Abstract
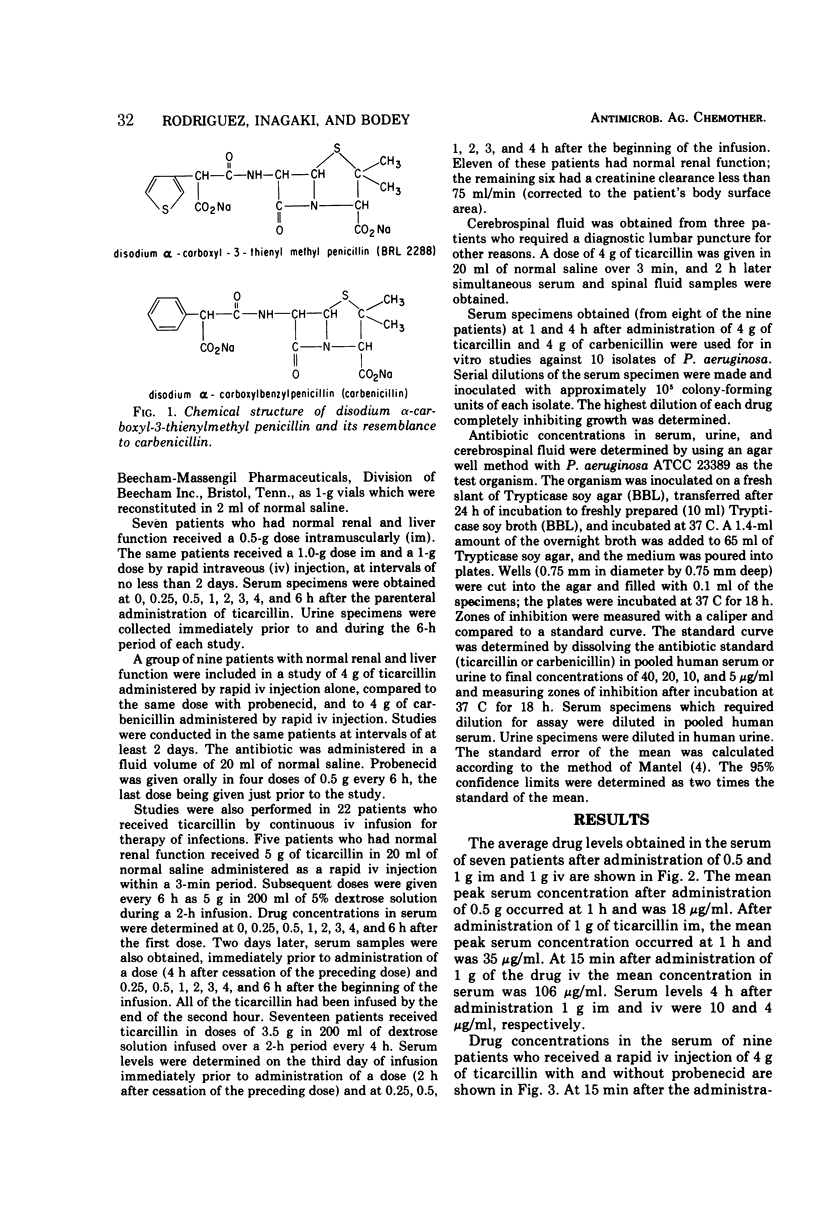
Clinical pharmacological studies of ticarcillin (α-carboxyl-3-thienylmethyl penicillin, BRL-2288) were conducted in patients with metastatic cancer and leukemia.After administration of 0.5 g intramuscularly, 1 g intramuscularly, and 1 g intravenously, the mean peak concentrations in serum were 18, 35, and 106 μg/ml, respectively. Greater than 80% of ticarcillin was excreted in the urine during the subsequent 6-h period. The mean concentrations in the serum of patients 15 min after they received an intravenous injection of 4 g of ticarcillin with and without probenecid were 508 and 519 μg/ml, respectively. Serum levels were determined in patients who received ticarcillin for therapy of infection in doses of 5 g every 6 h. The mean drug concentration in serum 15 min after the rapid administration of the first dose was 433 μg/ml. Subsequent doses were given during a 2-h infusion and the study was repeated 2 days later. The average initial serum level (4 h after the completion of the preceding dose) was 19 μg/ml, and the mean serum level at 15 min was 213 μg/ml. Drug concentrations in the serum of patients receiving ticarcillin by infusion in doses of 3.5 g every 4 h were also determined. In patients with normal renal function, the average initial serum level (2 h after completion of the preceding dose) was 49 μg/ml and the mean level at 15 min was 210 μg/ml. Drug concentrations in the serum of patients with impaired renal function were considerably higher. No detectable levels of ticarcillin were found in the cerebrospinal fluid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Deerhake B. In vitro studies of alpha-carboxyl-3-thienylmethyl penicillin, a new semisynthetic penicillin. Appl Microbiol. 1971 Jan;21(1):61–65. doi: 10.1128/am.21.1.61-65.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Terrell L. M. In vitro activity of carbenicillin against gram-negative bacilli. J Bacteriol. 1968 May;95(5):1587–1590. doi: 10.1128/jb.95.5.1587-1590.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Whitecar J. P., Jr, Middleman E., Rodriguez V. Carbenicillin therapy for pseudomonas infections. JAMA. 1971 Oct 4;218(1):62–66. [PubMed] [Google Scholar]
- Neu H. C., Swarz H. Carbenicillin: clinical and laboratory experience with a parenterally administered penicillin for treatment of Pseudomonas infections. Ann Intern Med. 1969 Nov;71(5):903–911. doi: 10.7326/0003-4819-71-5-903. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Burnett J., Rolinson G. N. -carboxy-3-thienylmethylpenicillin (BRL 2288), a new semisynthetic penicillin: in vitro evaluation. Antimicrob Agents Chemother (Bethesda) 1970;10:390–395. [PubMed] [Google Scholar]



