Abstract
The effect of dehydrated thermal treatment on the tilapia scale gelatin films was investigated to improve their water resistance and mechanical properties. The gelatin extracted from tilapia scales was mainly composed of β-chain, α-chain and their degraded products with imino acid content of 21.2 %. When the films prepared from tilapia scale gelatin were heated at 80 °C, no significant changes in the properties of films were observed. As heating temperature was increased to 100 or 120 °C, the tensile strength of films was increased gradually with increasing thermal treatment time, while film solubility and protein solubility were decreased. Based on the SDS-PAGE analysis and the protein solubility of the gelatin films in various protein denaturant solutions, it was found that the cross-linking in the gelatin film network between β-chain and α-chain could be induced by heating at 120 °C. It was revealed that the main interactions involved in the gelatin film formation were changed from ionic bonds and hydrogen bonds to hydrophobic interactions and covalent bonds, leading to improve water resistance properties of films.
Keywords: Edible gelatin films, Tilapia scales, Dehydrated thermal treatment, Water resistance ability, Mechanical properties
Introduction
Synthetic plastic films have been widely used as food packaging materials, due to their beneficial properties such as strength, lightness and transparency. However, almost all plastics are non-biodegradable, and their excessive use has led to serious environmental problems. Moreover, there is an increasing worry about the potential risk that plasticizer such as phthalate used in plastics can leach out into foodstuffs. Therefore, there is an urgent need to find substitute such as renewable resources to produce edible or biodegradable films that can provide barrier and mechanical protection for food products with a purpose to reduce the use of food plastic packaging materials.
Simultaneously, the output of fresh-water fish in China increases rapidly and has reached up to 21.8 million tons in 2011 (Anonymous 2012). Along with the change in dietary habits of consumers, a trend of utilizing fresh-water fish is to prepare steaks, fillets and surimi products. As a result, a large amount of byproducts are generated in the fish processing factories. Nevertheless, fish byproducts e.g. scales are presently generally discarded in China, representing a very large resource without being utilized properly or responsibly. Moreover, improper disposal of fish scales can cause serious environmental pollution. Fish scales are rich in collagen and gelatin, which have been widely used in food industries as ingredients to improve properties of foods and also can be used for encapsulation and edible film formation (Karim and Bhat 2009). It has been reported that fish gelatin exhibits excellent film-forming property among biopolymers including sarcoplasmic protein, myofibrillar protein, soy protein, whey protein, polysaccharides and lipids (Jongjareonrak et al. 2006; Muyonga et al. 2004; Shiku et al. 2003, 2004). Although there are many reports about the extraction and characterization of gelatin from fish scales (Sreejith et al. 2014; Wangtueai and Noomhorm 2009; Zhang et al. 2011), most previous studies concerning fish gelatin based films were mainly limited to fish skin. Therefore, preparation of edible gelatin films from fish scales not only can efficiently increase their potential value, but also provide a new source for edible film production.
Fish gelatin films present poorer mechanical properties than mammalian gelatin films and some synthetic films such as oriented polypropylene (OPP) films (Gómez-Guillén et al. 2009; Jongjareonrak et al. 2006; Shiku et al. 2003), resulting in the restriction of their wide application as packaging materials. Consequently, several studies have been carried out with an attempt to improve the performance of gelatin films by addition of cross-linking agents such as aldehydes (Bigi et al. 2001), transglutaminase or 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide (Kołodziejska and Piotrowska 2007; Yi et al. 2006). However, the toxicity and high cost of some agents have limited their practical application. In comparison, physical cross-linking methods, such as heat treatment, UV and γ irradiation (Jo et al. 2005; Matsuda et al. 2002), could thus be used as an alternative method to improve properties of gelatin films from the standpoint of food safety and economy. The effect of heat treatment on the properties of fish gelatin films was only carried out through heating film-forming solution (Hoque et al. 2010). There is little information concerning dehydrated thermal treatment of films on the properties of gelatin films prepared from fish scales.
Therefore, the present study was undertaken to extract and characterize gelatin from tilapia scales which are one of the most serious fresh-water fish processing wastes. The effect of dehydrated thermal treatment on the properties of edible films based on tilapia scale gelatin was also evaluated.
Materials and methods
Materials
The scales were removed from fresh tilapia (Tilapia zillii) during fish filleting in Xiamen Tongan Yuanshui Sea Products Co., Ltd., Fujian province, China. The tilapia scales were brought to the laboratory on ice within 1 h. Upon arrival, scales were washed with tap water, then blast frozen at −40 °C and stored at −18 °C during the study.
Gelatin extraction
Gelatin was eхtracted from tilapia scales according to the method of Limpisophon et al. (2009) with some modifications. Scales were soaked in a miхed solution containing 0.05 M NaOH and 25 % alcohol with a scale/solution ratio of 1:4 (w/v), then placed at 10 °C overnight to remove lipids and non-collagenous proteins. Alkaline treated scales were washed with tap water to achieve the neutral pH. Demineralisation of tilapia scales was carried out using 5 volumes of 0.05 M HCl solution for 2 h at room temperature. Then, the demineralised tilapia scales were washed to neutral pH with tap water. After pretreatment, the swollen scales were soaked in distilled water at 80 °C for 1 h to extract the gelatin. The extract was centrifuged at 15,000×g, 20 °C for 30 min with a refrigerated centrifuge (Avanti J-25; Beckman, California, USA). The supernatant of gelatin extract was freeze-dried and the gelatin powder obtained was stored at −18 °C until use.
Characterization of tilapia scale gelatin
Determination of proximate components
Tilapia scale gelatin powder was subjected to proximate analyses including moisture, crude protein and ash contents according to the AOAC methods (AOAC 2005).
Determination of amino acid and protein subunit compositions
Gelatin powder was hydrolyzed under reduced pressure in 6.0 M HCl at 110 °C for 24 h. After hydrolysis, HCl was removed and the amino acid composition of an aliquot was analyzed by a HITACHI model 835-50 high speed amino acid analyzer (HITACHI Co., Tokyo, Japan). The content of hydroxyproline in the same sample was determined by a hydroxyproline assay kit (Nanjing JianCheng Bioengineering Institute, Nanjing, China). Additionally, the protein subunit composition of scale gelatin was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970).
Preparation of gelatin films
Gelatin powder was dissolved with distilled water at 60 °C for 30 min to obtain the final protein concentration of 2 % (w/v) determined by the Lowry method (Lowry et al. 1951). The solution was added with glycerol as a plasticizer at the concentration of 20 % (w/w) of protein. The air bubbles were removed from film-forming solution (FFS) before casting by a hybrid miхer (UM-113; Unix Co., Tokyo, Japan). Then FFS was poured onto a rimmed silicone resin plate (50 × 50 mm) placing on a level surface and dried in an environmental chamber (PSX-330H; Laifu Technology Co., Ltd., Ningbo, China) at 25 ± 0.5 °C and 50 ± 5 % relative humidity (RH) for 24 h. Then the resulting films were manually peeled off.
Dehydrated thermal treatment of gelatin films
To investigate the effect of dehydrated thermal treatment on the properties of scale gelatin films, films were dehydrated at 80, 100 or 120 °C with a ventilated oven (DHG-9070A; Pudong Laifeng scientific instruments Co., Ltd., Shanghai, China) for 0.5 h, 1 h, 2 h, 4 h, 6 h, respectively.
Gelatin films assays
Preparation of samples
Prior to physical properties analyses, gelatin films were conditioned at 50 ± 5 % RH and 25 ± 0.5 °C for 48 h. For differential scanning calorimetry (DSC) analyses, films were ground into powder under liquid nitrogen and then conditioned in a dessicator containing dried silica gel for more than 2 weeks at room temperature to obtain the most dehydrated samples.
Mechanical properties
The film thickness was measured using a micrometer (Thickness Gauge; Ozaki MFG Co., Tokyo, Japan) to the nearest 0.001 mm at five random locations of the films. Tensile strength (TS) and elongation at break (EAB) were measured using a texture analyzer (TMS-PRO; Food Technology Co., USA) with 100 N load cell. The tests were operated according to the method described by Iwata et al. (2000) with a slight modification. Two rectangular strips (width 15 mm; length 45 mm) were cut from each gelatin film to measure the mechanical properties. Initial grip separation and mechanical crosshead speed were set at 30 mm and 60 mm/min, respectively. TS (MPa) was calculated by dividing the maximum load (N) necessary to pull the sample film apart by the cross-sectional area (m2). EAB (%) was calculated by dividing film elongation at the moment of rupture by the initial grip length of sample and multiplied by 100. Ten samples of each film type were used for testing.
Color
Color values of gelatin films were determined using a CIE colorimeter (WSC-S; Shanghai Precision & Scientific Instrument Co., China). Color values of the gelatin films were expressed as L* (lightness/brightness), a* (redness/greenness) and b* (yellowness/blueness).
Total soluble matter (TSM) and protein solubility (PS)
The TSM of gelatin films was determined using the method reported by Núñez-Flores et al. (2012) with a slight modification. The conditioned film samples (50 mm × 50 mm) were weighed and placed in the conical flasks containing 10 ml distilled water with 0.1 % (w/v) sodium azide. The flasks were sealed and then shaken gently and continuously at 30 °C for 24 h. Undissolved debris film matter was dried at 105 °C for 24 h. The weight of solubilized dry matter was calculated by subtracting the weight of insolubilized dry matter from the initial weight of dry matter. Actual protein content of gelatin films was measured according to the Kjeldahl method (AOAC 2005). Protein concentration in the solvent was measured using the Lowry method (Lowry et al. 1951), with a commercial Reagent (Bio-Rad Laboratories, Hercules, USA). Protein solubility was expressed as percentage of total protein in the films which were solubilized.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
Protein patterns of gelatin films were analyzed using SDS-PAGE according to the method described by Laemmli (1970) with a slight modification. A 4 % stacking gel and 6 % separating gel were used. Gels were stained with 0.025 % Coomassie Brilliant Blue R-250 (Merck, Darmstadt, Germany) in methanol/acetic acid/water (5:10:85 %, v/v/v), then destained in methanol/acetic acid/water (30:10:60 %, v/v/v). PageRulerTM Protein Ladder (Fermentas Life Sciences, Hanover, MD, USA) ranging from 10 to 200 kDa was used as a standard protein marker.
Protein subunits solubilized in various solvents
Gelatin films were solubilized in three different solutions at pH 7 according to the method of Pérez-Mateos et al. (1997) About 50 mg of film powders were homogenized at 16,000 rpm with 5 mL of S1 (0.6 M NaCl) for 2 min using a high speed homogenizer (Fluko FA25; Shanghai, China). The resulting homogenized solution was centrifuged at 15,000×g for 30 min. The pellet obtained was homogenized in S2 (0.6 M NaCl + 1.5 M urea) by the same process, and then again in S3 (0.6 M NaCl + 8 M urea). The protein concentration of supernatants was determined by the method of Lowry et al. (1951) using a commercial Assay Reagent (Bio-Rad Laboratories, Hercules, USA). The protein subunits of supernatants were determined by SDS-PAGE (Laemmli 1970). The samples (about 5 μg) were applied to sample wells and electrophoresed along with a protein standard.
DSC
Thermal properties of gelatin films were determined using differential scanning calorimeter (DSC-1, Mettler Toledo instruments Co., Zurich, Switzerland). Temperature calibration was performed using the Indium thermogram. The film powder (5–10 mg) was accurately weighed into aluminum pans and sealed, then scanned over the range of from −25 to 200 °C at a basic heating rate of 10 °C/min. An empty aluminum was used as a reference. The glass transition temperature (Tg) of gelatin films were obtained from the inflexion point of reversible heat flow the thermograms in the second scan.
Statistical analysis
Statistical analysis on a completely randomized experimental design was performed using the General Linear Model procedure in the SPSS computer program (SPSS Statistical Software, Chicago, USA). One-way analyses of variance (ANOVA) were carried out and mean comparisons were processed by Tukey-Kramer Honestly Significant Difference (HSD) test. Significance is defined at p < 0.05.
Results and discussion
Characterization of tilapia scale gelatin
The contents of moisture, crude protein and ash of tilapia scale gelatin extracted in this study were 12.3, 85.6 and 1.9 %, respectively. The amino acid composition of gelatin from tilapia scales is also determined and given in Table 1. The glycine content was most abundant, which was similar to that of gelatins from sardine, red sea bream and Japanese sea bass scales (Nagai et al. 2004). The imino acid (proline and hydroxyproline) content of tilapia scale gelatin was 212 residues per 1,000 amino acid residues, which was similar to that of warm-water fish gelatins (Nagai et al. 2004; Avena-Bustillos et al. 2006) and higher than that of tilapia skin gelatin (Weng et al. 2013). Furthermore, the content of alanine was also remarkably high in the tilapia scale gelatin. It has been reported that gelatin with higher content of imino acid and alanine exhibited better viscoelastic properties and ability to develop the triple-helix structures, which were positively relative to the film-forming properties of gelatin (Giménez et al. 2009).
Table 1.
Amino acid composition of gelatin from tilapia scales
| Amino acids | Residues/1,000 residues |
|---|---|
| Glycine | 336 |
| Proline | 142 |
| Alanine | 148 |
| Hydroxyproline | 70 |
| Glutamic acid | 66 |
| Arginine | 56 |
| Aspartic acid | 41 |
| Lysine | 23 |
| Serine | 27 |
| Leucine | 13 |
| Threonine | 12 |
| Phenylalanine | 10 |
| Valine | 19 |
| Tyrosine | 12 |
| Methionine | 5 |
| Isoleucine | 8 |
| Histidine | 5 |
As well, it is known that the molecular weight distribution and amino acid composition of gelatin contribute to the film-forming ability of gelatin (Gómez-Guillén et al. 2009), thus the protein patterns of tilapia scale gelatin were determined using SDS-PAGE. As shown in Fig. 1, proteins with molecular weight of around 230, 120 and 110 kDa were identified in the tilapia scale gelatin, which were respectively regarded as β, α1, α2 chains of type I collagen (Duan et al. 2009). Another two protein bands (~160 and ~80 kDa) were also observed in tilapia scale gelatin. It is worth noting that a similar phenomenon in tilapia skin gelatin protein fractions have been considered to be degraded from intrachain peptide bonds (Zhou et al. 2006).
Fig. 1.

SDS-PAGE patterns of gelatin extracted from tilapia scales. β: β-chain; α1: α1-chain; α2: α2-chain. M: Protein marker
Physical properties of tilapia scale gelatin films
Effects of dehydrated thermal treatment on the total soluble matter (TSM) and protein solubility (PS) of scale gelatin films were determined (Table 2). Gelatin films from tilapia scales had less TSM than gelatin films from deep water fish skin (Núñez-Flores et al. 2012). No change was observed in either TSM or PS when gelatin films were heated at 80 °C. However, the TSM and PS of gelatin films were decreased with increasing thermal treatment time at 100 or 120 °C. It has been reported that thermal treatment might lead to form cross-linking of collagen between carboxyl and primary amino side groups in the drying condition at elevated temperature (Weadock et al. 1983). When proteins were strongly involved in the film network, PS of protein films was decreased (Rhim et al. 2000). Therefore, the lower TSM and PS are mostly ascribable to the cross-linking in the tilapia scale gelatin films induced by thermal treatment (Table 2).
Table 2.
Effect of thermal treatment of films on the total soluble matter (TSM) and protein solubility (PS) of tilapia scale gelatin films
| Heating temperature (°C) | Heating time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | 6.0 | ||
| TSM (%) | 80 | 54.80 ± 0.96a | 55.48 ± 0.95a | 54.86 ± 0.35a | 55.29 ± 0.24a | 55.47 ± 0.22a | 55.04 ± 0.02a |
| 100 | 54.80 ± 0.96a | 52.34 ± 2.88ab | 51.14 ± 0.50ab | 52.66 ± 2.83ab | 50.01 ± 2.99b | 45.32 ± 4.53b | |
| 120 | 54.80 ± 0.96a | 46.62 ± 1.05b | 36.10 ± 1.60c | 34.47 ± 1.57c | 33.53 ± 1.47c | 28.16 ± 0.09d | |
| PS (%) | 80 | 64.90 ± 1.66a | 64.89 ± 2.53a | 63.86 ± 2.60a | 63.56 ± 1.85a | 66.25 ± 3.02a | 66.57 ± 2.96a |
| 100 | 64.90 ± 1.66a | 66.25 ± 2.08a | 59.95 ± 1.96b | 61.19 ± 1.31b | 54.49 ± 2.53c | 45.01 ± 2.83c | |
| 120 | 64.90 ± 1.66a | 60.64 ± 2.21b | 52.82 ± 1.74c | 44.68 ± 1.63d | 38.12 ± 1.21e | 28.35 ± 1.05f | |
Any two means in the same row followed by the same letter are not significantly different (p > 0.05)
Data are expressed as mean ± standard deviation
Table 3 shows thickness, tensile strength (TS) and elongation at break (EAB) of tilapia scale gelatin films heated at 80 °C, 100 °C, 120 °C, respectively. No changes were found in the thickness of scale gelatin films irrespective of heating temperature. The TS of gelatin films without heating were 42.08 MPa, which was higher than that of shark skin gelatin films (TS = 27.29 MPa) (Limpisophon et al. 2009) and tilapia skin gelatin films (TS = 37.51 MPa) (Weng et al. 2013), due to the higher imino acid content (Karim and Bhat 2009). Furthermore, the TS of tilapia scale gelatin films was almost close to that of OPP films (TS = 50.7 MPa) (Shiku et al. 2003), suggesting that the gelatin films prepared from tilapia scales are a potential alternative to replace synthetic polymer films and may be applied as novel food packaging materials. When gelatin films were dehydrated at 80 °C, no changes in the TS of films were observed. In the case of 100 or 120 °C thermal treatment, the TS of gelatin films were increased with increasing thermal treatment time, while increased TS of films heated at 120 °C was higher than that of 100 °C, suggesting that the strength of films was mainly depended on dehydrated temperature. On the other hand, the EAB of films did neither change with heating at 80 nor 100 °C, while decreased by heating at 120 °C.
Table 3.
Effect of thermal treatment of films on the thickness, tensile strength (TS) and elongation at break (EAB) of tilapia scale gelatin films
| Heating temperature (°C) | Heating time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | 6.0 | ||
| Thickness (μm) | 80 | 33.15 ± 3.05a | 34.88 ± 5.81a | 34.30 ± 7.35a | 33.88 ± 5.45a | 33.90 ± 6.83a | 34.85 ± 6.13a |
| 100 | 33.15 ± 3.05a | 32.23 ± 4.17a | 33.82 ± 2.93a | 32.01 ± 2.68a | 35.18 ± 3.26a | 32.38 ± 2.14a | |
| 120 | 33.15 ± 3.05a | 35.05 ± 3.04a | 33.42 ± 3.30a | 35.48 ± 2.97a | 33.78 ± 3.25a | 34.71 ± 2.23a | |
| TS (MPa) | 80 | 42.08 ± 4.03a | 46.61 ± 2.98a | 46.99 ± 3.67a | 46.56 ± 4.69a | 47.50 ± 4.81a | 48.00 ± 4.78a |
| 100 | 42.08 ± 4.03a | 46.91 ± 4.08ab | 47.33 ± 4.64ab | 49.97 ± 3.31b | 50.06 ± 2.96b | 51.06 ± 4.68b | |
| 120 | 42.08 ± 4.03a | 49.18 ± 2.60b | 50.17 ± 3.43b | 56.02 ± 3.33c | 55.09 ± 2.29c | 61.52 ± 3.39d | |
| EAB (%) | 80 | 48.05 ± 4.89a | 48.25 ± 4.37a | 46.94 ± 3.85a | 48.28 ± 4.40a | 49.29 ± 3.65a | 48.13 ± 4.25a |
| 100 | 48.05 ± 4.89a | 45.38 ± 3.18a | 47.56 ± 4.64a | 48.55 ± 3.98a | 47.69 ± 4.00a | 45.09 ± 4.88a | |
| 120 | 48.05 ± 4.89b | 46.63 ± 4.57b | 42.93 ± 3.89ab | 41.58 ± 4.35ab | 36.13 ± 3.74a | 34.75 ± 4.50a | |
Any two means in the same row followed by the same letter are not significantly different (p > 0.05)
Data are expressed as mean ± standard deviation
The effect of dehydrated thermal treatment on color of scale gelatin films is shown in Table 4. The L*, a*, b* values of gelatin films without heating were 90.07, −1.02 and 2.12, respectively, which approaches to those of white standard (L* = 91.86, a* = −0.88, b* = 1.42), indicating that colorless films could be prepared from fish scale gelatin. Lower a* value and higher b* value were accompanied with heating on gelatin films irrespective of dehydrated temperature, suggesting that slight Millard reaction might be occurred during the dehydrated process (Manzocco et al. 2000). The results are similar to the change in color of soy protein film (Gennadios et al. 1996) and wheat gluten film (Hernandez-Munoz et al. 2004) induced by heating.
Table 4.
Effect of thermal treatment of films on the color of tilapia scale gelatin films
| Heating temperature (°C) | Heating time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | 6.0 | ||
| L* | 80 | 90.07 ± 0.18a | 89.96 ± 0.08a | 89.99 ± 0.06a | 89.99 ± 0.12a | 89.83 ± 0.42a | 90.04 ± 0.08a |
| 100 | 90.07 ± 0.18a | 90.08 ± 0.40a | 90.01 ± 0.17a | 89.86 ± 0.55a | 90.38 ± 0.10a | 89.92 ± 0.21a | |
| 120 | 90.07 ± 0.18a | 89.60 ± 0.30a | 89.70 ± 0.16a | 89.87 ± 0.19a | 89.86 ± 0.19a | 89.84 ± 0.19a | |
| a* | 80 | −1.02 ± 0.05a | −1.04 ± 0.05a | −1.10 ± 0.03b | −1.13 ± 0.03b | −1.17 ± 0.01c | −1.25 ± 0.04c |
| 100 | −1.02 ± 0.05a | −1.11 ± 0.02b | −1.18 ± 0.01bc | −1.24 ± 0.02c | −1.23 ± 0.04c | −1.28 ± 0.04c | |
| 120 | −1.02 ± 0.05a | −1.14 ± 0.02b | −1.20 ± 0.10b | −1.24 ± 0.02bc | −1.29 ± 0.03c | −1.32 ± 0.04c | |
| b* | 80 | 2.12 ± 0.14a | 2.25 ± 0.04a | 2.27 ± 0.02a | 2.34 ± 0.07ab | 2.36 ± 0.04b | 2.39 ± 0.05b |
| 100 | 2.12 ± 0.14a | 2.24 ± 0.06ab | 2.34 ± 0.10b | 2.45 ± 0.11bc | 2.48 ± 0.09bc | 2.60 ± 0.13c | |
| 120 | 2.12 ± 0.14a | 2.46 ± 0.12b | 2.55 ± 0.03b | 2.59 ± 0.10b | 2.81 ± 0.10c | 2.87 ± 0.09c | |
Any two means in the same row followed by the same letter are not significantly different (p > 0.05)
Data are expressed as mean ± standard deviation
It is obvious from Tables 2 and 3 that the physical properties of tilapia scale gelatin films were improved markedly when heating at 120 °C. Therefore, the dehydrated temperature of 120 °C was preferably selected for studying the mechanism of dehydrated thermal treatment effect.
Mechanism of dehydrated thermal treatment on gelatin films
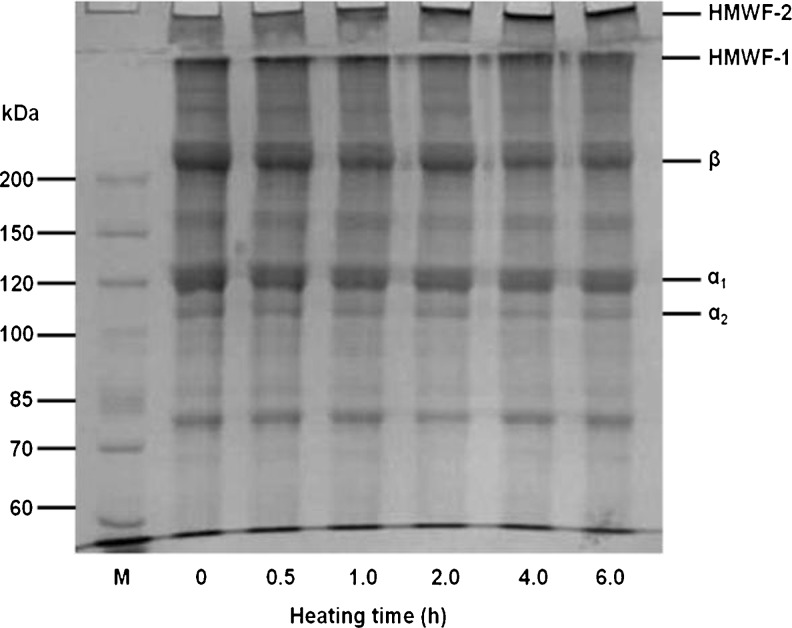
SDS-PAGE patterns of protein subunits in the tilapia scale gelatin films with or without thermal treatment at 120 °C were determined and depicted in Fig. 2. Compared with protein composition of tilapia scale gelatin (Fig. 1), it was clear that no protein bands disappeared or newly appeared in the gelatin films without thermal treatment. However, when gelatin films were heated at 120 °C, the band intensity of α1, β and high molecular weight fractions (HMWF) at the top of separating gel (HMWF-1) gradually reduced with the concomitant appearance of HMWF that were too large to enter the stacking gel (HMWF-2). This suggested that the cross-linking reaction might take place among the component of α1, β and HMWF-1 by heating at 120 °C, resulting in the lower TSM in water (Table 2) and higher film strength (Table 3). In general, proteins are sensitive to changes with temperature which could degrade, denature and cross-link proteins. However, no degraded protein subunit composition was observed in Fig. 2, indicating that dehydrated thermal treatment of films could be suitable to improve the properties of fish scale gelatin films.
Fig. 2.
SDS-PAGE patterns of tilapia scale gelatin films dehydrated at 120 °C. HMWF: High molecular weight fraction; β: β-chain; α1: α1-chain; α2: α2-chain. M: Protein marker
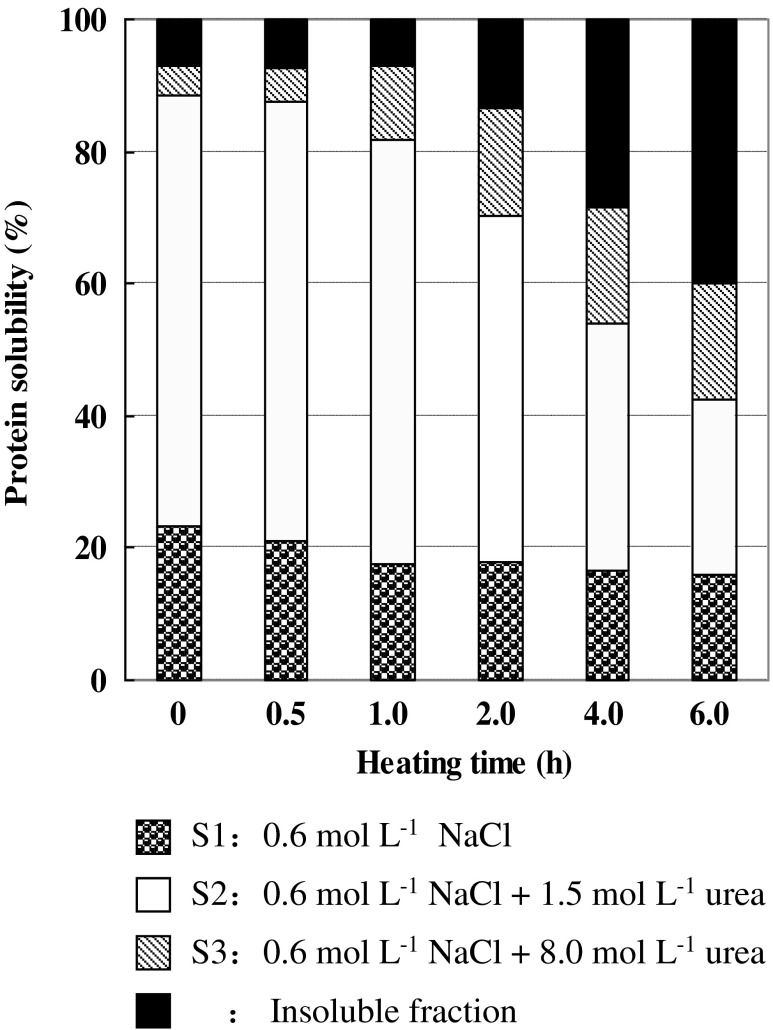
The film formation from proteins is believed to involve a tri-dimensional network of protein molecules by ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide bonds (Weng et al. 2007). The protein solubility of gelatin films in the following three different denaturing solutions was determined to reveal the associative forces involved in the formation of gelatin films dehydrated by 120 °C thermal treatment (Fig. 3). The denaturing solutions employed in this study were a 0.6 M NaCl solution (S1) that disrupts ionic bonds, a 0.6 M NaCl and 1.5 M urea solutions (S2) that disrupts hydrogen bonds, and a 0.6 M NaCl and 8 mol L−1 urea solution (S3) that disrupts hydrophobic interactions (Pérez-Mateos et al. 1997). Gelatin films without dehydrated thermal treatment were almost solubilized in S2, suggesting that the gelatin films are formed mainly through ionic bonds and hydrogen bonds. When gelatin films were heated at 120 °C, the protein fraction soluble in S2 was decreased with increasing heat treatment time, but the protein fraction soluble in S3 and insoluble fraction was increased, indicating that hydrophobic interactions and non-disulfide covalent bond mainly accounted for the forces involved in the formation of gelatin films. The results were in good agreement with those tendencies of film solubility in water (Table 2) and mechanical properties of films (Table 3).
Fig. 3.
Protein solubility of tilapia scale gelatin films dehydrated at 120 °C in different protein denaturant solutions
To further understand the film-forming characteristics of tilapia scale gelatin, the protein fractions of gelatin films solubilized in different denaturing solutions was analyzed using SDS-PAGE (Fig. 4). In the case of gelatin films without heating, the band intensity of α1, α2, β and some lower molecular weight composition of 100 and ~80 kDa were observed in S1. The protein fractions soluble in S2 were mainly composed of α, β, approximately 160 kDa fractions and HMWF. However, there was no protein bands observed in the S3. These results suggested that ionic bonds and hydrogen bonds were responsible to the interaction of protein fractions in the scale gelatin film formation. On the other hand, with increasing dehydrated thermal treatment time at 120 °C, the molecular weight fractions of higher than α2 reduced in S1 and S2 with appearance of them in S3, suggesting that those protein fractions involved in the gelatin film formation via hydrophobic interactions after dehydrated thermal treatment.
Fig. 4.
SDS-PAGE of soluble protein fractions of tilapia scale gelatin films dehydrated at 120 °C. HMWF: High molecular weight fraction; β: β-chain; α1: α1-chain; α2: α2-chain; S1: 0.6 mol L−1 NaCl; S2: 0.6 mol L−1 NaCl + 1.5 mol L−1 urea; S3: 0.6 mol L−1 NaCl + 8 mol L−1 urea. M: Protein marker
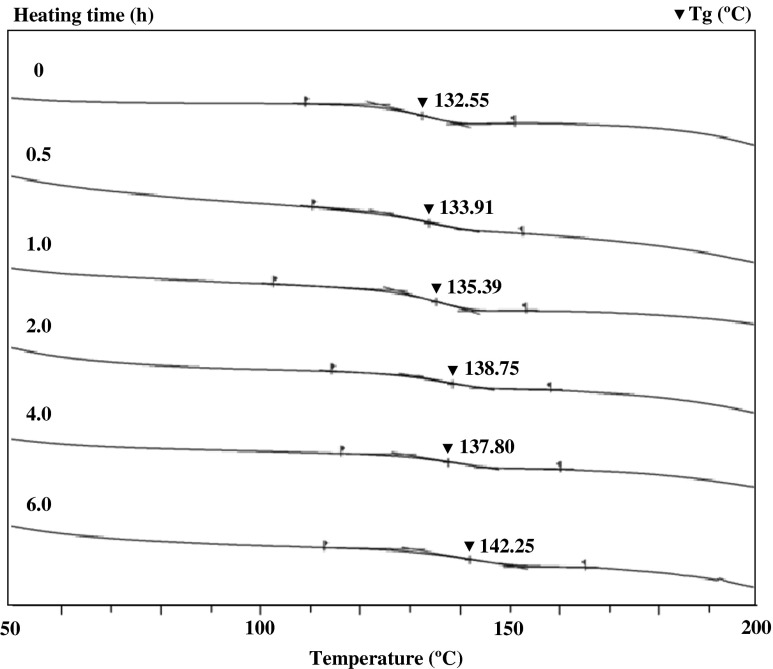
To determine the influence of cross-linking induced by heating on the thermal stability of films prepared from scale gelatin, DSC analyses were carried out on gelatin films whether heated at 120 °C or not (Fig. 5). Tg is an important parameter for determining the stability of edible biodegradable polymers (Ghanbarzadeh and Oromiehi 2009), and its value was found to have a positive relationship with the TS of protein films (Pommet et al. 2005). Thus, the strong films were obtained from tiliapia scale gelatin in the present study due to the high Tg of films. On the other hand, the Tg of scale gelatin films increased with increasing thermal treatment time at 120 °C (Fig. 5), probably attributed to the HMWF formation (Fig. 3) through covalent bonds and hydrophobic interactions induced by dehydrated thermal treatment of films.
Fig. 5.
DSC thermograms of tilapia scale gelatin films dehydrated at 120 °C. Tg: Glass transition temperature
Based on the results obtained in this study, the water resistance ability, mechanical properties and thermal stability of tilapia scale gelatin films could be enhanced with dehydrated thermal treatment of films at 100 °C or above. These results agree with earlier findings by Weng et al. (2013) who worked on edible films based on tilapia skin gelatin. However, the effect of dehydrated thermal treatment on the properties of tilapia scale gelatin films was more markedly than that of tilapia skin gelatin films.
Conclusion
Transparent colorless gelatin films with superior mechanical properties could be prepared from tilapia scales. The water resistance and mechanical properties of scale gelatin films were improved with dehydrated thermal treatment above 100 °C. Based on the results of SDS-PAGE and DSC, analysis of main interactions involved in the structure of gelatin films, it was found that HMWF in the gelatin films was formed through covalent bonds induced by heating of films at 120 °C, thus improving the thermal stability and mechanical properties of films. Present results show that tilapia scales can be used as an alternative source to prepare edible films, and their water resistance and mechanical properties can be improved by heating of films above 100 °C.
Acknowledgments
This work was sponsored by National Natural Science Fund (31271984) and Xiamen Science and Technology Project (3502Z20123025).
References
- Anonymous . China fisheries yearbook. Beijing: China Agricultural Press; 2012. [Google Scholar]
- AOAC (Association of Official Analytical Chemists) In: Official methods of analysis. Williams S, editor. Washington: AOAC; 2005. [Google Scholar]
- Avena-Bustillos RJ, Olsen CW, Olson DA, Chiou B, Yee E, Bechtel PJ, McHugh TH. Water vapor permeability of mammalian and fish gelatin films. J Food Sci. 2006;71:E202–E207. doi: 10.1111/j.1750-3841.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- Bigi A, Cojazzi G, Panzavolta S, Rubini K, Roveri N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials. 2001;22:763–768. doi: 10.1016/S0142-9612(00)00236-2. [DOI] [PubMed] [Google Scholar]
- Duan R, Zhang J, Du X, Yao X, Konno K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio) Food Chem. 2009;112:702–706. doi: 10.1016/j.foodchem.2008.06.020. [DOI] [Google Scholar]
- Gennadios A, Ghorpade VM, Weller CL, Hanna MA. Heat curing of soy protein films. Am Soc Agric Biol Eng. 1996;39:575–579. doi: 10.13031/2013.27537. [DOI] [Google Scholar]
- Ghanbarzadeh B, Oromiehi AR. Thermal and mechanical behavior of laminated protein films. J Food Eng. 2009;90:517–524. doi: 10.1016/j.jfoodeng.2008.07.018. [DOI] [Google Scholar]
- Giménez B, Gómez-Estaca J, Alemán A, Gómez-Guillén MC, Montero MP. Physico-chemical and film forming properties of giant squid (Dosidicus gigas) gelatin. Food Hydrocoll. 2009;23:585–592. doi: 10.1016/j.foodhyd.2008.07.003. [DOI] [Google Scholar]
- Gómez-Guillén MC, Pérez-Mateos M, Gómez-Estaca J, López-Caballero E, Giménez B, Montero P. Fish gelatin: a renewable material for developing active biodegradable films. Trends Food Sci Technol. 2009;20:3–16. doi: 10.1016/j.tifs.2008.10.002. [DOI] [Google Scholar]
- Hernandez-Munoz P, Villalobos R, Chiralt A. Effect of thermal treatments on functional properties of edible films made from wheat gluten fractions. Food Hydrocoll. 2004;18:647–654. doi: 10.1016/j.foodhyd.2003.11.002. [DOI] [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J Food Eng. 2010;96:66–73. doi: 10.1016/j.jfoodeng.2009.06.046. [DOI] [Google Scholar]
- Iwata K, Ishizaki S, Handa A, Tanaka M. Preparation and characterization of edible films from fish water-soluble proteins. Fish Sci. 2000;66:372–378. doi: 10.1046/j.1444-2906.2000.00057.x. [DOI] [Google Scholar]
- Jo C, Kang H, Lee NY, Kwon JH, Byun MW. Pectin- and gelatin-based film: effect of gamma irradiation on the mechanical properties and biodegradation. Radiat Phys Chem. 2005;72:745–750. doi: 10.1016/j.radphyschem.2004.05.045. [DOI] [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006;20:492–501. doi: 10.1016/j.foodhyd.2005.04.007. [DOI] [Google Scholar]
- Karim AA, Bhat R. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009;23:563–576. doi: 10.1016/j.foodhyd.2008.07.002. [DOI] [Google Scholar]
- Kołodziejska I, Piotrowska B. The water vapour permeability, mechanical properties and solubility of fish gelatin–chitosan films modified with transglutaminase or 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and plasticized with glycerol. Food Chem. 2007;103:295–300. doi: 10.1016/j.foodchem.2006.07.049. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Limpisophon K, Tanaka M, Weng WY, Abe S, Osako K. Characterization of gelatin films prepared from under-utilized blue shark (Prionace glauca) skin. Food Hydrocoll. 2009;23:1993–2000. doi: 10.1016/j.foodhyd.2009.03.014. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2000;11:340–346. doi: 10.1016/S0924-2244(01)00014-0. [DOI] [Google Scholar]
- Matsuda S, Se N, Iwata H, Ikada Y. Evaluation of the antiadhesion potential of UV cross-linked gelatin films in a rat abdominal model. Biomaterials. 2002;23:2901–2908. doi: 10.1016/S0142-9612(01)00418-5. [DOI] [PubMed] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus) Food Chem. 2004;85:81–89. doi: 10.1016/j.foodchem.2003.06.006. [DOI] [Google Scholar]
- Nagai T, Izumi M, Ishii M. Fish scale collagen. Preparation and partial characterization. Int J Food Sci Technol. 2004;39:239–244. doi: 10.1111/j.1365-2621.2004.00777.x. [DOI] [Google Scholar]
- Núñez-Flores R, Giménez B, Fernández-Martín F, López-Caballero ME, Montero MP, Gómez-Guillén MC. Role of lignosulphonate in properties of fish gelatin films. Food Hydrocoll. 2012;27:60–71. doi: 10.1016/j.foodhyd.2011.08.015. [DOI] [Google Scholar]
- Pérez-Mateos M, Lourenço H, Montero P, Borderías AJ. Rheological and biochemical characteristics of high-pressure- and heat-induced gels from blue white (Micromesistius poutassou) muscle proteins. J Agric Food Chem. 1997;45:44–49. doi: 10.1021/jf960185m. [DOI] [Google Scholar]
- Pommet M, Redl A, Guilbert S, Morel MH. Intrinsic influence of various plasticizers on functional properties and reactivity of wheat gluten thermoplastic materials. J Cereal Sci. 2005;42:81–91. doi: 10.1016/j.jcs.2005.02.005. [DOI] [Google Scholar]
- Rhim JW, Gennadios A, Handa A, Weller CL, Hanna MA. Solubility, tensile, and color properties of modified soy protein isolate films. J Agric Food Chem. 2000;48:4937–4941. doi: 10.1021/jf0005418. [DOI] [PubMed] [Google Scholar]
- Shiku Y, Hamaguchi PY, Tanaka M. Effect of pH on the preparation of edible films based on fish myofibrillar proteins. Fish Sci. 2003;69:1026–1032. doi: 10.1046/j.1444-2906.2003.00722.x. [DOI] [Google Scholar]
- Shiku Y, Hamaguchi PY, Benjakul S, Visessanguan W, Tanaka M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004;86:493–499. doi: 10.1016/j.foodchem.2003.09.022. [DOI] [Google Scholar]
- Sreejith S, Samant MP, Jakhar JK, Kothari DC, Venkateshwarlu G. Modeling the impact of extraction conditions on functional properties of gelatin from scales of blackspotted croaker (Protonibea diacanthus) Proc Natl Acad Sci India Sect B Biol Sci. 2014 [Google Scholar]
- Wangtueai S, Noomhorm A. Processing optimization and characterization of gelatin from Lizardfish (Saurida spp.) scales. LWT J Food Sci Technol. 2009;42:825–834. doi: 10.1016/j.lwt.2008.11.014. [DOI] [Google Scholar]
- Weadock K, Olson RM, Silver FH. Evaluation of collagen crosslinking techniques. Artif Cells Blood Substit. 1983;11:293–318. doi: 10.3109/10731198309118815. [DOI] [PubMed] [Google Scholar]
- Weng WY, Hamaguchi PY, Osako K, Tanaka M. Properties of edible surimi film as affected by heat treatment of film-forming solution. Food Sci Technol Res. 2007;13:391–398. doi: 10.3136/fstr.13.391. [DOI] [Google Scholar]
- Weng W, Wu F, Osako K, Su W. Dehydrated thermal treatment improving properties of edible films from tilapia skin gelatin. Trans CSAE. 2013;29:284–291. [Google Scholar]
- Yi J, Kim Y, Bae H, Whiteside W, Park H. Influence of transglutaminase-induced cross-linking on properties of fish gelatin films. J Food Sci. 2006;71:376–383. doi: 10.1111/j.1750-3841.2006.00191.x. [DOI] [Google Scholar]
- Zhang F, Xu S, Wang Z. Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod Process. 2011;89:185–193. doi: 10.1016/j.fbp.2010.05.003. [DOI] [Google Scholar]
- Zhou P, Mulvaney SJ, Regenstein JM. Properties of Alaska pollock skin gelatin: a comparison with tilapia and pork skin gelatins. J Food Sci. 2006;71:313–321. doi: 10.1111/j.1750-3841.2006.00065.x. [DOI] [Google Scholar]