Abstract
Significance of preharvest salicylic acid (SA) treatments on maturity, quality and postharvest life of grape cv. Flame Seedless were studied during two years. The experiment was performed on 12-year old own rooted, grapevines planted at 3 m × 3 m spacing trained on overhead system. Vines were treated with aqueous solutions of SA (0.0, 1.0, 1.5 and 2.0 mM) at pea stage and at veraison. After harvesting, clusters were divided into two lots in which one was subjected to initial quality evaluation, while the other was stored in cold room (3–4 °C, 90-95 % RH) for evaluation of postharvest quality. SA at the dose of 1.5 and 2.0 mM hastened berry maturity by 3 to 5 days, produced less compact bunches alongside larger berries in contrast to control and the lowest dose. The same doses effectively maintained peel colour, higher firmness, lower pectin methyl esterase activity and electrolyte leakage alongside suppressing degradation of TSS and TA during cold storage. These two doses also exhibited higher efficacy on maintaining anthocyanins, phenols and organoleptic properties while reducing weight loss, rachis browning and decay incidence. Correlation analysis demonstrated that many quality parameters are interdependent. In conclusion, preharvest spray of 1.5 mM SA proved to be an effective means of improving quality and extending postharvest life of grape cv. Flame Seedless.
Keywords: Quality, Salicylic acid, Shelf life, Table grapes, Vitis vinifera L
Introduction
Viticulture is one of major horticultural industries in the world with the area exceeding 70,860.22 km2 (FAOstat 2011). India stands 9th among grape producing countries of the world producing 1.24 million tonnes (FAOstat 2011) of grapes annually, out of which 78 % is used as table grapes. Grape (Vitis vinifera L.) variety Flame Seedless has been recommended by the Punjab Agricultural University quite recently for commercial cultivation in Punjab and adjoining states of North India. Berries of Flame Seedless are light red coloured, ripen during first fortnight of June and has higher total soluble solids (TSS) and lower juice acidity. However, berry size of this variety is medium and the colour development within the cluster is uneven. If the berry size and colour quality could be improved further, the crop would fetch premium prices raising its economic value.
Attempts have been made to improve its quality by adopting cultural practices such as crop load management along with foliar application of ethephon (an ethylene releasing compound—2-chloroethyl phosphonic acid). However, ethylene is a growth retardant that would pose negative impact on important physical properties of the berries such as size, weight and firmness. Furthermore, since it releases ethylene, can drift and get absorbed by the leaves, causing yellowing and defoliation. This would adversely affect vine productivity in the current season and sprouting in the following season (Roberto et al. 2012). On the other hand, the concentration commonly needed for colour improvement often reduces fruit firmness (Peppi et al. 2006) and also increase berry shatter (Zoffoli et al. 2009) during subsequent postharvest period. Although grape is a non climacteric fruit with a relatively low physiological activity, perishable nature of the berry as indicated by weight loss, softening, degradation of colour, increased berry shatter, rachis browning, decay development and degradation of organoleptic, properties impair its long term storability. Moreover, as harvesting of grapes in Punjab coincides with rainy season a bulk of the produce comes to the market within short span of time creating a glut. Therefore, it is imperative to develop a compatible strategy that would improve physical quality parameters of berries, more importantly berry size, colour and firmness, alongside maintaining postharvest quality during subsequent long term storage.
Salicylic acid (SA) or ortho-hydroxyl benzoic acid is an endogenous plant growth regulator of phenolic nature and classified as a growth promoter. It has been found to play a key role in the regulation of plant growth, development and enhance plant vigour under biotic and abiotic stresses (Hayat et al. 2010). It is an important secondary metabolite in grape berries and plays an essential role in determining berry quality such as colour, flavour, astringency and bitterness (Chamkha et al. 2003). As evidenced by recent research reports, SA can enhance physical properties of fruits such as size (Marzouk and Kassem 2011), weight (Elwan and El-Hamahmy 2009) and firmness (Srivastava and Dwivedi 2000; Zhang et al. 2003; Shafiee et al. 2010). In addition, SA was found to hasten maturity of both climacteric and non climacteric fruits like strawberry (Karlidag et al. 2009) and tomato (Mady 2009; Yıldırım and Dursun 2009). Additionally, SA positively affect on reducing fruit respiration, ethylene biosynthesis (Srivastava and Dwivedi 2000) weight loss, decay and softening rate (Babalar et al. 2007; Shafiee et al. 2010) during storage. Therefore, considering the positive effects of SA on fresh horticultural commodities, the present study was conducted to investigate the effects of preharvest treatments of SA on berry maturity, initial harvest quality and maintaining the quality during subsequent long term cold storage of grapes cv. Flame Seedless.
Materials and methods
Experimental procedure
The experiment was performed on 12-year old own rooted grapevine (Vitis vinifera L.) cv. Flame Seedless planted at 3 m × 3 m spacing (440 plants per acre) trained on bower (overhead) system in the vineyard of “New Orchard”, Department of Fruit Science, Punjab Agricultural University, Ludhiana, Punjab, India during two consecutive crop seasons of 2012 and 2013. Twenty vines (5 per treatment) having a girth diameter of 7.22 ± 1.02 cm which bear 60–80 canes (4 buds per cane) were chosen and uniform cultural practises were adopted each season as per the recommendations (Anon 2010). Aqueous solutions of SA (0.0 – control, 1.0, 1.5 and 2.0 mM - Sigma Aldrich Co., USA) were prepared by dissolving them in a small amount of ethanol and bringing to the final volume of 25 L with water. A surfactant Tween 20® (polyoxy ethylenesorbitan monolaurate polyethylene glycol, Sigma Aldrich Co., USA) at the rate of 0.1 % was added to obtain better retention and penetration of SA solution. The prepared solutions were sprayed directly to the clusters of vines (5 L per vine) at pea stage (4–5 mm diameter berry size, two weeks after fruit set) and at veraision (approximately 10 % of the berries of 50 % of the clusters become soft and at colour break) by a sprayer machine until runoff in the morning on a sunny day.
At commercial maturity (TSS ≥ 16 obrix), bunches were harvested manually, packed in plastic crates of 18–20 kg capacity and transported to the postharvest laboratory of the department immediately. Well coloured, properly formed bunches that are free from any visible defects were selected and were divided into two lots out of which one was utilized for evaluation of initial harvest quality (0 day). From the other lot, 2 kg of grapes were packed in low density polyethylene film (LDPE, 37.5 μ) packages each containing in-package dual release SO2 generator pad (Grapage® - Sodium Metabisulphite – 40 %, JK Enterprises, Pune, India). These primary packages were put in to 2 kg capacity CFB boxes and stored in the cold room (3–4 °C, 90–95 % RH) for evaluation of postharvest quality. During cold storage, the measurements were repeated at 30, 45 and 60 up to 75 days.
Maturity, yield and physical properties evaluation
Time taken to attain veraision and harvest maturity (TSS ≥ 16 obrix and ≈ 60 % clusters per vine well coloured) were recorded based on number of days after fruit set (DAFS). After harvesting cluster weight, length and diameter were measured from 10 randomly selected clusters from each vine. Number of clusters per vine were counted and multiplied by average cluster weight to record the yield per vine. The observation on berry characters were recorded on the basis of 50 berries taken from 10 bunches. The 50 berries were first weighed to obtain mean berry weight and then placed in a trough so that their ends or equators are gently touched to measure mean berry length and diameter, respectively. Specific gravity (SG) was measured by water displacement method and calculated by: SG (g/cm3) = weight of the fruit (g) /volume of the fruit (cm3). Peel colour of berries was measured as CIE L*, a*, b* colour scale values using Hunter lab colour difference meter (ColorFlex® EZ, USA). Berry firmness was measured using a Texture Analyzer (TA + HDi® Stable Micro Systems, UK) equipped with a HDP/90 platform and 5 kg load cell. The measurement was made on the equatorial position of the berry with 4 mm probe at a test speed of 1 mm/s to a constant compression distance of 1 mm. The readings were expressed as g/force (Rolle et al. 2011). Membrane electrolyte leakage was measured as described by Jiang et al. (2001) using a conductivity meter (ELICO® CM 180, cell constant 1.04) and the results were expressed as μS/100 g fresh weight (FW).
Anthocyanins, phenols and enzymatic activity of pectin methyl esterase
Total anthocyanin content of berries was determined as described by Ranganna (1986) with the extraction solvent ethanolic HCL and absorbance was noted at 535 nm wavelength by spectrophotometer (Spectronic 200+, Thermo Scientific). Total phenols were determined by Folin-Ciocalteu method, based on colourimetric oxidation/reduction reaction of phenols (Slinkard and Singleton 1977). Phenol extraction was carried out with 80 % ethanol and the absorbance was measured at 765 nm by spectrophotometer (Spectronic 200+, Thermo Scientific) against a blank. The results were expressed as mg of gallic acid equivalents/100 g FW using a gallic acid standard curve. The enzymatic activity of pectin methyl esterase was carried out with 5 g of tissue sample as per the AOAC (2005). The volume of 0.02 N NaOH consumed to adjust the pH to 7.5 was recorded and the results were expressed as μg/g FW.
Determination of other chemical properties
For analysis of other chemical parameters, 50 berries from each replicate were squeezed and the juice obtained was filtered through a cheese cloth. Total soluble solids (TSS) was measured by a temperature compensated digital refractometer (Atago PAL-1, model 3810, Japan) and expressed as obrix. Titratable acidity (TA) was determined as per AOAC (2005) and expressed as grams of tartaric acid equivalents/100 ml of juice. TSS/TA ratio was calculated by dividing the values of TSS by TA.
Weight loss, berry shatter and decay incidence
Physiological loss in weight (PLW) was recorded by subtracting final weight from the initial weight of the clusters and then expressed as percent weight loss with reference to the initial weight. Percent berry shatter was calculated for each box by dividing the weight of free berries from the total weight of packed clusters. Decay incidence was expressed as a proportion (by weight) of rotten berries relative to total weight of berries within each box.
Evaluation of rachis browning and organoleptic quality
Rachis condition was rated according to Crisosto et al. (2002), as described. 1 = healthy, entire rachis including the pedicels are green and healthy, 2 = slight, rachis in good condition, but noticeable browning of pedicels, 3 = moderate, browning of pedicels and secondary rachis, 4 = severe, pedicels, secondary and primary rachis completely brown. The berries were rated for organoleptic quality by a panel of 30 judges on the basis of colour, texture, flavour and overall acceptability. A nine point hedonic scale described by Amerine et al. (1965) was used for its inference.
Experimental design and analysis
The field experiment was performed according to randomized complete block design with five blocks while the postharvest experiment was carried out as complete randomized design (CRD) with three replicates. Data were analyzed for variance by using SAS (V 9.3, SAS Institute Inc., USA). When interactions between treatments were significant (P ≤ 0.05), the effect of each treatment was determined separating means by Least Significant Difference (LSD). Pearson correlation coefficient was calculated for some selected parameters to assess the nature and extent of relationship between them. Data of rachis browning and organoleptic quality was analyzed by Kruskal Wallis test and Friedman test respectively, using MINITAB 15 software (Minitab Inc., USA).
Results and discussion
Maturity, yield and physical properties
Clusters treated with 1.5 and 2.0 mM SA attained veraison 3 days earlier in 2012 and 5 days earlier in 2013 seasons compared to the control (0.0 mM SA). Berries in both these treatments ripened early and developed more uniform colour, than the control in both the seasons. Grapes treated with 1.0 mM SA attained harvest maturity on the same day as the control in 2012, but 2 days earlier in 2013. SA treatment increased photosynthetic pigments and total carbohydrates (Mady 2009), and promotes translocation of sugars from leaves to fruit (Elwan and El-Hamahmy 2009) which can be postulated that it hastens maturity. Early yields when treated with SA have been reported for strawberry (Karlidag et al. 2009) and tomato (Mady 2009; Yıldırım and Dursun 2009).
Significant increase in cluster weight, length, breadth and yield were observed in both seasons when vines were sprayed with 1.5 or 2.0 mM SA, compared to control and 1.0 mM SA (Table 1). Berry weight, length and breadth were prominently higher in clusters treated with these two higher doses (1.5 and 2.0 mM) of SA on the contrary to control and the lowest dose (1.0 mM) (Table 2). Evidently, grapes treated with either 1.5 or 2.0 mM SA produced less compact bunches (as indicated by higher cluster length and breadth) alongside larger berries in contrast to control. SA enhances bioproductivity of crops by increasing leaf area, photosynthetic pigments and subsequently rate of photosynthesis (Hayat et al. 2010). Positive effects of SA on these parameters might account for improved cluster and berry physical properties. This is an advantage because best price of table grapes are always fetched by properly shaped clusters with large berries both in domestic and export markets. None of the SA treatments could affect berry specific gravity significantly in two crop seasons examined (Table 2).
Table 1.
Cluster weight, length, breadth and yield per vine of grape cv. Flame Seedless subjected to different treatments of salicylic acid during two consecutive crop seasons of 2012 and 2013
| Concentration (mM) | Cluster weight (g) | Cluster length (cm) | Cluster breadth (cm) | Yield (kg vine−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| 0.0 | 220.0d | 500.0d | 21.3b | 23.2c | 8.33b | 13.13b | 25.02c | 30.00d |
| 1.0 | 312.3c | 620.7b | 19.3c | 29.0b | 12.67a | 13.33b | 31.33b | 35.65b |
| 1.5 | 351.0b | 662.3a | 23.3a | 32.8a | 12.33a | 19.83a | 34.60a | 38.99a |
| 2.0 | 359.2a | 643.6c | 23.8a | 33.0a | 12.33a | 20.33a | 35.92a | 33.49c |
| P | 0.001 | 0.001 | 0.003 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| LSD | 6.36 | 21.6 | 1.44 | 2.03 | 0.86 | 0.87 | 0.04 | 1.73 |
Means in a column with the same letter are not significantly different (at P ≤ 0.05) according to LSD. Each value represent mean of 3 replicates (n = 10).
Table 2.
Berry weight, length, breadth and specific gravity of grape cv. Flame Seedless subjected to different treatments of salicylic acid during two consecutive crop seasons of 2012 and 2013
| Concentration (mM) | Berry weight (g) | Berry length (cm) | Berry breadth (cm) | Specific gravity(g cm−3) | ||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| 0.0 | 1.56c | 2.38c | 1.28b | 1.71b | 1.29b | 1.59c | 1.09a | 1.11a |
| 1.0 | 1.46d | 2.97b | 1.26b | 1.87a | 1.19c | 1.65b | 1.12a | 1.09a |
| 1.5 | 1.69b | 3.33a | 1.54a | 1.93a | 1.48a | 1.77a | 1.00a | 0.99a |
| 2.0 | 1.90a | 3.00b | 1.55a | 1.98a | 1.49a | 1.76a | 1.13a | 1.12a |
| P | 0.0001 | 0.0001 | 0.0001 | 0.008 | 0.0001 | 0.0001 | NS | NS |
| LSD | 0.04 | 0.18 | 0.02 | 0.13 | 0.02 | 0.05 | - | - |
Means in a column with the same letter are not significantly different (at P ≤ 0.05) according to LSD. Each value represent mean of 3 replicates (n = 50 for berry weight, length and breadth; n = 10 for specific gravity)
NS not significant
Firmness
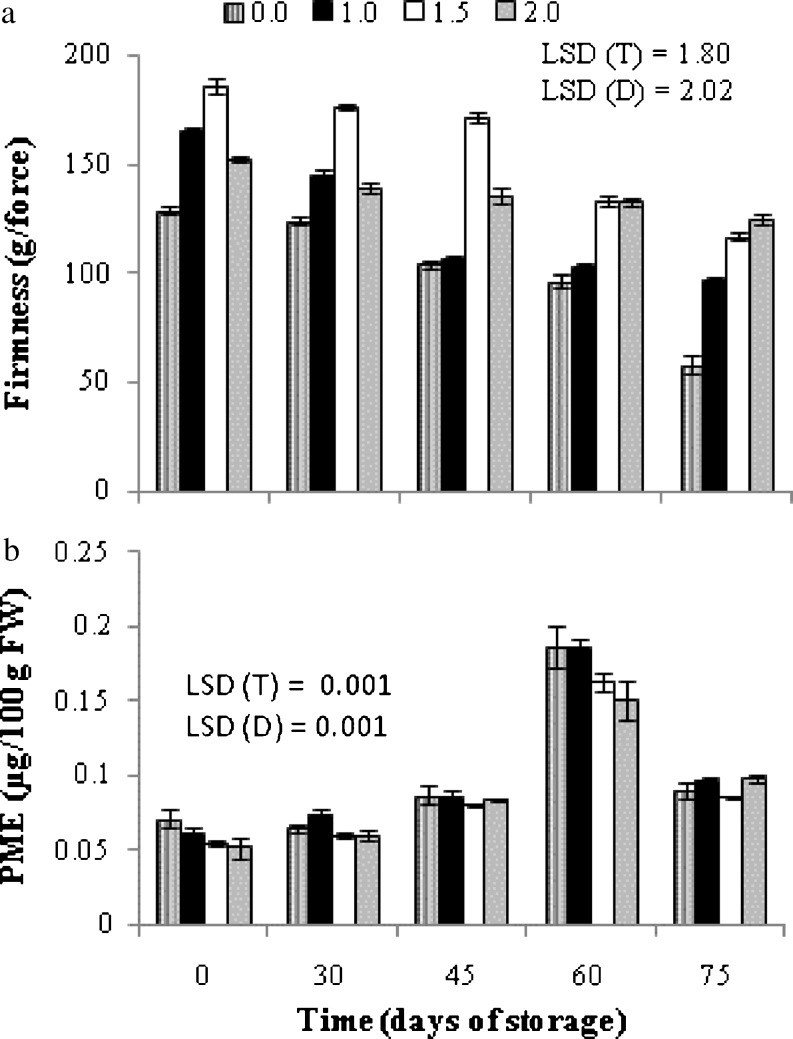
Berry firmness (Fig. 1a) declined during storage at low temperature despite treated or control. However, firmness was always significantly higher in SA treated berries than in control group out of which clusters sprayed with 1.5 and 2.0 mM SA were the most effective. At the end of 75 days of cold storage period, SA treated fruits maintained almost 2 fold higher firmness on the contrary to control. Firmness is one of the main indicators in judging the quality of table grapes for fresh consumption. Grapes lose their firmness by loss of water and/or by changes in their structure/composition during postharvest storage (Bermstein and Lustig 1981). SA prevents fruit softening by affecting activities of major cell wall degrading enzyme such as cellulase, polygalactouronase and xylanase (Srivastava and Dwivedi 2000; Zhang et al. 2003). Rapid softening of fruits during ripening was simultaneous with rapid decrease in endogenous SA (Srivastava and Dwivedi 2000). The correlation analysis (Table 3) in our experiment showed that firmness is negatively correlated with physiological loss in weight (PLW), enzymatic activity of pectin methyl esterase (PME) and membrane electrolyte leakage (MEL) indicating the influence of biophysical and biochemical properties in maintaining berry firmness during storage.
Fig. 1.
Variation in berry firmness (panel a) and enzymatic activity of pectin methyl esterase (PME, panel b) of grape cv. Flame Seedless during cold storage (3–4 °C, 90–95 % RH) in relation to different treatments of salicylic acid. Results represent pooled data of two seasons in 2012 and 2013.Vertical bars represent ± S.E. of means for 6 replicates (3 per season), (0.0, 1.0, 1.5 and 2.0 are salicylic acid concentrations in mM)
Table 3.
Relationship between some selected quality parameters of grape cv. Flame Seedless treated with different concentrations of salicylic acid during 75 days of cold storage (3–4 °C, 90–95 % RH) in two consecutive crop seasons of 2012 and 2013
| Variables compared | Pearson correlation coefficient (r) | |
|---|---|---|
| 2012 | 2013 | |
| Firmness vs. PLW | − 0.42** | − 0.59** |
| Firmness vs. PME | − 0.71** | − 0.68** |
| Firmness vs. MEL | − 0.83** | − 0.83** |
| Lightness vs. TAC | − 0.27** | − 0.37** |
| a* vs. TAC | 0.62** | 0.62** |
| Berry shatter vs. PLW | 0.77** | 0.79** |
| Decay incidence vs.PLW | 0.71** | 0.75** |
| Decay incidence vs.TPC | − 0.60** | − 0.58** |
| MEL vs. PME | 0.63** | 0.62** |
| PLW vs. MEL | 0.44** | 0.42** |
PLW physiological loss in weight, PME enzymatic activity of pectin methyl esterase, MEL membrane electrolyte leakage, TAC total anthocyanin content, TPC total phenol content
**Significant at P < 0.01
Enzymatic activity of pectin methyl esterase
Irrespective of treated or control, pectin methyl esterase (PME) activity of grape berries increased slowly up to 45 days and then exhibited a sharp rise coming to a peak on 60 days of storage (DOS) thereafter showed a sharp fall for the next 15 days (Fig. 1b). However, lower PME activity was shown by the fruit treated with 1.5 and 2.0 mM SA in contrast to control and the lowest dose (1.0 mM) up to 60 DOS. Pectic substances, cellulose, and hemicellulose are the major cell wall polysaccharides, some of which are depolymerized during ripening leading to fruit softening. In many fruits, including grapes, pectin degradation is occurred initially by the action of PME. It catalyses the hydrolysis of methyl-ester groups from galacturonosyl residues and plays important roles in determining the extent to which demethylated polygalacturonans are accessible to degradation by polygalacturonases (Barnavon et al. 2001). Therefore, the positive effect of SA on suppression of fruit softening enzymes might account for firmer berries in the treated group as compared to control.
Peel colour
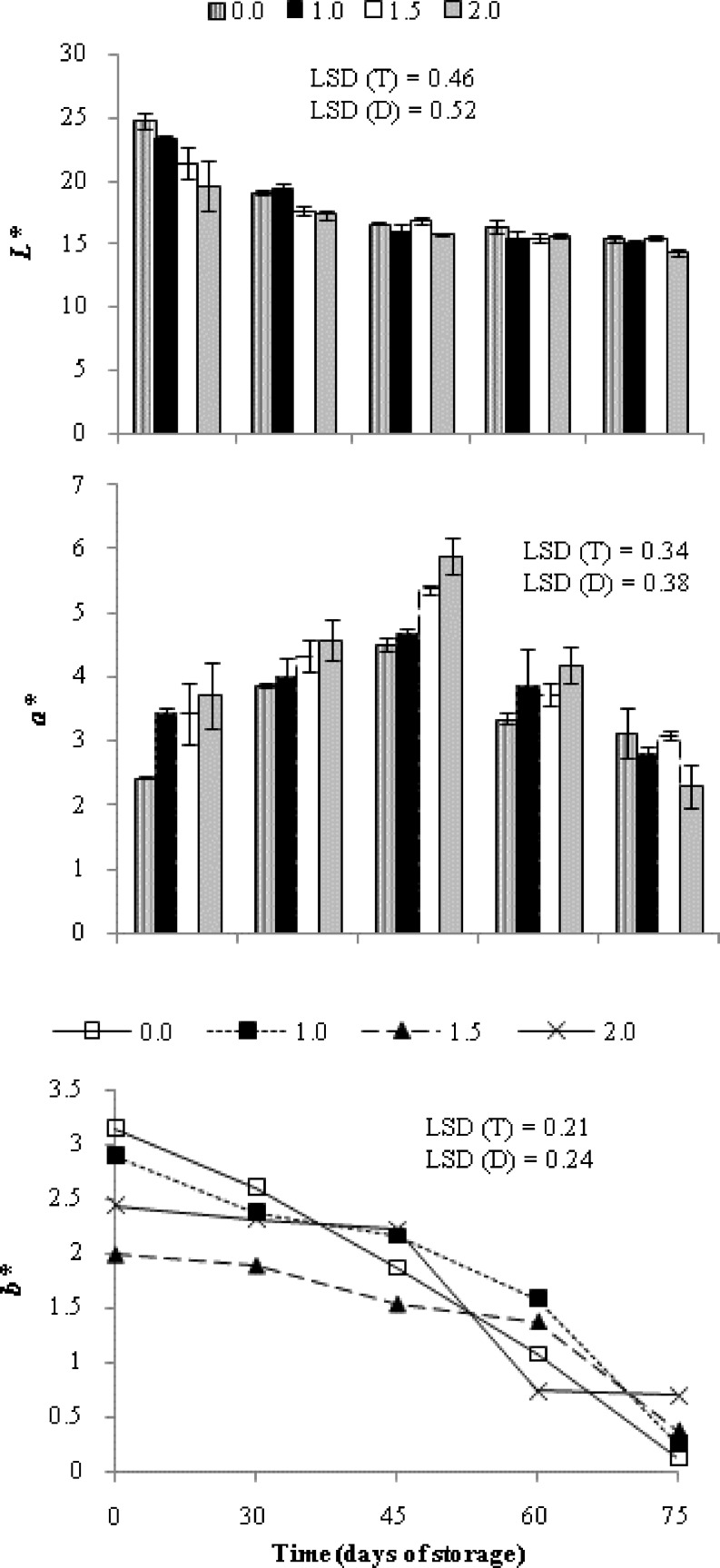
As Flame Seedless berries mature, their colour changes from a relatively pure green to yellow, and eventually to red. At harvest, SA treatment recorded a significantly lower L* and b* values along with higher a* value (Fig. 2) such that fruit appears darker and redder in contrast to control group. During 75 days of cold storage, berry lightness decreased continuously irrespective of treatments. Clusters treated with 1.5 and 2.0 mM SA effectively maintained higher a* (redness) and lower b* (blueness) values up to 60 DOS on the contrary to control and 1.0 mM SA. The results are in line with Shafiee et al. (2010) who reported that SA treatment does not diminish the colour brightness of strawberry.
Fig. 2.
Variation in peel colour (L *, a * and b *) of grape cv. Flame Seedless during cold storage (3–4 °C, 90–95 % RH) in relation to different treatments of salicylic acid. Results represent pooled data of two seasons in 2012 and 2013.Vertical bars represent ± S.E. of means for 6 replicates (3 per season, n = 30 berries), (0.0, 1.0, 1.5 and 2.0 are salicylic acid concentrations in mM)
Anthocyanins
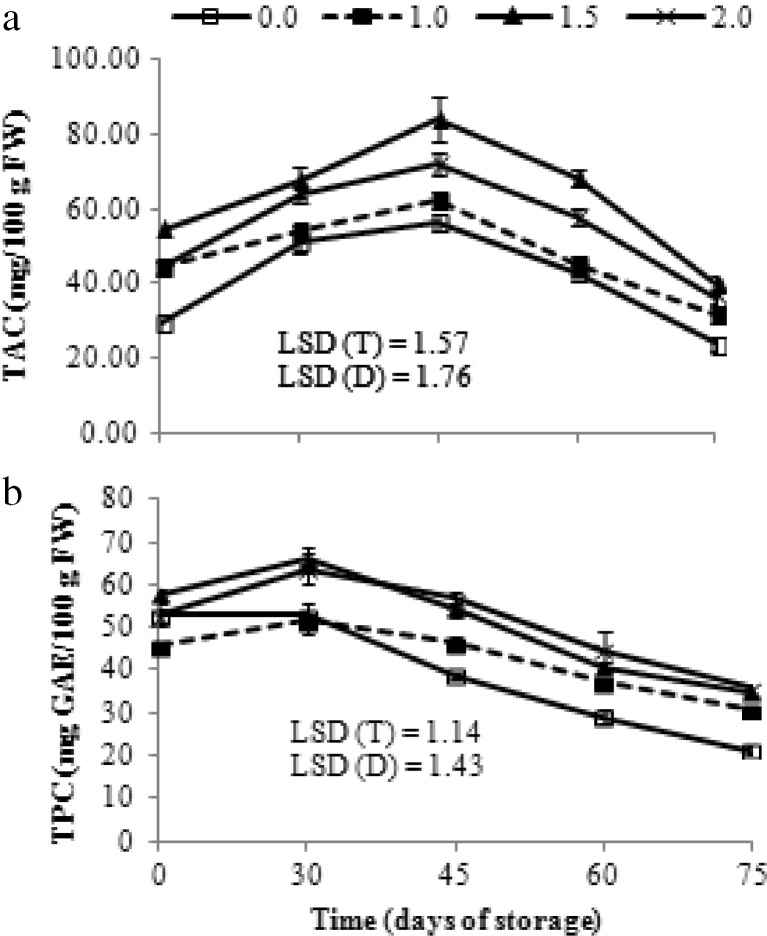
The pigment responsible for red colouration of grapes is anthocyanins and in cv. Flame Seedless cyanidin-3-glucoside is the most abundant pigment (Fernandez-Lopez et al. 1998). These pigments are mainly confined to the skin and, the external colour and anthocyanic profile are closely linked (Fernandez-Lopez et al. 1998). Total anthocyanin content (TAC) of grape berries displayed an increasing trend up to 45 days and then declined gradually (Fig. 3a) during rest of the period. All three concentrations of SA had significantly higher TAC on the contrary to control in which significant reduction in these pigments were detected during prolonged storage. Clusters sprayed with 1.5 mM SA demonstrated higher efficacy in suppressing the degradation of TAC followed by 2.0 mM and 1.0 mM doses. The results are in accordance with previous work (Jamali et al. 2013). According to correlation analysis (Table 3), lightness (L*) vs. TAC showed significant negative correlation whereas, a* vs. TAC showed significant positive correlation. The results comply with Peppi et al. (2006) who reported that lightness has a highly significant inverse relationship with anthocyanins. As skin anthocyanins rises lightness decreases while a* increases such that fruit appears darker.
Fig. 3.
Variation in total anthocyanin content (TAC, panel a) and total phenol content (TPC, panel b) of grape cv. Flame Seedless during cold storage (3–4 °C, 90–95 % RH) in relation to different treatments of salicylic acid. Results represent pooled data of two seasons in 2012 and 2013.Vertical bars represent ± S.E. of means for 6 replicates (3 per season), (0.0, 1.0, 1.5 and 2.0 are salicylic acid concentrations in mM), GAE: gallic acid equivalents
Phenols
Total phenol content (TPC) of all grape samples with or without SA treatment first increased, reached a peak value at 30 day, then decreased gradually during rest of the cold storage period (Fig. 3b). Preharvest SA treatment significantly affected total phenol content (TPC) of berries in dose dependent manner of which the highest concentration maintained the highest TPC. Promotion of phenolic compounds with foliar application of SA has been observed by Sarikhani et al. (2010) and Jamali et al. (2013). Generally, phenolic compounds are an important group of grapes secondary metabolites and strongly influence the berry quality such as colour, flavour, bitterness, astringency and berry functional properties (Chamkha et al. 2003). They are also involved in enhancing antimicrobial properties of grape berries. In our experiment, correlation analysis showed that there is significant negative correlation between TPC and decay incidence (Table 3).
TSS, TA and TSS/TA ratio
All three doses of SA displayed lower TSS contents compared to control at harvest (day 0) (Table 4). During 75 days of cold storage, clusters treated with 1.5 and 2.0 mM SA effectively retarded the degradation of TSS on the contrary to control in which dramatic reduction of TSS was detected after 45 DOS. Our results are in accordance with previous work (Tareen et al. 2012). TA content of the grapes initially declined up to 45 days in cold storage then showed an increasing trend during next 30 days (Table 4). Higher two doses (1.5 and 2.0 mM) of SA effectively retarded the rate of reduction in TA and this was obvious at each storage interval in contrast to the lowest dose (1.0 mM ) and the control. The highest TSS/TA ratio was observed in 2.0 mM SA treated berries at harvest (Table 4) while there was no significant difference observed among two lower doses (1.0 and 1.5 mM) and the control on this date. Increase in TSS/TA ratio was prominent in control group up to 45 DOS which then declined rapidly, whereas SA treated samples effectively suppressed the deviation of TSS/TA from its initial values for up to 60 DOS. SA suppress rate of respiration and ethylene biosynthesis (Srivastava and Dwivedi 2000; Zhang et al. 2003; Babalar et al. 2007) which might account for retardation of ripening related changes.
Table 4.
Total soluble solids (TSS), titratable acidity (TA) and TSS/TA ratio of Flame Seedless grape berries subjected to different treatments of salicylic acid at initial harvest (day 0) and during cold storage (3–4 °C, 90–95 % RH)
| Parameter | Time (days of storage) | ||||
|---|---|---|---|---|---|
| 0 | 30 | 45 | 60 | 75 | |
| TSS (obrix) | |||||
| Concentration (mM) | |||||
| 0.0 | 18.6a | 18.8a | 19.1a | 15.9d | 15.2c |
| 1.0 | 16.8c | 17.6d | 17.9d | 17.1c | 15.8b |
| 1.5 | 17.3b | 18.3c | 18.5c | 17.4b | 16.1a |
| 2.0 | 17.5b | 18.4b | 18.7b | 17.8a | 16.0a |
| LSD(P < 0.05) | 0.38 | 0.09 | 0.19 | 0.13 | 0.12 |
| TA(g of tartaric acid equiv./100 ml juice) | |||||
| Concentration (mM) | |||||
| 0.0 | 0.75a | 0.58c | 0.52b | 0.60c | 0.60b |
| 1.0 | 0.67b | 0.58c | 0.53b | 0.61c | 0.60b |
| 1.5 | 0.68b | 0.65a | 0.60a | 0.64b | 0.62a |
| 2.0 | 0.63b | 0.63b | 0.59a | 0.68a | 0.62a |
| LSD(P < 0.05) | 0.06 | 0.03 | 0.02 | 0.02 | 0.01 |
| TSS/TA ratio | |||||
| Concentration (mM) | |||||
| 0.0 | 24.80b | 32.41a | 36.73a | 26.50b | 25.33a |
| 1.0 | 25.07b | 30.34b | 33.77b | 28.03a | 26.33a |
| 1.5 | 25.44b | 28.15c | 30.83c | 27.19ab | 25.97a |
| 2.0 | 27.78a | 29.21b | 31.69bc | 26.18b | 25.81a |
| LSD(P < 0.05) | 2.21 | 1.30 | 2.13 | 1.23 | 1.25 |
Means in a column with the same letter are not significantly different (at P < 0.05) according to LSD. Each value represent mean of 6 replicates (3 per season) during two seasons of 2012 and 2013, respectively. (n = 50).
Physiological loss in weight
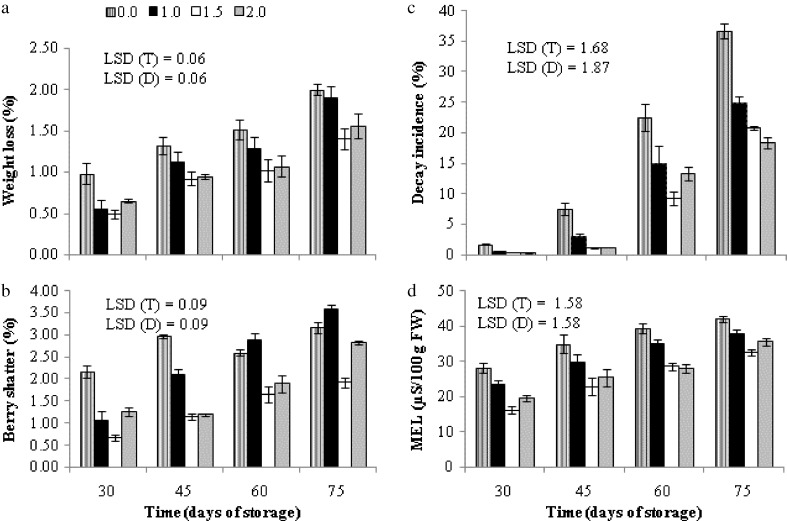
The physiological loss in weight (PLW) of all grape clusters with or without SA treatment increased during 75 days in cold storage (3–4 °C, 90–95 % RH), but the rate of increase is different among the treatments (Fig. 4a). All three concentrations of SA exhibited a comparatively lower rate of PLW on the contrary to control and by the end of 75 days of cold storage period, cumulative weight loss of control berries was 5.82 % of their initial weight while this percentage in 1.0, 1.5 and 2.0 mM SA treated clusters were 4.88, 3.83 and 4.23 respectively. SA as an electron donor produces free radicals which prevent normal respiration thus leading to lower weight loss (Shafiee et al. 2010). Further, SA can decrease respiration through inhibition of ethylene biosynthesis or action (Srivastava and Dwivedi 2000). SA also cause decrease respiration rate and fruit weight loss by closing stomata.
Fig. 4.
Variation in physiological loss in weight (panel a) berry shatter (panel b), decay incidence (panel c) and membrane electrolyte leakage (MEL, panel d) of grape cv. Flame Seedless during cold storage (3–4 °C, 90–95 % RH) in relation to different treatments of salicylic acid. Results represent pooled data of two seasons in 2012 and 2013.Vertical bars represent ± S.E. of means for 6 replicates (3 per season, n = 2 kg clusters), (0.0, 1.0, 1.5 and 2.0 are salicylic acid concentrations in mM)
Berry shatter
The percent berry shatter was significantly affected (P < 0.0001) by SA treatment (Fig. 4b). All SA treated clusters exhibited a significantly lower shatter percentage compared to the control up to 45 DOS. Thereafter, percent berry shatter of clusters that received 1.0 mM SA increased dramatically and from 60 days onwards it showed no significant difference with control. The grape clusters sprayed with 1.5 and 2.0 mM SA maintained a significantly lower shatter percentage up to 60 DOS and after this point clusters treated with the highest dose (2.0 mM) of SA showed significantly higher shatter percentage. By the end of experiment, percent cumulative berry shatter of control (0.0), 1.0, 1.5 and 2.0 mM samples were 10.90, 9.62, 5.38 and 7.16 respectively. Correlation analysis (Table 3) showed that there is a strong positive correlation between PLW and berry shatter indicating that water loss causes not only berry softening but also berry shattering. Effects of SA on inhibition of ethylene biosynthetic enzymes are well documented (Srivastava and Dwivedi 2000; Zhang et al. 2003; Babalar et al. 2007). The suppression of formation of ethylene which is the primary trigger of abscission process (Taiz and Zeiger 2010) might cause reduction in berry shatter in treated clusters in contrast to control.
Decay incidence
With the treatments of SA, decay incidence was significantly low up to 30 DOS (<0.5 %) whereas, in control samples percent decay incidence was ≈ 2 % at this stage (Fig. 4c). After 45 DOS, it increased dramatically despite the treatments. However, SA treated berries maintained lower rate of decay development in comparison to control throughout the storage period, out of which 1.5 and 2 mM SA displayed higher efficacy. Preharvest treatment of SA was found to increase β-1, 3-glucanase, phenylalanine ammonia-lyase (PAL) and peroxidase (POD) activity (Yao and Tian 2005) and other defense related compounds such as chitinase, and glutathione (Cao et al. 2006) which might account on suppression of diseases by SA. Lower decay incidence of strawberry (Babalar et al. 2007; Shafiee et al. 2010) and plums (Luo et al. 2011) have been observed with treatments of SA.
Membrane electrolyte leakage
One of the common features accompanying chilling and senescence is increased membrane permeability, expressed as increasing leakage of ions which is used as an indicator of membrane damage. All SA treated grape clusters exhibited a significantly lower membrane electrolyte leakage (MEL) rate, compared to the control (Fig. 4d). Among three concentrations examined, SA at the dose of 1.5 mM displayed a higher potential of inhibiting MEL on the contrary to other two doses. By the end of 60 days, MEL rate of control and 1.0 mM SA were ≥ 35 μS/100 g FW while this rate in berries treated with 1.5 and 2.0 mM SA were ≤ 28 μS/100 g FW. Our results are supported by the findings of Luo et al. (2011) who observed a lower MEL in ‘Qingnai’ plums (Prunus salicina) when treated with SA.
Rachis browning
Rachis quality of grape bunches has been gaining more interest among producers and exporters because of its high impact on the cluster freshness that determines consumer perception (Balic et al. 2012). SA at the concentrations of 1.5 and 2.0 mM effectively repressed rachis browning up to 60 DOS on the contrary to control in which noticeable browning of pedicels and secondary rachises were observed at this stage (Table 5). Evidently, the results showed that, SA has a better potential in achieving beneficial effects against rachis browning of detached grape clusters. This improvement might account by the direct effect of SA on suppression of ethylene production, which is the main hormone triggering chlorophyll loss and colour fading (Taiz and Zeiger 2010). Moreover, positive effect of SA on reducing water loss and suppression of polyphenol oxidase enzyme activity (Lu et al. 2011; Tareen et al. 2012) might also account for maintaining healthy rachis in SA treated clusters.
Table 5.
Rachis browning and organoleptic rating of Flame Seedless berries subjected to different treatments of salicylic acid at initial harvest (day 0) and during cold storage (3–4 °C, 90–95 % RH)
| Parameter | Time (days of storage) | ||||
|---|---|---|---|---|---|
| 0 | 30 | 45 | 60 | 75 | |
| Rachis browninga | |||||
| Concentration (mM) | |||||
| 0.0 | 1.0 | 1.8 | 2.9 | 3.5 | 4.0 |
| 1.0 | 1.0 | 1.2 | 2.1 | 3.3 | 3.8 |
| 1.5 | 1.0 | 1.1 | 1.8 | 3.0 | 3.6 |
| 2.0 | 1.0 | 1.1 | 1.8 | 3.0 | 3.9 |
| P | 0.08 NS | 0.03 | 0.02 | 0.02 | 0.05 |
| Organoleptic ratingb | |||||
| Concentration (mM) | |||||
| 0.0 | 9.0 | 7.35 | 6.37 | 5.29 | 2.91 |
| 1.0 | 9.0 | 8.10 | 7.01 | 6.37 | 4.68 |
| 1.5 | 9.0 | 8.67 | 8.11 | 7.48 | 5.33 |
| 2.0 | 9.0 | 8.76 | 8.21 | 7.13 | 5.28 |
| P | 0.08 NS | 0.001 | 0.001 | 0.001 | 0.01 |
a1 = healthy, entire rachis including the pedicels are green and healthy, 2 = slight, rachis in good condition, but noticeable browning of pedicels, 3 = moderate, browning of pedicels and secondary rachis, 4 = severe, pedicels, secondary and primary rachis completely brown
b9 = extremely desirable, 8 = very much desirable, 7 = moderately desirable, 6 = slightly desirable, 5 = neither desirable nor undesirable, 4 = slightly undesirable, 3 = moderately undesirable, 2 = very much undesirable, 1 = extremely undesirable
SA treatments suppressed decline in sensory quality significantly (Table 5) during prolonged cold storage (3–4 °C, 90–95 % RH) on the contrary to control. Berries in the grape clusters treated by 1.5 and 2.0 mM SA had higher overall acceptability rating in the taste panel, at each storage interval. However, berries which received the lowest dose of SA (1.0 mM) had lower scores from 60 days onwards exhibiting parallel effects as that of control sample mainly because of development of off flavours. Organoleptic properties of berries in the control group reduced drastically after 45 DOS, as a consequence of poor texture and development of off flavours.
Conclusions
At initial harvest, salicylic acid (SA) hastened maturity of Flame Seedless grapes by 2 to 5 days, reduced cluster compactness, increased berry size and firmness significantly. During subsequent cold storage (3–4 °C, 90–95 % RH), SA demonstrated higher efficacy on maintaining peel colour, stabilizing anthocyanins, reducing the rate of berry softening, rachis browning alongside effectively suppressing other ripening related changes. Moreover, it maintained higher phenol content, reduced decay incidence, enzymatic activity of pectin methyl esterase and membrane electrolyte leakage. On the whole, SA at the dose of 1.5 mM improved berry physicochemical properties at harvest and extended the postharvest life of table grape cv. Flame Seedless by 15 days on the contrary to control, which was commercially acceptable only up to 45 days.
References
- Amerine MA, Pangborn RM, Roessler EB (1965) Principles of sensory evaluation of food. In: Food science and technology monographs, Academic Press, New York, p 338–339
- Anon (2010) Package of practices for cultivation of fruits. Punjab Agricultural University, Ludhiana, Punjab, India, p 63–74
- AOAC (2005) Official method of analysis of AOAC International. 18th edn, Suite 500 481, North frederick avenue, Gaithersburg, Maryland 20877–2417. USA
- Babalar M, Asghari M, Talaei A, Khosroshahi A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of selva strawberry fruit. Food Chem. 2007;105:449–453. doi: 10.1016/j.foodchem.2007.03.021. [DOI] [Google Scholar]
- Balic I, Moreno A, Sanhueza D, Huerta C, Orellana A, Defillippi BG, Campos-Vagas R. Molecular and physiological study of postharvest rachis browning of table grape cv. Red globe. Postharvest Biol Tec. 2012;72:47–56. doi: 10.1016/j.postharvbio.2012.05.005. [DOI] [Google Scholar]
- Barnavon L, Docoa T, Terrierb N, Ageorgesb A, Romieub C, Pellerina P. Involvement of pectin methyl-esterase during the ripening of grape berries: partial cDNA isolation, transcript expression and changes in the degree of methyl-esterification of cell wall pectins. Phytochemistry. 2001;58:693–701. doi: 10.1016/S0031-9422(01)00274-6. [DOI] [PubMed] [Google Scholar]
- Bermstein Z, Lustig I. A new method of firmness measurement of grape berries and other juicy fruits. Vitis. 1981;20:15–21. [Google Scholar]
- Cao J, Zeng K, Jiang W. Enhancement of postharvest disease resistance in Ya Li pear (Pyrus bretschneideri) fruit by salicylic acid sprays on the trees during fruit growth. Eur J Plant Pathol. 2006;114:363–370. doi: 10.1007/s10658-005-5401-8. [DOI] [Google Scholar]
- Chamkha M, Cathala B, Cheynier V, Douillard R. Phenolic composition of champagnes from Chardonnay and Pinot Noir vintages. J Agr Food Chem. 2003;51:3179–3184. doi: 10.1021/jf021105j. [DOI] [PubMed] [Google Scholar]
- Crisosto CH, Garner D, Crisosto G. Carbon dioxide-enriched atmospheres during cold storage limit losses from Botrytis but accelerate rachis browning of Red Globe table grapes. Postharvest Biol Tec. 2002;26:181–189. doi: 10.1016/S0925-5214(02)00013-3. [DOI] [Google Scholar]
- Elwan MWM, El-Hamahmy MAM. Improved productivity and quality associated with salicylic acid application in greenhouse pepper. Sci Hortic. 2009;122:521–526. doi: 10.1016/j.scienta.2009.07.001. [DOI] [Google Scholar]
- FAOstat (2011) FAO statistical database, Food and agricultural organization of the United Nations. http://www.faostat.org/site/340/ Date cited: 2013-03-13
- Fernandez-Lopez J, Almela L, Munoz JA, Hidalgob V, Carreno J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Res Int. 1998;31:667–672. doi: 10.1016/S0963-9969(99)00043-5. [DOI] [Google Scholar]
- Hayat Q, Hayat S, Irfan M, Ahmad A. Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot. 2010;68:14–25. doi: 10.1016/j.envexpbot.2009.08.005. [DOI] [Google Scholar]
- Jamali B, Eshghi S, Taffazoli E. Vegetative growth, yield, fruit quality and fruit and leaf composition of strawberry cv. ‘Pajaro’ as influenced by salicylic acid and nickel sprays. J Plant Nutr. 2013;36:1043–1055. doi: 10.1080/01904167.2013.766803. [DOI] [Google Scholar]
- Jiang Y, Shiina T, Nakamura N, Nakahara A. Electrical conductivity evaluation of postharvest strawberry damage. J Food Sci. 2001;66:1392–1396. doi: 10.1111/j.1365-2621.2001.tb15220.x. [DOI] [Google Scholar]
- Karlidag H, Yildirim E, Turan M. Exogenous applications of salicylic acid affect quality and yield of strawberry grown under antifrost heated greenhouse conditions. J Plant Nutr Soil Sci. 2009;172:270–276. doi: 10.1002/jpln.200800058. [DOI] [Google Scholar]
- Lu X, Sun D, Li Y, Shi W, Sun G. Pre- and post-harvest salicylic acid treatments alleviate internal browning and maintain quality of winter pineapple fruit. Sci Hortic. 2011;130:97–101. doi: 10.1016/j.scienta.2011.06.017. [DOI] [Google Scholar]
- Luo Z, Chen C, Xie J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Postharvest Biol Tec. 2011;62:115–120. doi: 10.1016/j.postharvbio.2011.05.012. [DOI] [Google Scholar]
- Mady MA. Effect of foliar application with salicylic acid and vitamin E on growth and productivity of tomato (Lycopersicon esculentum Mill.) plant. J Agric Sci Mansoura Univ. 2009;34:6735–6746. [Google Scholar]
- Marzouk HA, Kassem HA. Improving yield, quality and shelf life of Thompson seedless grapevine by preharvest foliar applications. Sci Hortic. 2011;130:425–430. doi: 10.1016/j.scienta.2011.07.013. [DOI] [Google Scholar]
- Peppi MC, Fidelibus MW, Dokoozlian N. Abscisic acid application timing and concentration affect firmness, pigmentation and colour of flame seedless grapes. Hortscience. 2006;41:1440–1445. [Google Scholar]
- Ranganna S (1986) Anthocyanin. In: Handbook of analysis and quality control of fruit and vegetable products. Tata McGrow Hill Publishing Co.Ltd, 7 west Patel Nagar, New Delhi, 110 008. pp 94–99
- Roberto SR, Marinho de Assis A, Yamamoto LY, Miotto LCV, Sato AJ, Renata K, Werner G. Application timing and concentration of abscisic acid improve color of ‘Benitaka’ table grape. Sci Hortic. 2012;142:44–48. doi: 10.1016/j.scienta.2012.04.028. [DOI] [Google Scholar]
- Rolle L, Simone G, Vincenzo G, Vittorino N. Comparative study of texture properties characteristics, and chemical of ten white table-grape varieties. Am J Enol Vitic. 2011;62:49–56. doi: 10.5344/ajev.2010.10029. [DOI] [Google Scholar]
- Sarikhani H, Sasani-Homa R, Bakshi D. Effect of salicylic acid and SO2 generator pad on storage life and phenolic contents of grape (Vitis vinifera L. Bidaneh Sefid and Bidaneh Ghermez) Acta Horticult. 2010;877:1623–1630. [Google Scholar]
- Shafiee M, Taghave TS, Babalar M. Application of SA to nutrient solution combined with postharvest treatments (hot water, SA and Ca dipping) improved postharvest fruit quality of strawberry. Sci Hortic. 2010;124:40–45. doi: 10.1016/j.scienta.2009.12.004. [DOI] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–53. [Google Scholar]
- Srivastava KM, Dwivedi UN. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000;158:87–96. doi: 10.1016/S0168-9452(00)00304-6. [DOI] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2010) Ethylene In: Plant physiology, International edn, Sinauer Associates Inc., Publishers, Sunderland, Massachusetts USA, pp 519–537
- Tareen MJ, Abbasi NA, Hafiz IA. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci Hortic. 2012;142:221–228. doi: 10.1016/j.scienta.2012.04.027. [DOI] [Google Scholar]
- Yao H, Tian S. Effects of pre and postharvest applications of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit storage. Postharvest Biol Tec. 2005;35:253–262. doi: 10.1016/j.postharvbio.2004.09.001. [DOI] [Google Scholar]
- Yıldırım E, Dursun A. Effect of foliar salicylic acid applications on plant growth and yield of tomato under greenhouse conditions. Acta Horticult. 2009;807:395–400. [Google Scholar]
- Zhang Y, Kunsong C, Zhang S, Ferguson I. The role of salicylic acid in postharvest ripening of kiwi fruit. Postharvest Biol Tec. 2003;28(1):67–74. doi: 10.1016/S0925-5214(02)00172-2. [DOI] [Google Scholar]
- Zoffoli JP, Latorre BA, Naranjo P. Pre-harvest applications of growth regulators and their effect on postharvest quality of table grapes during cold storage. Postharvest Biol Tec. 2009;51:183–192. doi: 10.1016/j.postharvbio.2008.06.013. [DOI] [Google Scholar]