catfish (Pangasius hypophthalmus) and fully grown fresh button mushroom were procured from local market, Mangalore. The fish were between 1,400 and 1,500 g in weight. The fresh fish were transferred to the processing hall in iced condition with a ratio of 1:1 (w/w). The fish was thoroughly washed in chilled water for further process. The button mushroom was processed by rinsing with clean water for 3 to 4 times and chopped coarsely until a uniform size ranged from 2 to 5 mm is obtained.
Preparation of fish patty
The fish was dressed so as to remove scale, fins, head and entrails. The dressed fish was thoroughly washed with chilled water and excess water was drained. The fish meat separation was carried out by reciprocatory meat picking machine SG, model of Toyo Seikan Kaisha Ltd, Japan. The separated meat was minced in order to reduce the particle size using meat mincer (M-3, Type-42, supplied by Toyo Seikan Kaisha Limited, Japan). The mincing operation were carried out in two stages, the first stage using bigger perforations disc (5–6 mm dia) and second stage in smaller perforated disc (2 mm dia).
After washing button mushroom were subjected for chopping to get uniform particle size of 2–5 mm diameter. Then the boiled potatoes were made into paste manually. Spices like black pepper, clove, cinnamon, short and long chilli, cardamom and coriander were powdered using grinder. Ginger and garlic were cleaned in potable water and made into paste. The addition of ingredients to the fish mince was carried out manually. The composition of the mix is given in the Table. 1. The mixed fish paste with mushroom was moulded into round shape using a mould. The average weight of individual fish patties was 33 g, with a thickness of 15 mm in height and 50 mm in diameter. Patty was divided into two batches i.e., treated patty (TP) and control patties (CP) and packed in a polythene bags. They were stored in a refrigerator (6 ± 2 °C). The samples were drawn in triplicates for each analysis with an interval of 3 days.
Table 1.
Ingredients used for preparation of fish patties
| Sl. No. | Ingredients (%) | CP | TP |
|---|---|---|---|
| 1. | Fish mince | 79.0 | 64 |
| 2. | Button mushroom | Nil | 15 |
| 3. | Potato | 10 0.0 | 10 0.0 |
| 4. | Soya flour | 4.0 | 4.0 |
| 5. | Breadcrumbs | 1.0 | 1.0 |
| 6. | Ginger | 1.0 | 1.0 |
| 7. | Garlic | 1.0 | 1.0 |
| 8. | Salt | 0.9 | 0.9 |
| 9. | Chilli | 0.8 | 0.8 |
| 10. | Sugar | 0.5 | 0.5 |
| 11. | Chilled water | 0.3 | 0.3 |
| 12. | vegetable oil | 0.2 | 0.2 |
| 13. | Polyphosphate | 0.2 | 0.2 |
| 14. | Cinnamon | 0.2 | 0.2 |
| 15. | Coriander | 0.2 | 0.2 |
| 16. | Black pepper | 0.15 | 0.15 |
| 17. | Cardamom | 0.1 | 0.1 |
| 18. | Clove | 0.07 | 0.07 |
The nutritional composition of button mushroom (moisture, proteins, fat, carbohydrates and ash) were estimated using the standard methods of AOAC (2005). Total carbohydrates were calculated by difference. The total dietary fiber was expressed by subtracting the protein and ash contents from the residue by following the method demonstrated by Prosky et al. (1998)
The Biochemical composition of fish patty such as moisture content was determined by weight difference between the homogenized sample before and after oven-drying for 16 h at 100 ± 1 °C (AOAC 2000) and crude fat and ash content were estimated by using AOAC (2005) method and crude protein was determined by using Microkjeldahl method by AOAC (1990). The results were expressed as percentage.
The pH of fish patties were measured using digital pH meter (Systronix μ pH system 361) following AOAC (2005) method. Oxidative stability of stored fish patties was measured using titrimetric determination using standard methodologies. The primary stage of oxidation was estimated by peroxide value (AOCS 1999) and expressed as milli eq. of O2 per kg of fat, thiobarbituric acid reacting substances (TBARS) (Tarladgis et al. 1960) of sample was estimated spectrophotometrically (Systronics Vis double beam spectro 1203, Ahmedabad, India) and expressed as mg malonaldehyde per kg of sample and free fatty acids (Link, 1959) were determined in samples and expressed as mg% as oleic acid. Total volatile base nitrogen (TVB-N) and trimethylamine (TMA) contents of each sample were estimated using the Conway micro diffusion assay according to the method of Conway and Byrne (Conway and Byrne 1936). Total plate counts were determined by the pour plate method, using Plate Count Agar (PCA, Oxoid, CM463) containing 0.5 % NaCl as the medium. Plates were incubated at 35 °C for 48 h (Harrigan and Mccanee 1976). Microbiological data were transformed into logarithms of the number of colony forming units (CFU/ml). Aerobic spore formers were estimated according to the procedures described in APHA (1992).
Sensory quality of Pangasius fish patties were evaluated by minimum of 12 trained panelists, using a nine point hedonic scale (1-dislike extremely to 9-like extremely) for product acceptability. Panelists scored for appearance, colour, taste, texture, odour and overall acceptability. During the evaluation sessions, the samples were coded by a letter and presented in random order
All the analysis was carried out in triplicate. The data were subjected to analysis of variance (ANOVA) and the significant differences were mentioned as p < 0.05, and the means were separated with Duncan’s multiple range tests and expressed as mean values ± standard deviation (SD) using SPSS (2000) Statistical Package for Social Science. software version 16.0 (SPSS Inc., Illinois, USA).
Results and discussion
Nutritional composition
The result of the nutritional composition of button mushroom is represented in Table 2. The moisture content is observed to be more i.e., 81.79 % followed by 29.29 % of protein content based on dry weight basis. This value is very close to the percentage range with those reported previously by Sharma and Vaidya (2011). Mushrooms contain a high moisture percentage depending on the mushroom species and other parameters related to harvest, growth, culinary and storage conditions (Guillamón et al. 2010). Mattila et al. (2001) have reported that mushrooms are reported to be a good source of protein, which is in accordance with the present study. The button mushrooms revealed lower fat content i.e., 2.22 % and showed highest ash content of 7.12 %. Carbohydrate was calculated discounting protein, ash and fat content was abundant macronutrient, which was observed to be 20.59 %. Total fiber content was observed to be 24.56 %. Manzi et al. (2004) reported that a very high level of total fiber in Agrocybe aegerita, Agaricus bisporus, P. seryngii and P. ostreatus, which is similar to the present study.
Table 2.
Nutritional composition of fresh button mushroom (Agaricus bisporus)
| Sl. No. | Parameters | Percentage |
|---|---|---|
| 1 | Moisture* | 81.79 ± 0.36 |
| 2 | Crude protein | 29.29 ± 0.20 |
| 3 | Crude fat | 2.22 ± 0.25 |
| 4 | Carbohydrates | 20.57 ± 0.27 |
| 5 | Fibre | 24.56 ± 0.09 |
| 6 | Ash | 7.12 ± 0.19 |
Data indicate analyses of triplicates ± standard deviation
*All factors except the moisture were calculated based on dry weight
The treated and untreated patties were stored under refrigerated temperature at 6 ± 2 °C and analysed over a period of 12 days for control and 20 days for treated patties. There were significant (p < 0.05) differences in proximate composition between CP and TP. Protein, lipid and ash contents were observed to be higher in CP whereas moisture content was found to be higher in TP. The decrease in the moisture content during the refrigerated storage (6 ± 2 °C) is represented in Tables 3 and 4. The initial moisture content of CP and TP were observed to be 70.05 % and 74.11 %, respectively. Wan Rosli et al. (2011) have reported similar decrease in moisture content during refrigerated storage, which could be probably due to the inability of mushroom fiber to create a tridimensional matrix within the patties due to the high moisture content. The button mushroom used in the present work was observed to have high moisture content (81.79 %). The ash content was increased in the control patties from 2.37 to 3.93 % whereas in case of treated patties it increased marginally from 2.13 to 3.6 %.
Table 3.
Changes in biochemical composition of treated fish patties during refrigerated storage (6 ± 2 °C)
| Sl. No. | Parameters (g%) | Treated (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | 20 | ||
| 1 | Moisture | 74.11 ± 0.09a | 72.01 ± 0.03b | 73.25 ± 0.09c | 72.75 ± 0.09c | 71.39 ± 0.25b | 70.27 ± 0.05d |
| 2 | Crude Fat | 7.3 ± 0.06a | 7.6 ± 0.05a | 7.83 ± 0.16b | 8.25 ± 0.07c | 9.27 ± 0.14d | 9.94 ± 0.14d |
| 3 | Crude Protein | 16.26 ± 0.19a | 16.02 ± 0.06a | 15.81 ± 0.07b | 15.01 ± 0.06c | 14.68 ± 0.17c | 13.68 ± 0.17d |
| 4 | Ash | 1.08 ± 0.04a | 1.29 ± 0.09b | 1.43 ± 0.15b | 1.65 ± 0.05b | 1.94 ± 0.05cb | 2.35 ± 0.05d |
Data explain analyses of triplicates ± standard deviation and a-d Different letters within the column are significantly different (p < 0.05)
Table 4.
Changes in biochemical composition of control fish patties during refrigerated storage (6 ± 2 °C)
| Sl. No. | Parameters (g%) | Control (days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | 16 | 20 | ||
| 1 | Moisture | 70.05 ± 0.12a | 68.12 ± 0.09b | 65.22 ± 0.08c | 65.01 ± 0.13c | * | |
| 2 | Crude Fat | 9.14 ± 0.13a | 9.45 ± 0.04b | 9.82 ± 0.25cb | 9.95 ± 0.21 cd | * | |
| 3 | Crude Protein | 20.46 ± 0.16a | 19.93 ± 0.18b | 19.15 ± 0.27b | 16.23 ± 0.05d | * | |
| 4 | Ash | 1.77 ± 0.04a | 2.21 ± 0.05b | 2.51 ± 0.04b | 3.13 ± 0.09c | * | |
*spoilt
Data indicate analyses of triplicates ± standard deviation and a-d Different letters within the column are significantly different (p < 0.05)
Changes in biochemical composition
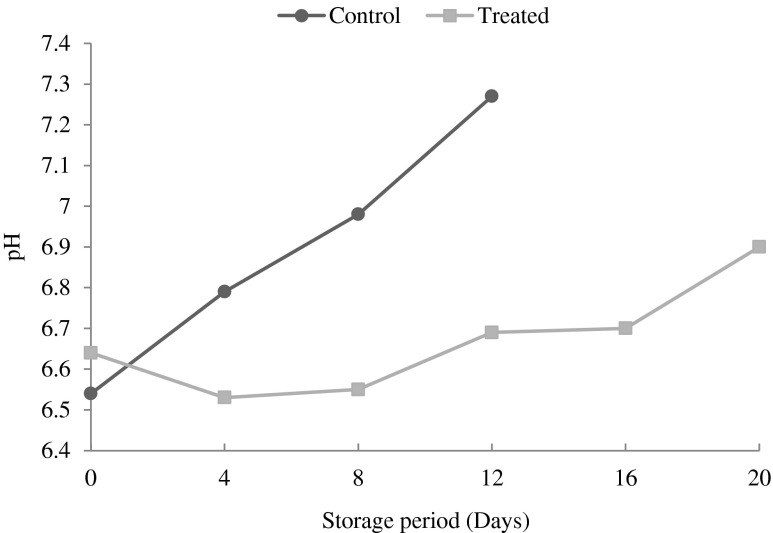
The pH value is related to the post-mortem evolution of the fish flesh and is influenced by the species, diet, seasons, level of activity or stress during the catch as well as category of muscle (Periago et al. 2005). The initial pH of the treated patties was 6.64 and 6.54 in control patties (CP) Fig. 1. Changes in pH values during the refrigerated storage of fish and fishery product were observed by Fan et al. (2009) and Song et al. (2010). The pH in CP increased extremely to 7.27 at the end of 12 days. The increase of pH value might be attributed to the increase in volatile bases (e.g., ammonia and trimethylamine) produced by either endogenous or microbial enzymes (Manat et al. 2005). However the pH content of TP increased slightly during the study period and observed to be 6.90 at the end of 20 days of refrigerated storage.
Fig. 1.
Changes in pH of control and treated patties stored during refrigerated storage (6 ± 2 °C)
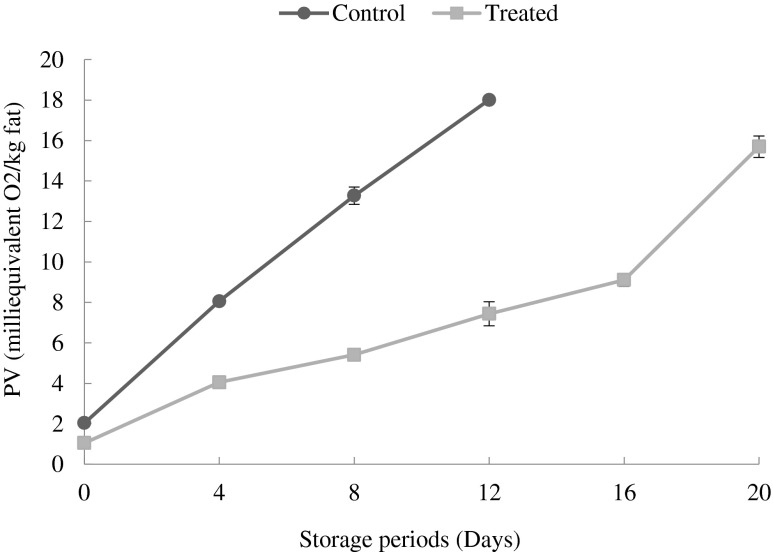
Changes in peroxide value (PV)
In the present study, the peroxide value (PV) was employed for determining the formation of primary lipid oxidation products during the storage period (Fig. 2). The results indicated that the PV of the CP increased significantly (p < 0.05) from an initial value of 2.04 to 18.01 meq.O2 / kg of fat at the end of 12 days when compared to TP it was only 7.44 meq. O2 / kg of fat indicating that, the TP were still in good condition. This could be attributed to the antioxidant properties of mushroom that were incorporated at a level of 15 %. Yang and Maguer (2002) reported that phenols such as tocopherol, found in mushrooms are known to have effective antioxidant properties. Thiansilakul et al. (2012) reported that the total phenols in the extracts of mushroom ranged from 14 to 21 mg / g. The results showed that the button mushroom was effective in retarding the production of peroxide / hydroperoxides radicals in TP during the refrigerated storage. However, at the end of 20 days the PV in TP were observed to be 15.70 meq.O2 / kg of fat, indicating that, a gradual increase in PV of patties with mushroom were in propagation stage of lipid oxidation with a lower rate of decomposition of hydroperoxides formed.
Fig. 2.
Changes in peroxide value of control and treated patties stored during refrigerated storage (6 ± 2 °C) Bars indicate standard deviations from triplicate determinations
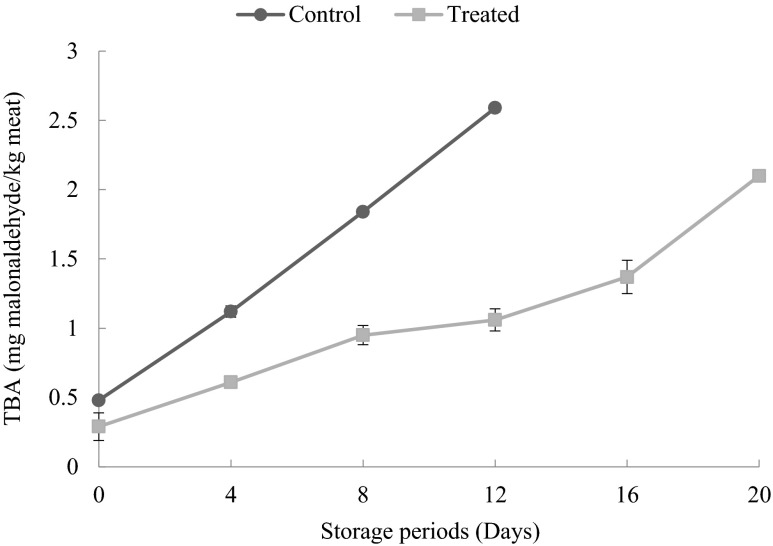
Changes in thiobarbituric acids (TBA)
Thiobarbituric acid (TBA) is widely used as an indicator of degree of lipid oxidation, and the presence of TBA reactive substances is due to the second stage of auto-oxidation during which peroxides are oxidized to aldehydes and ketones (Lindsay 1991). In this study, changes in TBA value are shown in Fig. 3. The initial TBA value of CP was observed to be 0.48 mg malonaldehyde / kg of meat and showed an increased to 2.59 mg malonaldehyde / kg of meat at the end of 12 days, whereas in the case of TP the TBA values raised slowly from 0.29 mg malonaldehyde / kg of meat and on 16th day it was only 1.37 mg malonaldehyde / kg of meat indicating the TP were still in good condition but at the end of 20 days it reached a maximum of 2.10 mg malonaldehyde /kg of meat. The increase in TBA value during the refrigerated storage may be attributed to the partial dehydration of product and to the increased oxidation of unsaturated fatty acids. This observation of the increase of the TBA value during refrigerated storage is in agreement with results reported by Yanar and Fenercioglu (1998) for fish balls made from carp and by Gelman and Benjamin (1988) for minced meat of silver carp. The TBA values of control patties (CP) rose continuously and reached 2.59 mg malonaldehyde / kg of meat on 12th day of refrigerated storage, which is usually regarded as the limit, beyond which the fish will normally develop an objectionable odour and taste (Connell 1990). Hence it can be understood from the present study that the keeping quality of TP was better than CP i.e., up to 16 days, beyond which the sample started to degrade in its quality.
Fig. 3.
Changes in thiobarbituric acid values of control and treated patties during refrigerated storage (6 ± 2 °C) Bars indicate standard deviations from triplicate determinations
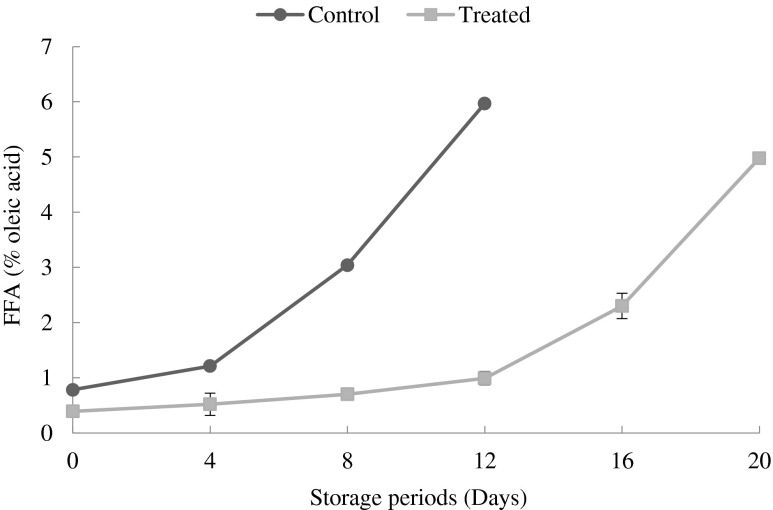
Changes in free fatty acids (FFA)
The free fatty acid is a suitable means for assessment of fish quality during storage and can be used as a quality index of fish and other food products (Hui and Tung 1997). It is well known that FFA is a result of enzymatic decomposition of lipid in fish and fisheries products (Hardy 1980). Pacheco-Aguilar et al. (2000) have reported that, lipases and phospholipases hydrolyze glycerol-fatty acid to release free fatty acids. Free fatty acids have been shown to oxidize faster than their triacylglycerols (Labuza et al. 1969; Yanishlieva-Maslarova 1985) and faster than ethyl and methyl esters (Miyashita and Takagi 1986). Thus the free fatty acids exert a pro-oxidative effect. This effect is due to complex formation between hydroperoxides and carboxyl groups through a hydrogen bond, which results in an accelerated decomposition of hydroperoxides into free radicals (Miyashita and Takagi 1986). Bimbo (1998) has suggested a maximum acceptable value of 5 % as quality specifications for crude fish oil. In the present study increase in free fatty acid content was not detected during the 0 day however changes were observed to start from 4th day, where the CP and TP showed FFA content of 1.21 and 0.52 mg % of Oleic acid respectively. The level of FFA for the CP reached its maximum of 5.97 mg % of Oleic acid at the end of the 12 days as shown in Fig. 4. It was observed that there was considerable difference (p < 0.05) in FFA between the CP and TP on 12th day as TP recorded only 0.99 mg % of Oleic acid. The lower FFA was observed in TP while the highest FFA was found for the CP. The FFA values increased gradually with an increasing storage period. Lipid hydrolysis developed at a slower rate in the patties added with button mushroom, which could be attributed to the effect of phenolic compounds present in it. A number of studies on mushroom have been focused on biological activities of phenolic compound as a potential antioxidant and free radical scavengers (Marja and Anu 1999). Presence of phenols in A. bisporus made this macro fungus a good candidate for formulating antioxidant products. The study showed that, the FFA level of TP reached close to 5 % (i.e. 4.98 mg % of Oleic acid) only on 20th day indicating that the addition of mushroom extends the keeping quality of fish patty when compared to the control sample.
Fig. 4.
Changes in free fatty acid values of control and treated patties during refrigerated storage (6 ± 2 °C) Bars indicate standard deviations from triplicate determinations
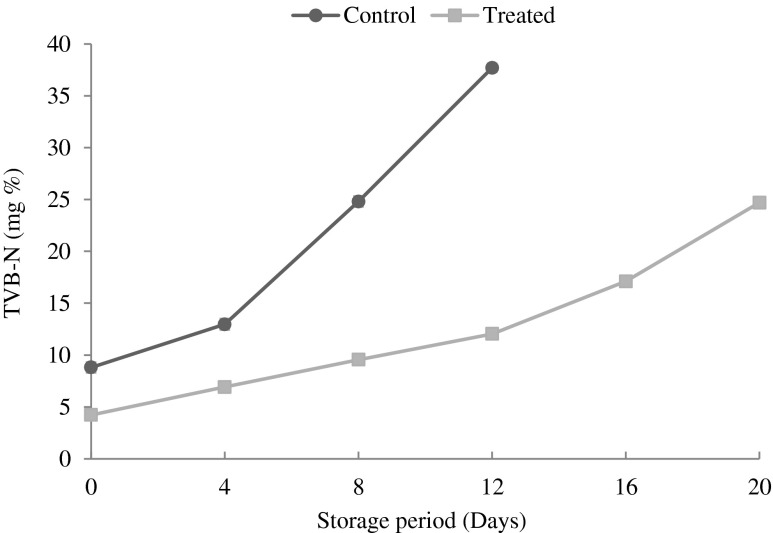
Changes in total volatile base nitrogen (TVB-N):
Total volatile base nitrogen (TVB-N), is mainly composed of ammonia, primary, secondary and tertiary amines (Beatty 1938) as a result of degradation of proteins and non-protein nitrogenous compounds, which is chiefly caused by microbial activity (Ruiz-Capillas and Moral 2005). TVB-N is widely used as an indicator for quality deterioration of meat (Olafsdottir et al. 1997). A level of 35 to 40 mg % of fish muscle is usually regarded as spoiled (Lakshmanan 2000). In the present study, changes in TVB-N are shown in Fig. 5. The results showed that, the values increased gradually in all samples during the refrigerated storage. Initially TVB-N values were observed to be 8.80 mg % for the control patties and 4.23 mg % for treated patties. TVB-N value increased regularly from 8.80 mg % and reached a maximum of 37.70 mg % in CP at the end of 12 days of storage. Similarly Kose et al. (2009) found that, TVB-N value for whiting burgers during refrigerated storage increased from the initial value of 2.02 mg % to maximum value of 42.03 mg %. In the case of TP the values ranged between 4.23 and 24.70 mg % at the end of 20th day of refrigerated storage. Lang (1983) reported that the quality classification of fish and fishery products based on TVB-N values would be ‘high quality’ up to 25 mg %; ‘good quality’ up to 30 mg %; ‘limit of acceptability’ up to 35 mg %, and ‘spoilt’ above 35 mg %. On the 12th day of storage, CP crossed the limit of acceptance (37.70 mg %) whereas TP were in good condition. This increase was significantly lower in treated TP when compared to CP. This can be attributed to either a more rapidly reduced bacterial population or decreased capacity of bacteria for oxidative deamination of non-protein nitrogen compounds or both (Banks et al. 1980)
Fig. 5.
Changes in total volatile base nitrogen of control and treated patties during refrigerated storage (6 ± 2 °C) Bars indicate standard deviations from triplicate determinations
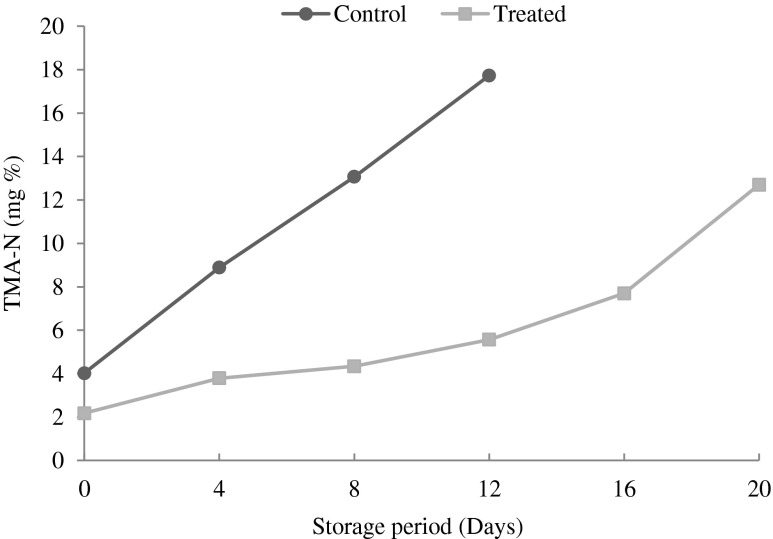
Changes in trimethylamine nitrogen (TMA-N):
TMA-N is often used as an index in assessing the shelf-life and keeping quality of fishery products because of their rapid accumulation in the meat under refrigerated condition (Ocano-Higuera et al. 2009). The TMA-N production in fish tissue during refrigerated storage could be used as an indicator of bacterial activity and it is an accepted measure for deterioration. The pungent odour of spoilt fish has been often related to the presence of TMA, also with the number of spoilage organisms present in many fish species (El-Marrakchi et al. 1990). The acceptable limit of TMA-N for fishery product is 10 to 15 mg / 100 g (Connell 1975). In the present study, the production of TMA-N followed a similar pattern to TVB-N under refrigerated storage, where a significant increase of TMA values was observed (P < 0.05) with time (Fig. 6). TMA-N content of control patties and treated patties on the initial day was 4.02 mg % and 2.18 mg % respectively. TMA-N content gradually increased up to 16.73 mg % for control patties on the 12th day of storage indicating the spoilage whereas in the case of treated patties it was 12.70 mg % on 20th day of storage.
Fig. 6.
Changes in trimethyl amine nitrogen of control and treated patties during refrigerated storage (6 ± 2 °C) Bars indicate standard deviations from triplicate determinations
Changes in microbiological characteristics
Microbial growth during storage will be depended on the preservation conditions. Microbial activity leads to prominent unpleasant changes in aroma of fishery products due to metabolism of amino acids into biogenic amines, sulfides, organic acids and other compounds (Gram and Dalgaard 2002). Microorganisms associated with aquatic products usually reflect the microbial population in their environment. Changes of total plate counts (TPC) during the 20 days of refrigerated storage of treated fish patties and 12 days for control patties are presented in Table 5. The results showed that microbiological growth was significantly (p < 0.05) influenced by the addition of the button mushroom. Initial TPC for both control patties and treated patties were 4.7 × 102 cfu /g and 3.2 × 102 cfu / g respectively. The fish muscle is known to be sterile when caught, but it is quickly contaminated by surface as well as intestinal bacteria, along with contamination from the aquatic environment, equipment, human handling and product formulation (Li et al. 2012). The microbial count of both the patties increased slowly during the initial storage days with a gradual increment in later stage. Sini et al. (2008) noticed an initial decline in total plate count followed by gradual increase in sausages prepared by using rohu (Labio rohita) meat during refrigerated storage. The low count at the initial days of refrigerated storage may be related to the low temperature, which could have resulted in low metabolic rate of bacteria owing to stressful conditions due to low temperature (Ghorban 2002).
Table 5.
Changes in total plate counts and spore formers of control and treated patties during refrigerated storage (6 ± 2 °C)
| Storage days | TPC (cfu / g) | Spores (No. / g) | ||
|---|---|---|---|---|
| CP | TP | CP | TP | |
| 0 | 4.70 × 102 ± 1.24a | 3.20 × 102 ± 1.48a | 3.80 × 101 ± 0.28a | 3.20 × 101 ± 1.03a |
| 4 | 2.62 × 103 ± 1.45b | 6.40 × 102 ± 1.93a | 1.20 × 102 ± 1.63b | 5.30 × 101 ± 1.65a |
| 8 | 1.30 × 105 ± 1.03c | 9.80 × 102 ± 0.83a | 8.24 × 102 ± 0.43c | 9.60 × 101 ± 1.43a |
| 12 | 8.40 × 106 ± 1.87d | 1.39 × 103 ± 1.43c | 7.50× 103 ± 1.43 c | 2.26 × 102 ± 1.71b |
| 16 | * | 2.25 × 104 ± 1.22c | * | 8.13 × 102 ± 1.03c |
| 20 | 1.20 × 106 ± 0.33d | 9.70 × 102 ± 1.73c | ||
*spoilt
Data indicate analyses of triplicates ± standard deviation and a-d Different letters within the row are significantly different (p < 0.05)
TPC in treated fish patties samples rose continuously after 12th day of refrigerated storage and reached about 1.20 × 106 cfu / g on the 20th day of storage. The increase in TPC in fish products during refrigerated storage has been demonstrated by Lyon and Reddmann (2000). TPC of TP was found to be the same as CP during the end of the refrigerated storage, but initially it was observed to increase slowly when compared to control samples. The significant reduction in TPC observed in treated patties could be attributed to the inhibitory effect of mushroom on spoilage bacteria. Antimicrobial activity of button mushroom must have been due to the presence of essential bioactive components including catechin, caffeic acid and rutin. Catechin which is one of the phenolic components has been found to exhibit antimicrobial activity (Shimamura et al. 2007). Caffeic acid and rutin has been shown to exhibit antimicrobial activity (Baise et al. 2002). Further storage resulted in the increase in bacterial count which could be related to the growth of Psychrotrophic bacterial population in the fish patties.
Aerobic spore formers also increased during the refrigerated storage period (Table 5.) The aerobic spore count increased from 3.20 × 101 to 9.70 × 102 cfu / g in treated patties and 3.80 × 101 to 7.50 × 10 3 cfu / g in control patties till end of the storage periods.
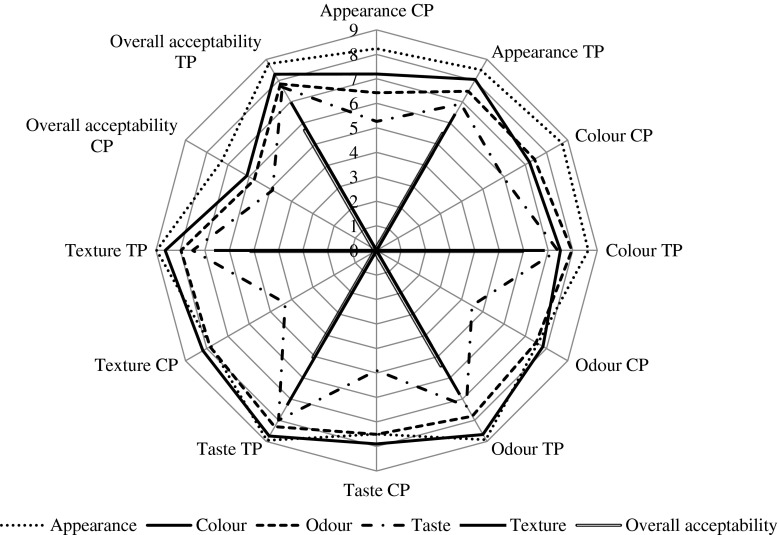
Changes in sensory characteristics
The Sensory evaluation is a scientific discipline that evokes, measures, analyses and interprets reactions to those characteristics of food and materials as they are perceived by the senses of vision, odour, taste, touch and hearing (Sidel and Stone, 2006). The acceptability of fish and fishery products during refrigerated storage depends on the changes in their sensory attributes, which were evaluated in terms of appearance, colour, taste, texture, odour and overall acceptability (Fig. 7). The results of organoleptic evaluation of CP and TP showed a decreasing trend in the overall acceptability during refrigerated storage. According to the statistical analysis, there were no significant differences in appearance of all groups during the storage. But there were significant differences (p < 0.05) in colour, flavor and texture between the TP and CP. This could be attributed to formation of some volatile low molecular weight compounds, lipid oxidation and protein degradation during storage (Undeland and Lingnert 1999) and (Pawar 2011). The sensory properties of TP received a higher score than CP, where CP was acceptable up to 12 days and TP was in good condition during the whole 16 days of refrigerated storage. From the data, we can observe that TP could retain good quality characteristics in terms of sensory assessment. In addition the result of sensory evaluation is supported by the results of biochemical analyses.
Fig. 7.
Changes in sensory scores of fish patties during refrigerated storage at (6 ± 2 °C) CP: Control Patties TP: Treated Patties
Conclusion
The biochemical and sensory qualities indicated that the TP was in good condition up to 16 days and started deteriorating 20th day onwards, whereas the CP was acceptable only up to 12 days. It can be concluded that, addition of spices in patties such as ginger, garlic, pepper, clove and cinnamon offers the preservative effect due to their antioxidant and antimicrobial properties whereas the compounds present in button mushroom viz., β-glucans, Catechin, and Vitamin C etc. provides a synergetic effect to the product. Therefore it can be recommended that, addition of 15 % of button mushroom to Sutchi catfish patty not only increases the nutritional quality but also improves the shelf life of patties under refrigerated storage.
Acknowledgement
The authors wish to express their sincere thanks to the Dean, College of Fisheries, Mangalore for providing facilities to conduct this work. Authors also thank the Head, Department of Fish Processing Technology for extending support to conduct this work. The financial assistance from All India Coordinated Research Project on Post Harvest Technology, CIPHET (ICAR) Ludhiana, India, in carrying out this study is gratefully acknowledged.
Contributor Information
Prakash Chandra Nayak, Phone: 0484-2421990, Email: nayakpc420@gmail.com.
C. V. Raju, Phone: 0484-2421990, Email: cvraj@hotmail.com, Email: cvrajfish@gmail.com
I. P. Lakshmisha, Phone: 0484-2421990, Email: iplaxmish@gmail.com
Rajkumar Ratankumar Singh, Phone: 0484-2421990, Email: singhratan14@gmail.com.
Faisal Rashid Sofi, Phone: 0484-2421990, Email: sofi.faisal@gmail.com.
References
- Anon (2010) Alaska pollock. http://en.wikipedia.org/wiki/Alaska_Pollock. Accessed 6 April 2010
- AOAC . Official methods of analysis. 18. Washington: Association of Official Analytical Chemists; 1990. [Google Scholar]
- AOAC . Official methods of analysis. 18. Washington: Association of Official Analytical Chemists; 2005. [Google Scholar]
- AOAC (2000) Official method of analysis 934.01 (17th edn) Volume I. Association of Official Analytical Chemists, Inc., Maryland, USA
- AOCS . Official methods and recommended practices of American Oil Chemists Society. 5. Champaign: A.O.C.S; 1999. [Google Scholar]
- APHA Recommended methods for the microbial examination of foods. Broadway: Am Public Health Assoc. 1992;19:181–188. [Google Scholar]
- Baise HP, Walker TS, Stermitz FR, Hufbauer RS, Vivanco JM. Enantiometric dependent phytotoxic and antimicrobial activity of catechin; a rhizosecreted racemic mixture from Centaurea maculosa (spotted knapweed) Plant Physiol. 2002;128:1127–1135. doi: 10.1104/pp.011019. [DOI] [PubMed] [Google Scholar]
- Banks H, Nickelson R, Finne G. Shelf life studies on carbon dioxide packaged finfish from Gulf of Mexico. J Food Sci. 1980;45:157–162. doi: 10.1111/j.1365-2621.1980.tb02566.x. [DOI] [Google Scholar]
- Beatty S. Studies of fish spoilage. The origin of trimethylamine produced during the spoilage of cod muscle press juice. J Fish Res Board Can. 1938;4:63. doi: 10.1139/f38-008. [DOI] [Google Scholar]
- Bimbo AP (1998) Guidelines for characterizing food grade fish oil. Int News Fats oils Relat Mater 9: 473--483
- Chockchaisawasdee S, Namjaidee S, Pochana S, Stathopoulos CE. Development of fermented oyster-mushroom sausage. Asian J Food Agro-Ind. 2010;3:35–43. [Google Scholar]
- Connell JJ. Control of fish quality. Farham: Survey, VK, Fishing News (Books) Ltd; 1975. [Google Scholar]
- Connell JJ (1990) Control of fish quality (3rd ed.). Oxford, UK: Fishing News Books
- Conway EJ, Byrne A. An absorption apparatus for the micro-determination of certain volatile substances. I. The micro determination of ammonia, J of. Biochemistry. 1936;27:419–429. [PMC free article] [PubMed] [Google Scholar]
- Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;124:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- El-Marrakchi A, Bennour M, Bouchriti N, Hamama A, Tagafait H. Sensory, chemical, and microbiological assessments of Moroccan sardines (Sardina pilchardus) stored in ice. J Food Protect. 1990;53:600–605. doi: 10.4315/0362-028X-53.7.600. [DOI] [PubMed] [Google Scholar]
- Fan WJ, Sun JX, Chen YC, Qiu J, Zhang Y, Chi YL. Effects of chitosan coating on quality and shelf life of silver carp during frozen storage. Food Chem. 2009;115:66–70. doi: 10.1016/j.foodchem.2008.11.060. [DOI] [Google Scholar]
- FAO (2010) Statistical analysis year book. Available: http://www.fao.org/fi/ economics/ess/syb/en/
- Gelman A, Benjamin E. Characteristics of mince from pond-bred silver carp (Hypophthalmichthys molitrix) and preliminary experiments on its use in sausages. J Sci Food Agric. 1988;47:225–241. doi: 10.1002/jsfa.2740470210. [DOI] [Google Scholar]
- Ghorban ZG (2002) Estimation of microbiological and chemical variations in minced fish processing of Atlantic Pollock (Pollachius vireous) Final Project Report. Iranian Fisheries (Shilat) Research Organization 1--30
- Gram L, Dalgaard P (2002) Fish spoilage bacteria-Problems and solutions. Curr Opin Biotechnol 13:262–266 [DOI] [PubMed]
- Guillamón E, García-Lafuente A, Lozano M, D’Arrigo M, Rostagno MA,, Villares A,, Martínez JA, Edible mushrooms: role in the prevention of cardiovascular diseases. Fitoterapia. 2010;81:715–723. doi: 10.1016/j.fitote.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Hardy R. Fish lipids. In: Connell JJ, editor. Advances in fish science and technology. Farnham: Fishing News Book Ltd; 1980. pp. 103–111. [Google Scholar]
- Harrigan WF, Mccanee ME. Laboratory methods in food and dairy microbiology. London: Academic press; 1976. [Google Scholar]
- Hui ZZ, Tung CL. Rapid near-infrared spectroscopic method for the determination of free fatty acid in fish and its application in fish quality assessment. J Agric Food Chem. 1997;45:9–12. [Google Scholar]
- Kose S, Balaban MO, Boran M, Boran G. The effect of mincing method on the quality of refrigerated whiting burgers. Int J Food Sci Technol. 2009;44:1649–1660. doi: 10.1111/j.1365-2621.2009.01984.x. [DOI] [Google Scholar]
- Labuza TP, Tsuyuki H, Karel M. Kinetics in linoleate oxidation in model systems. J Am Oil Chem Soc. 1969;46:409–416. doi: 10.1007/BF02545625. [DOI] [PubMed] [Google Scholar]
- Lakshmanan PT. Fish spoilage and quality assessment. In: Iyer TSG, Kandoran MK, Thomas M, Mathew PT, editors. Quality assurance in seafood processing. Cochin: Soc Fish Technol; 2000. pp. 26–4. [Google Scholar]
- Lang K. Der fluchtige basenstickstoff (TVB-N) bei im binnenland in den verkehr gebrachten frischen seeficchen. 11. Mitteilung. Archiv fu¨r Lebensmittelhygiene. 1983;34:7–10. [Google Scholar]
- Lee CM. Technical strategies for development of formulated seafood products from fish mince. In: Shahidi F, Jones Y, Kitts DD, editors. Seafood safety, processing and biotechnology. Lancaster: Technomic Publishing Company Inc; 1997. [Google Scholar]
- Li T, Hu W, Li J, Zhang X, Zhu J, Li X. Coating effects of tea polyphenol and rosemary extract combined with chitosan on the storage quality of large yellow croaker (Pseudosciaena crocea) Food Control. 2012;25:101–106. doi: 10.1016/j.foodcont.2011.10.029. [DOI] [Google Scholar]
- Lindsay RC (1991) Flavour of fish. In: 8th World Congress of Food Science and Technology, Toronto, Canada, September 29 –4th October
- Lyon WJ, Reddmann CS. Bacteria associated with processed crawfish and potential toxin production by Clostridium botulinum type E in vacuum packaged and aerobically packaged crawfish tails. J Food Protect. 2000;63(12):1687–1696. doi: 10.4315/0362-028x-63.12.1687. [DOI] [PubMed] [Google Scholar]
- Manat C, Soottawat B, Wonnop V, Cameron F. Changes of pigments and colour in sardine (Sardinella gibbosa) and mackerel (Rastrelliger kanagurta) muscle during iced storage. Food Chem. 2005;93:607–617. doi: 10.1016/j.foodchem.2004.10.035. [DOI] [Google Scholar]
- Manzi P, Marconi S, Aguzzi A, Pizzoferrato L (2004) Commercial mushrooms: nutritional quality and effect of cooking. Food Chem 84:201–206
- Marja PK, Anu IH (1999) Activities of plant extracts containing phenolic compounds. J Agric Food Chem 47:3954--3962 [DOI] [PubMed]
- Mattila P, Könkö K, Eurola M, Pihlava J-M, Astola J, Vahteristo L, Hietaniemi V, Kumpulainen J, Valtonen M, Piironen V. Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J Agric Food Chem. 2001;49:2343–2348. doi: 10.1021/jf001525d. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Takagi T. Study on the oxidative rate and prooxidant activity of free fatty acids. J Am Oil Chem Soc. 1986;63:1380–1384. doi: 10.1007/BF02679607. [DOI] [Google Scholar]
- Niki E, Shimaski H, Mino M. Antioxidantism- Free Radical and Biological Defense. Tokyo: Gakkai Syuppan Center; 1994. [Google Scholar]
- Ocaño Higuera VM, Márquez Ríos E, Canizales Dávila M, Castillo Yáñez FJ, Pacheco Aguilar R, Lugo Sánchez ME, García Orozco KD, GracianoVerdugo AZ, (2009) Postmortem changes in cazon fish muscle stored on ice. Food Chem 116: 933938
- Olafsdottir G, Martinsdottir E, Oehlenschlager J, Dalgaarg P, Undeland Method to evaluate fish freshness in research and industry. Trends Food Technol. 1997;8:258–265. doi: 10.1016/S0924-2244(97)01049-2. [DOI] [Google Scholar]
- Orban E, Nevigato T, Lena GD, Masci M, Irene Casini I, Gambelli L, Roberto Caproni R. New trends in the seafood market. Sutchi catfish (Pangasius hypophthalmus) fillets from Vietnam: nutritional quality and safety aspects. Food Chem. 2008;110:383–389. doi: 10.1016/j.foodchem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Pacheco-Aguilar R, Lugo-Sanchez ME, Robles-Burgueno MR. Postmortem biochemical and functional characteristic of Monterey sardine muscle stored at 0 °C. J Food Sci. 2000;65:40–47. doi: 10.1111/j.1365-2621.2000.tb15953.x. [DOI] [Google Scholar]
- Pagarkar AU, Joshi VR, Baug TE, Kedar JG (2011) Value addition is need of seafood industries. Fishcoops 8–14
- Pawar PP (2011) Preparation of battered and breaded product from freshwater fish (Catla catla). M F Sc thesis submitted to Konkan Krishi Vidyapeeth, Dapoli, Maharashtra state, India
- Periago MJ, Ayala MD, Lopez-Albors O, Abdel I, Martinez C, Garcia-Alcazar A. Muscle cellularity and flesh quality of wild and farmed sea bass, Dicentrarchus labrax L. Aqua. 2005;249:175–188. doi: 10.1016/j.aquaculture.2005.02.047. [DOI] [Google Scholar]
- Pinero MP, Parra K, Huerta-Leidenz N, de Moreno LA, Ferrer M, Araujo S, Barboza Y. Effect of oat’s soluble fibre (β-glucan) as a fat replacer on physical, chemical, microbiological and sensory properties of low-fat beef patties. Meat Sci. 2008;80:675–680. doi: 10.1016/j.meatsci.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Prosky L, As NG, Schweizer TF, DeVries JM, Furda I (1988) Determination of total dietary fiber in foods and food products: collaborative study. J Assoc Offic Anal Chem 71:1017--1023 [PubMed]
- Rathod NB, Pagarkar AU, Pujari KH, Gokhale NB, Joshi VR. Standardisation of recipe for fish cutlet product from Pangasianodon hypophthalmus. Ecol Environ Conserv. 2012;18(4):1–6. [Google Scholar]
- Reddy MA, Elavarasan A, Reddy DA, Bhandary MH. Suitability of reef cod (Epinephelus diacanthus) minced meat for preparation of ready to serve product. Adv Appl Sci Res. 2012;3(3):1513–1517. [Google Scholar]
- Ruiz-Capillas C, Moral A. Sensory and biochemical aspects of quality of whole bigeye tuna (Thunnus obesus) during bulk storage in controlled atmospheres. Food Chem. 2005;89(3):347–354. doi: 10.1016/j.foodchem.2004.02.041. [DOI] [Google Scholar]
- Sehgal HS, Sehgal GK, Thind SS, Kaur R. Development of “fish mince pakora” from a cultured carp species. Labeo rohita (Ham) J of Food Process Preserv. 2010;34:15–23. doi: 10.1111/j.1745-4549.2008.00263.x. [DOI] [Google Scholar]
- Sharma S, Vaidya D. White button mushroom (Agaricus bisporus): composition, nutritive value, shelf-life extension and value addition. Int J Food Ferm Technol. 2011;1(2):185–199. [Google Scholar]
- Shimamura T, Zhao WH, Hu ZQ. Mechanism of action and potential for use of tea catechin in an antiinfective agent. Anti-infectAgents Mech Chem. 2007;6:57–62. doi: 10.2174/187152107779314124. [DOI] [Google Scholar]
- Sidel JL, Stone H (2006) Sensory Science: Methodology. In: Hui YH (ed) Handbook of food science, technology & engineering, Vol I, CRC Press Taylor & Francis, USA, pp 3–1–3–10
- Sini TK, Santosh S, Joseph AC, Ravishankar CN. Changes in the characteristics of rohu fish (Labeo rohita) sausage during storage at different temperature. J Food Process Preserv. 2008;32:429–442. doi: 10.1111/j.1745-4549.2008.00188.x. [DOI] [Google Scholar]
- Song YL, Liu L, Shen HX, You J, Luo YK (2010) Effect of sodium alginate based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 1–8
- Tarladgis BG, Watts BM, Younathan MT. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J Am Oil Chem Soc. 1960;37:44–48. doi: 10.1007/BF02630824. [DOI] [Google Scholar]
- Thiansilakul Y, Benjakul S, Eric W, Grunwald EW, Richards MP. Retardation of myoglobin and haemoglobin-mediated lipid oxidation in washed bighead carp by phenolic compounds. Food Chem. 2012;134:789–796. doi: 10.1016/j.foodchem.2012.02.182. [DOI] [PubMed] [Google Scholar]
- Undeland I, Lingnert H. Lipid oxidation in fillets of herring (Clupea harengus) during frozen storage. Influence of pre-freezing storage. J Agric Food Chem. 1999;47:2075–2081. doi: 10.1021/jf980944w. [DOI] [PubMed] [Google Scholar]
- Vicente SJV, Torres EAFS. Formation of four cholesterol oxidation products and loss of free lipids, cholesterol and water in beef hamburgers as a function of thermal processing. Food Control. 2007;18:63–68. doi: 10.1016/j.foodcont.2005.08.009. [DOI] [Google Scholar]
- Wan Rosli WI, Solihah MA, Aishah M, Nik fakurudin NA, Mohsin SSJ. Colour, textural properties, cooking characteristics and fibre content of chicken patty added with oyster mushroom (Pleurotus sajor-caju) Int Food Res J. 2011;18:621–627. [Google Scholar]
- Wang T, Zhang J, Zhang X. Fish quality evaluation based on temperature monitoring in cold chain. Afr J Biotechnol. 2010;9(37):6146–6151. [Google Scholar]
- Yanar Y, Fenercioglu H. The utilization of carp (Cyprinus carpio) flesh as fish ball. Turk J Vet Anim Sci. 1998;23:361–365. [Google Scholar]
- Yang DC, Maguer ML. Mass transfer kinetics of osmotic dehydration of mushrooms. J Food Process Preser. 2002;16(3):215–231. doi: 10.1111/j.1745-4549.1992.tb00203.x. [DOI] [Google Scholar]
- Yanishlieva-Maslarova NV. Differences in the kinetics and mechanism of autoxidation of stearic acid and tristearin. Grasay Aceites. 1985;36:115–119. [Google Scholar]